Journal of Water Resource and Protection
Vol.4 No.9(2012), Article ID:22286,4 pages DOI:10.4236/jwarp.2012.49089
Reproduction of Atherina lagunae from the Tunis North Lake
1Unit of Marine Biology, Faculty of Sciences, University Campus, Tunis El Manar, Tunisia
2Laboratory of Analysis, Topology and Probability 7353, Aix-Marseille Universités, Marseille, France
3Laboratory of Ichthyology, University of Montpellier II, Montpellier, France
Email: *nediasakli@yahoo.fr
Received July 2, 2011; revised August 4, 2011; accepted August 16, 2011
Keywords: Atherina lagunae; Tunis North Lake; Tunisia; Reproduction; GSI
ABSTRACT
In this study, the reproduction of the sand smelt, Atherina lagunae, from the Tunis North Lake is investigated. The smallest mature female was 39 mm total length (TL). Fifty percent of the females were mature at 58 mm (TL). The yearly and monthly average gonadosomatic indexes (GSI) are presented. The average value of GSI for the 12-month period was respectively 3.02%, 2.57% and 2.8% for females, males and both sexes. The GSI began to increase in March (2.37% for females and 2.12% for males). The maximum GSI was recorded in May, 11.29% for females and 10.03% for males. The highest values were recorded between March and July, indicating the reproduction period of Atherina lagunae in the Tunis North Lake. Similar GSI values were recorded in sand smelt males from brackish lagoons of southern France.
1. Introduction
Atherina is a small inshore fish with many populations living in brackish, marine and freshwater. It is distributed in the Eastern Atlantic Ocean and Mediterranean Sea, extending to the south along the African coast into the Indian Ocean [1]. Sand smelts (members of the Atherina boyeri complex) are common in the Mediterranean and adjacent seas, and are also found in the north-east Atlantic from the Azores to the north-west coast of Scotland [1, 2]. Works on Atherina boyeri (Risso, 1810), in both the eastern and western Mediterranean Sea showed that the species includes two distinct groups of populations, a homogenous group of marine atherinids and a heterogeneous group of atherinids populating lagoons [2-5]. The identification of these two groups (Atherina boyeri sensu stricto and Atherina lagunae) is based both on meristic and molecular characters [5,6].
Atherina lagunae is a small, short-lived, euryhaline atherinid fish which mainly inhabits coastal lagoons and, more rarely, inland waters, over a wide range of salinities from freshwater to hypersaline conditions (110‰ maximum recorded) [7,8]. Morphologically, it displays several important characters that change in relation to habitat while also being capable of adjusting to wide ranges of environmental conditions [9-11]. In Tunisia, Atherina lagunae is found in several lagoons including the Tunis North Lake, a brackish coastal lagoon located between the Tunis city and the Tunis Golf. The surface area of this lake is 28 km2 and the average depth is 2 m; in some areas, the depth reaches 4 m. It is connected to the Mediterranean Sea by the Golf with Khereddine channel. This lake shows a high salinity (37‰), with a temperature varying from 13˚C in winter to 26˚C in summer. Some processes occurred in the Tunis North Lake from 1985 to 1988 to eliminate the sources of pollution [12,13].
Fish of the Atherina boyeri complex constitute a source of food in some regions including of Tunisian areas, therefore their biological and ecological traits must be known. That is not the case for several populations. For example, there is no information about the biology of the sand smelts applicable to the Tunis North Lake, and only some works describe spinal anomalies [12-14]. Also, the aim of this study is to provide basic information about some biological parameters of Atherina lagunae from this lagoon, including maturation and recruitment.
2. Materials and Methods
This work was carried out in the Tunis North Lake. The monthly average temperature varied from 10.3˚C in February to 28.7˚C in August. Salinity ranged from 18.9 practical salinity unit (psu) to 29 psu, with an average of 21.7 psu. We collected nearly two thousand four hundred specimens of A. lagunae (50% males and 50% females) from January 2004 to December 2005 in the Northwest of the Tunis North Lake (36˚50'N, 10˚12'E). The fish were caught with a drag net with a mesh size of 8 mm. Temperature and salinity were measured monthly during sampling.
