Journal of Cosmetics, Dermatological Sciences and Applications
Vol.1 No.4(2011), Article ID:8839,3 pages DOI:10.4236/jcdsa.2011.14027
Carnosine and N-Acetylcarnosine Induce Inhibition of UVB Erythema in Human Skin
![]()
Department of Systematic Pathology, Section of Dermatology University Federico II of Naples, Naples, Italy.
E-mail: *massimilianonino@yahoo.it
Received June 17th, 2011; revised August 5th, 2011; accepted August 27th, 2011.
Keywords: Carnosine, Human Skin, N-Acetylcarnosine, Inhibition of UVB Erythema
ABSTRACT
Background: Carnosine is a low molecular weight water soluble biological dipeptide, composed of alanine and histidine, present in a levorotatory form in mammalian tissues. Interesting activities are related to the detoxification from free radical species and byproducts of membrane lipids peroxidation. Objectives: The aim of the present study was to evaluate the photoprotective properties of carnosine and acetylated carnosine when applied to human skin. Materials and methods: Carnosine and N-acetylcarnosine at 0.5% solution in water were applied before and after UVB irradiation in twenty healthy volunteers with phototype 2 or 3. 9 patients were males and 11 females, 25 to 46 years of age. None of the patients had a positive case history for photodermatoses or had received any sun exposure. The minimal erythemal dose (MED) for UVB was determined before the study with a UVB Philips TL12 lamp with a radiance of 4 mW/cm2 and a 290 - 320 nm emission spectrum. Results: Carnosine solution obtained 3.6% reduction of erythema (compared to MED) and N-acetylcarnosine 7.3% reduction. Conclusions: An antioxidant capacity of N-acetylcarnosine and carnosine was shown, probably more significant with vehicles improving skin penetration of the substances through skin barrier. N-acetylcarnosine represents an interesting hydrophilic antioxidant for dermatological preparations.
1. Introduction
Carnosine (β-alanyl-l-hystidine) is a low molecular weight water soluble dipeptide commonly present in mammalian tissues, particularly in the cytosol of skeletal muscle cells. It is usually present in a levorotatory form. Several in vitro and in vivo studies have suggested that carnosine and homocarnosine (β-inobutyril-l-histidine) can act as scavengers of reactive oxygen species. Carnosine and its derivatives are potent and selective scavenger of membrane lipids peroxidation products.
Carnosine could act as the water soluble lipophilic counterpart of α-tocopherol protecting from oxidative stress. It has been proposed that carnosine could act as a natural scavenger of dangerous reactive aldehydes from the degradative oxidative pathway of endogenous molecules such as sugars, polyunsaturated fatty acids and proteins [1].
A study indicates that carnosine can protect mast cells from degranulation and histamine release [2]. Carnosine inhibits DNA-cross-linking in neurodegenerative disorders and inflammatory diseases. It has also been shown that glycosidic derivatives of carnosine and other histidinecontaining dipeptides-homocarnosine (gamma-aminobutyryl-l-histidine) and anserine (β-alanyl-3-methyl-l-histidine) have higher antioxidant effects than those of the corresponding free histidine-containing dipeptides and show resistance to carnosinase activity [3].
Interesting studies have been carried out on topical effects on skin: experiments with carnosine applied on mice skin showed that this dipeptide has immunomodulating properties and interesting immunoprotective action from UVB radiation [4]. N-acetylcarnosine has also become the interest of recent studies. Because an association between photo-oxidation of lens proteins and lipid peroxidation with the genesis of age-related cataract has been found, the efficacy of a topical antioxidant formulation including N-acetyl carnosine in the treatment of canine cataract has been studied.
The biological activities of N-acetylcarnosine and carnosine have postulated their use in ophthalmic and skin care products. Carnosine may possibly be included among functional cosmeceuticals and anti-wrinkle agents.
Carnosine is not normally present in the skin, and the effect of this substance appears particularly interesting toward UV photostimulation. The present study aimed to evaluate the antioxidant and photoprotective activities of carnosine and N-acetylcarnosine, when applied on human skin.
2. Materials and Methods
The 6-month study was carried out with 20 healthy volunteers phototype 2 or 3.
Nine patients were males and 11 females, 25 to 46 years of age. None of the patients had a positive case history for photodermatoses or had received any sun exposure.
The MED for UVB was determined before the study with a UVB Philips TL12 lamp with a radiance of 4 mW/cm2 and a 290 - 320 nm emission spectrum.
The preparations used were: 1) 0.5% carnosine in a water solution; 2) 0.5% N-acetylcarnosine in a water solution.
The procedure, illustrated in Table 1, was the following: on the skin of the left forearm of each subject 5 areas of 1 cm2 each were choosen and numbered 1 to 5 (Area 1 was proximal to the hand, Area 5 was distal to it); no substance was applied on Area 1; 0.2 ml of water were applied on Area 2; 0.2 ml of 0.5% carnosine on Area 3; 0.2 ml of 0.5% N-acetylcarnosine on Area 4 and no substance was applied on Area 5. All areas, except Area 1, were irradiated with twice individual MED obtaining a visible and perceptible erythema. Immediately after irradiation, the substances were again applied with the above described procedure; after 24 hours the grade of erythema was evaluated in each area.