Fresh specimens were transported to the laboratory where total length (TL) and standard length (SL) were measured to the nearest 0.01 mm and body weight to the nearest 0.01 g. All specimens were sexed. Gonads were examined to determine sex and reproductive stage. Gonads from sexually mature specimens were dissected and weighed. The gonadosomatic index (GSI) was calculated as follows: 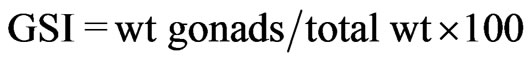 [15]. The monthly and annual GSI was determined first for females, males separately, then for both sexes. The GSI is particularly helpful in identifying days and seasons of spawning, as the ovaries of gravid females swiftly increase in size just prior to spawning.
[15]. The monthly and annual GSI was determined first for females, males separately, then for both sexes. The GSI is particularly helpful in identifying days and seasons of spawning, as the ovaries of gravid females swiftly increase in size just prior to spawning.
3. Results
Maturation, represented by the percentage of females with a GSI above the average yearly GSI of the total female sample significantly correlated with total length. The smallest adult female was 39.62 mm (TL) and 35.27 mm (SL) (Figure 1). Fifty percent of females were mature at 58.38 mm (TL). The average GSI for the 12- month period was respectively 3.02%; 2.57% and 2.8% for females, males and both sexes (Figure 2). The lowest values of GSI were recorded in December, 0.05% for male and 0.09% for female. The GSI began to rise in March (2.37% for females and 2.12% for males. The maximum GSI was recorded in May was 11.29% for females and of 10.03% for males (10.66% for both sexes). During the period March-June, over 90% of all females had an above average value GSI. From July, the GSI value decreases for both sexes. The percentage of new recruited was higher in July and August, 21% and 19%. The number decreased from September to November.
4. Discussion
The smallest mature female Atherina lagunae from the Tunis North Lake was 35.27 mm (LS). Similar values have been found in the Mauguio Lagoon (Languedoc, South of France) [16] and in the Guadalquivir River (Spain) [17]: 38 and 40 mm (SL) respectively. In Egypt, the species reached sexual maturity at 34 mm (SL), from Bardawil Lake [7] and 27 mm (SL) in the Suez Canal [18].
The GSI values of A. lagunae from the Tunis North Lake were relatively similar for both females and males. The GSI values began to increase in March (>2%) and reached a maximum in May (≥10%). After July, they were always lower than 2%. The reproduction period of these Tunisian lagoonal fish was similar to those of marine sand smelt, Atherina boyeri which reproduced in the second half of spring and early summer [19,20]. Similarly, the spawning season in the brackish lagoons of southern France extended from February to September, peaking in April-June [16]. More precisely, the higher GSI values of A. lagunae from the Tunis North Lake were similar to those recorded in sand smelts living in brackish lagoons of Languedoc (southern France), which represented a maximum GSI 10.60% in April [21]. Whereas, in the Camargue lagoon (southern France), the GSI increased rapidly from March to May, reaching a maximum value of 13.3%, then regresses from June for females and peaked in April (9.16%) for males [22]. The GSI values of A. lagunae from the Tunis North Lake were higher than those recorded in sand smelts from the Egyptian Suez Canal with a maximum of 5.3% for females and 4.5% for males [18] and from the estuary of
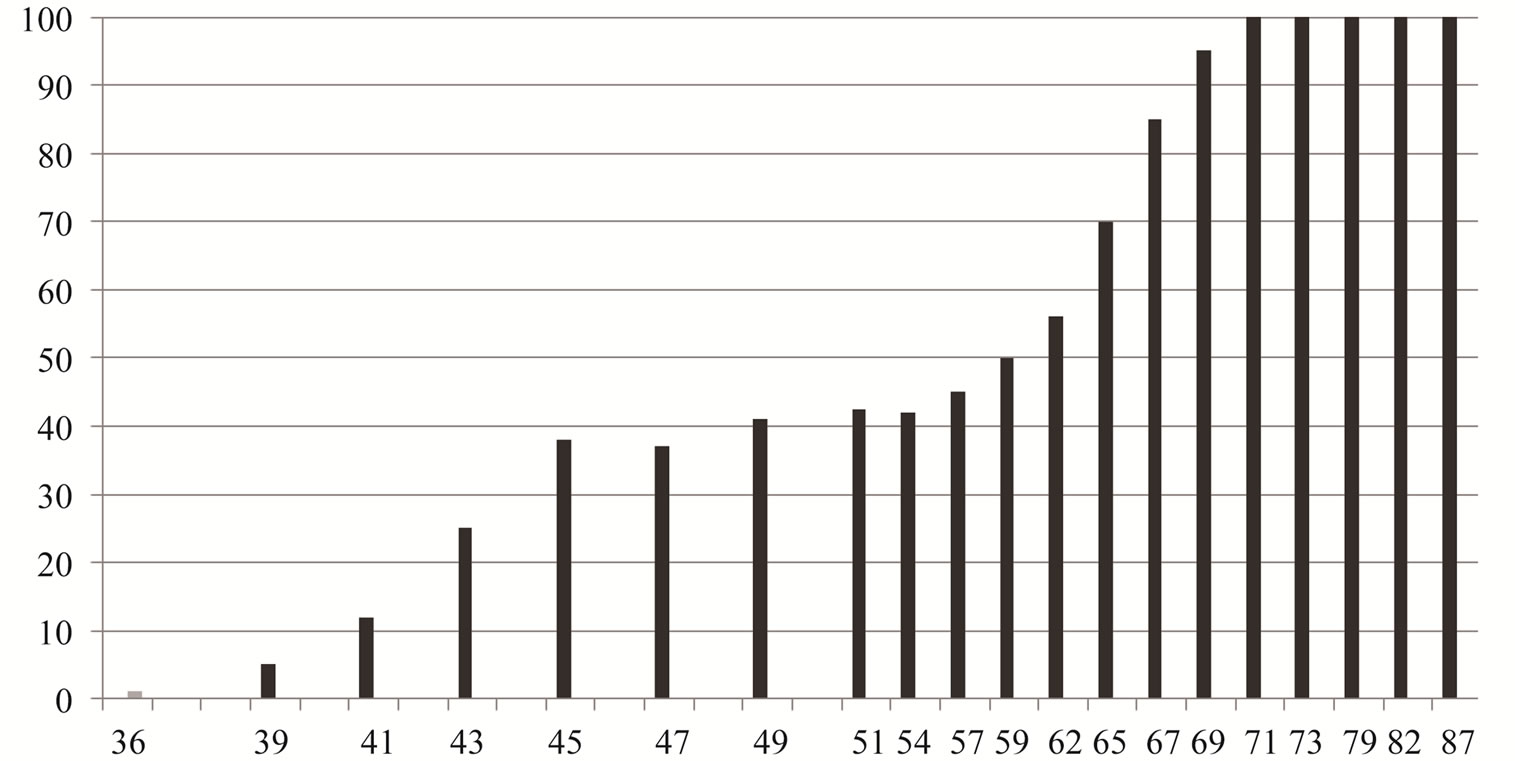
Figure 1. Percentage and length of mature sand smelt females from the Tunis North Lake.
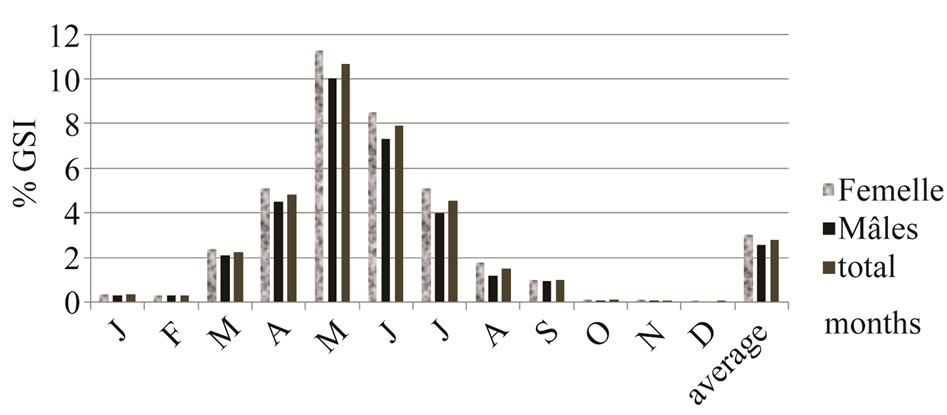
Figure 2. Average gonadosomatic index respectively of males, females, and both sexes in monthly samples of sand smelt and yearly GSI from the Tunis North Lake.
the Guadalquivir River (8% for females and 4% for males) [23].
Within the populations of Atherina lagunae, discrepancies can be found concerning the size of the smallest mature females and the maximum GSI values. Moreover, large differences can also be found concerning this last value between females and males. Based on all these parameters, the sand smelts of Tunis North Lake were close to those of the lagoons of southern France which was also suggested by molecular data [6]. On the basis of morphoanatomical parameters, the Atherina boyeri complex is viewed as a highly polymorphic complex. This could be a response to variations of environmental parameters. Indeed, species of this complex live generally in unstable ecosystems with the living conditions where temperature, salinity, turbidity, currents, and the quality and quantity of food vary. Therefore, reproduction characteristics are specific to each population [24]. The recruitment process is affected by the instability conditions [24] and success depends on the availability of appropriate surfaces, such as sublittoral algae for egg attachment [25]. It has also been reported that some environmental disturbances cause vertebral deformities [14,24,26].