Objective determination was obtained by an X-Rite 968 spectrocolorimeter. The scale used is known as L*a*b* and it is based on a tridimensional Cartesian axis system in which the L* axis shows the variations between white and black, the a* axis shows the variations between red and green and the b* axis those between yellow and blue. As erythema index was used the delta a* = a* of the skin radiated with (MED UVB)-a* of the skin treated with 0.5% carnosine or 0.5% N-acetylcarnosine, before and after the photostimulation. The skin color modifications were colorimetrically determined: the areas where carnosine (Area 3) and N-acetylcarnosine (Area 4) were applied were compared with control areas. Determinations were performed three times for each area.
3. Results
The erythema for carnosine showed 3.6% reduction related to MED (delta a* 0.30 and 1.74) and 7.3% reduction (delta a* 0.3 and 0.9) for N-acetylcarnosine (Figure 1).
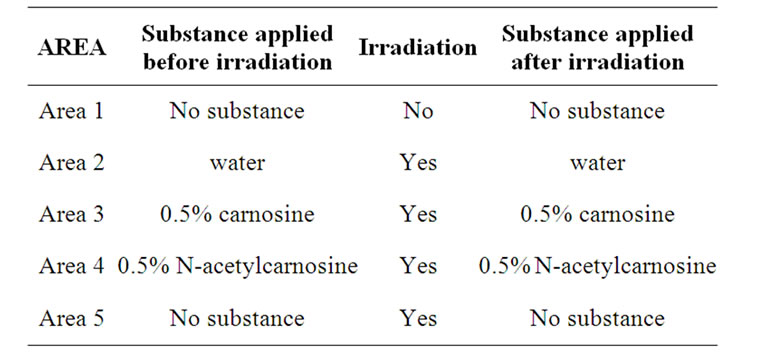
Table 1. Procedure of the study performed on the skin of the left forearm of the subjects.
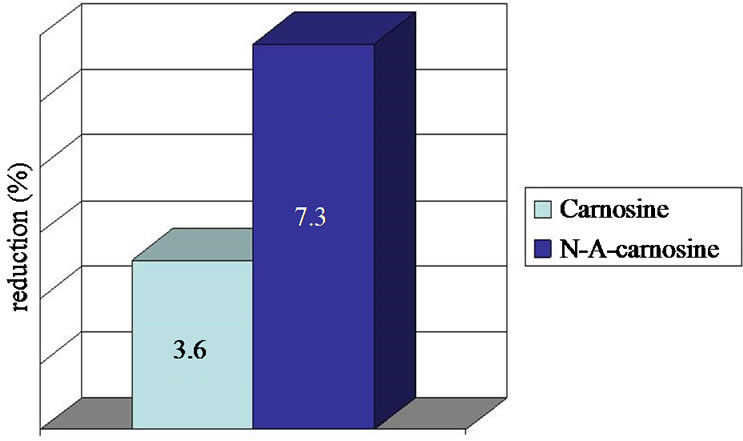
Figure 1. Erythema reduction compared to MED after application of carnosine and N-acetylcarnosine.
4. Conclusions
Our results show that carnosine and N-acetylcarnosine present an interesting effect of UVB photoprotection when applied on human skin. The very low permeability of the stratum corneum for water soluble substances in the integrity of the barrier is an important limiting factor [5]. In this study the substances were dissolved in water. Appropriate vehicles and excipients should be found for better skin absorption, modulated by enhancers systems.
REFERENCES
- A. Guiotto, A. Calderan, P. Ruzza and G. Borin, “Carnosine and Carnosine-Related Antioxidants: A Review,” Current Medicinal Chemistry, Vol. 12, No. 20, 2005, pp. 2293-2315. doi:10.2174/0929867054864796
- Y. Shen, S. Zhang, L. Fu, W. Hu and Z. Chen, “Carnosine Attenuates Mast Cell Degranulation and Histamine Release Induced by Oxygen-Glucose Deprivation,” Cell Biochemistry and Function, Vol. 26, No. 3, 2008, pp. 334-338. doi:10.1002/cbf.1447
- F. Bellia, A. M. Amorini, D. La Mendola, et al., “New Glycosidic Derivatives of Histidine-Containing Dipeptides with Antioxidant Properties and Resistant to Carnosinase Activity,” European Journal of Medicinal Chemistry, Vol. 43, No. 2, 2008, pp. 373-380. doi:10.1016/j.ejmech.2007.03.038
- V. E. Reeve, M. Bosnic and E. Rozinova, “Carnosine (Beta-Alanylhistidine) Protects from the Suppression of Contact Hypersensitivity by Ultraviolet B (280 - 320 nm) Radiation or by cis-Urocanic Acid,” Immunology, Vol. 78, No. 1, 1993, pp. 99-104.
- P. Santoianni, M. Nino and G. Calabrò, “Intradermal Drug Delivery by Low Frequency Sonophoresis (25 KHz),” Dermatology Online Journal, Vol. 10, No. 2, 2004, p. 24.