5. Conclusion
This study provides basic information about some biological parameters of Tunisian lagoonal sand smelts, including maturation and recruitment. However, as the variation of physicochemical parameters of the living environment including temperature and salinity, could explain the variations of the sand smelt life cycle, future studies must combine both analyses of biological characteristics of Atherina and of physicochemical parameters of their environment. Such works are particularly important, since these fish can be a significant food resource in some areas.
REFERENCES
- J.-P. Quignard and A. Pras, “Atherinidae,” In: P. J. P. Whitehead, M. L. Bauchot, J. C. Hureau, J. Nielsen and E. Tortonese, Eds., Fishes of the North-Eastern Atlantic and the Mediterranean, UNESCO, Paris, 1986, pp. 1207-1210.
- A. Kiener and C. J. Spillmann, “Contribution to the Systematic and Ecological Study of Atherines of the French Coasts,” Memoirs of the National Museum of Natural History, Series A, Vol. 60, No. 2, 1969, pp. 33-74.
- J. P. Marfin, “Problems Related to Polymorphism of Atherina Species boyeri Risso, 1810,” Cybium, Vol. 6, No. 4, 1982, pp. 19-26.
- M. Trabelsi and F. Kartas, “Contribution to the Study of Digital Characters of Sandsmelt Atherina boyeri Risso, 1810 in The Tunisian Coast,” Report of the International Commission for the Scientific Exploration of the Mediterranean Sea, Vol. 29, No. 4, 1985, pp. 187-189.
- M. Trabelsi, J. P. Quignard, J. A. Tomasini, M. Boussaid, B. Focant and F. Maamouri, “Discriminative Value of the Meristic Characteristics of Atherina boyeri Risso, 1810 Lagoon Populations,” Vie et Milieu, Vol. 52, No. 2-3, 2002, pp. 34-39.
- M. Trabelsi, M. A. Gilles, C. Fleury, J.-P. Quignard, M. Maamouri and E. Faure, “Atherina punctata and Atherina lagunae (Pisces, Atherinidae), New Species Found in the Mediterranean Sea. Article 2: Molecular Investigations of Three Atherinid Species,” Proceedings of the Academy of Sciences (Ser. III), Vol. 325, No. 11, 2002, pp. 1119- 1128.
- O. Gon and A. Ben-Tuvia, “The Biology of Boyer’s Sand Smelt, Atherina boyeri Risso in the Bardawil Lagoon on the Mediterranean Coast of Sinai,” Journal of Fish Biology, Vol. 22, No. 5, 1983, pp. 537-547. doi:10.1111/j.1095-8649.1983.tb04213.x
- P. A. Henderson and R. Bamber, “On the Reproductive Biology of the Sand Smelt Atherina boyeri Risso, 1810 (Pisces: Atherinidae) and Its Evolutionary Potential,” Biological Journal of the Linnean Society, Vol. 32, No. 4, 1987, pp. 395-415. doi:10.1111/j.1095-8312.1987.tb00440.x
- A. Kiener and C. J. Spillmann, “Supplementary Note to the Systematic Study on Ecological of Atherina boyeri Risso (Pisces, Cyprinidae) in the Current Zone Dispersion,” Newsletter of the National Museum of Natural History, Vol. 55, No. 41, 1972, pp. 563-580.
- A. Kiener and C. J. Spillmann, “Atherinidae,” In: J. C. Hureau and Th. Monod, Eds., Check-List of the Fishes of the North-Eastern Atlantic and of the Mediterranean (CLOFNAM), UNESCO, Paris, 1979, pp. 576-578.
- E. Tortonese, “Osteichthyes (Bony Fish) Part Two,” Fauna of Italy, Vol. 11, 1975, pp. 1-636.
- N. Ayed, E. Faure, J.-P. Quignard and M. Trabelsi, “Lordosis in Natural Population of Atherina lagunae from the Tunis North Lake,”Journal of the Society of Natural Sciences of Tunisia, Vol. 36, No. 1, 2010, pp. 1-12.
- N. Ayed, E. Faure, J.-P. Quignard and M. Trabelsi, “Determination of P, Ca, Zn, Cd and Pb Concentrations in Muscle, Gills, Liver, Gonads and Skeletons of Two Natural Populations of Atherina lagunae from the Tunis North Lake, Tunisia,” Journal of Water Resource and Protection, Vol. 3, No. 6, 2011. pp. 421-428. doi:10.4236/jwarp.2011.36052
- N. Ayed, E. Faure, J.-P. Quignard, F. Maamouri and M. Trabelsi, “Incidence of Kyphosis Deformities in Natural Population of Atherina lagunae (Trabelsi et al., 2002) from the Tunis North Lake, Tunisia,” Marine Biology, Vol. 153, No. 3, 2008, pp. 319-325. doi:10.1007/s00227-007-0813-y
- R. J. Wootton, “Ecology of Teleost Fishes,” Chapman and Hall, London, 1990.
- J. A. Tomasini, D. Collart and J. P. Quignard, “Female Reproductive Biology of the Sand Smelt in Brackish Lagoons of Southern France,” Journal of Fish Biology, Vol. 49, No. 4, 1996, pp. 594-612. doi:10.1111/j.1095-8649.1996.tb00057.x
- C. Fernandez-Delgado and J. A. Hernando, “Morphometric Relationships of Atherina boyeri Risso (Pisces: Atherinidae) of Zonar Lake (Cordoba, Spain),” Donana, Acta Vertebrata, Vol. 9, No. 2, 1982, pp. 13-25.
- M. M. Fouda, “Life History Strategies of Four Small-Size Fishes in the Suez Canal, Egypt,” Journal of Fish Biology, Vol. 46, No. 4, 1994, pp. 687-702. doi:10.1111/j.1095-8649.1995.tb01104.x
- I. Jardas, “Jadranska Ihtiofauna [Adriatic Ichthyofauna],” Školska Knjiga, Zagreb, 1996.
- A. Pallaoro, M. Franičević and S. Matić, “Age, Growth and Mortality of Big-Scale Sand Smelt, Atherina (Hepsetia) boyeri Risso, 1810 in the Pantana Lagoon, Croatia,” Periodicum Biologorum, Vol. 104, No. 2, 2002, pp. 175-183.
- J. A. Tomasini and T. Laugier, “Male Reproductive Strategy and Reserve Allocation in Sand Smelt from Brackish Lagoons of Southern France,” Journal of Fish Biology, Vol. 60, No. 3, 2002, pp. 521-531. doi:10.1111/j.1095-8649.2002.tb01681.x
- E. Rosecchi and A. J. Crivelli, “Study of a Sand Smelt (Atherina boyeri Risso 1810) Population Reproducing in Fresh Water,” Ecology of Freshwater Fish, Vol. 1, No. 2, 1992, pp. 77-85. doi:10.1111/j.1600-0633.1992.tb00076.x
- C. Fernandez-Delgado, J. A. Hernando, M. Herrera and M. Bellido, “Life History Patterns of the Sandsmelt Atherina boyeri Risso, 1810 in the Estuary of the Guadalquivir River, Spain,” Estuarine, Coastal and Shelf Science, Vol. 27, No. 6, 1988, pp. 697-706. doi:10.1016/0272-7714(88)90076-5
- V. Bartulović, B. Glamuzina, A. Conides, J. Dulčić, D. Lučić, J. Njire and V. Kožul, “Age, Growth, Mortality and Sex Ratio of Sand Smelt, Atherina boyeri Risso, 1810 (Pisces: Atherinidae) in the Estuary of the Mala Neretva River (Middle-Eastern Adriatic, Croatia),” Journal of Applied Ichthyology, Vol. 20, No. 5, 2004, pp. 427-430. doi:10.1111/j.1439-0426.2004.00560.x
- P. A. Henderson, A. W. H. Turnpenny and R. N. Bamber, “Long Term Stability of a Sand Smelt (Atherina presbyter Cuvier) Population Subject to Power Station Cropping,” Journal of Applied Ecology, Vol. 21, No. 1, 1984, pp. 1-10. doi:10.2307/2403034
- P. Tutman, B. Glamuzina, B. Skaramuca, V. Kožul, N. Glavić and D. Lučić, “Incidence of Spinal Deformities in Natural Populations of Sandsmelt, Atherina boyeri (Risso, 1810) in the Neretva River Estuary Middle Adriatic,” Fisheries Research, Vol. 45, No. 1, 2000, pp. 61-64. doi:10.1016/S0165-7836(99)00098-3
NOTES
*Corresponding author.

