Journal of Biomedical Science and Engineering
Vol.6 No.5(2013), Article ID:31902,7 pages DOI:10.4236/jbise.2013.65074
Controlled release of cisplatin and cancer cell apoptosis with cisplatin encapsulated poly(lactic-co-glycolic acid) nanoparticles
![]()
Department of Orthopaedic Surgery, University of Toledo, Toledo, USA
Email:*a.jayasuriya@utoledo.edu
Copyright © 2013 A. Champa Jayasuriya, Anthony J. Darr. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 11 February 2013; revised 1 April 2013; accepted 5 May 2013
Keywords: Nanoparticles, Cisplatin; Poly(Lactic-co-Glycolic Acid); Controlled Release; Cancer; Apopotosis
ABSTRACT
The goal of the present study is to utilize cis-diamminedichloroplatinum (cisplatin) loaded polymer nanoparticles (NPs) to give a controlled, extended, and local drug therapy for the treatment of cancer. We have used biodegradable and biocompatible poly(lactic-co-glycolic acid) (PLGA) to prepare the NPs by adjusting the double emulsion technique using poly(vinylalcohol) as a surface active agent. The PLGA NPs were characterized for particle size and shape, controlled release of cisplatin, and degradation. Cisplatin solubility in deionized water was increased up to 4 mg/mL by simply changing the solution parameters. Cisplatin encapsulated NPs were incubated in phosphate buffered saline (PBS) at 37˚C to study the release kinetics of cisplatin. Cisplatin was released in a sustained manner with less than 20% release during a 3-day period followed by 50% release during a 21- day period. A degradation study of PLGA NPs demonstrated the loss of spherical shape during a 21- day period. We also examined the cisplatin sensitive A2780 cell apoptosis when cells were incubated with cisplatin encapsulated PLGA NPs. A large number of cell apoptosis occurred as a result of cisplatin release from the PLGA NPs. These results suggest that cisplatin encapsulated PLGA NPs can be used to treat the cancer cells by injecting them into a localized site minimizing the side effects.
1. INTRODUCTION
Drug delivery is still considered a weak link in drug development, with more than 95% of all new potential therapeutics having poor pharmacokinetics [1]. Nearly all therapeutics currently on the market are delivered in a non-specific manner to the whole body resulting in unintentional side effects [1-7]. The delivery method for a drug can have a significant effect on the drug’s therapeutic efficacy. Conventional drug delivery methods result in sharp changes in systemic drug levels that can be harmful for the patients. When cancer patients are treated with chemotherapy, they experience severe toxic and adverse side effects from the non-specific treatments.
Controlled drug release can eliminate the problems associated with conventional therapy by allowing fewer administrations and still maintaining drug levels. In addition, controlled drug delivery can be used to treat the cancers by injecting the drug delivery system at solid cancer site [2-5]. Among various colloidal drug delivery systems, NPs represent a very promising approach [8-10]. Various types of drug carriers, such as liposomes and polymeric NPs, have been investigated for local delivery of numerous drugs [11-18]. Polymeric NPs possess some advantages over liposomes in terms of stability both during storage and in vivo environment [19]. Furthermore, these NPs provide the possibility of modulating the drug release profile, for example by controlling the polymer biodegradation. NPs are especially interesting due to their particle size which allows for transport of carriers across biological membranes and specific targeting by a possible surface modification [20,21].
Cis-diamminedichloroplatinum, (cisplatin) is one of the most active anticancer agents, and it is used in the treatment of several cancers such as ovarian, testicular, osteosarcoma, head and neck, and small cell lung cancer [22]. Cisplatin must be used in limited short-term, highdose treatments for cancer because of its nephrotoxi- city and ototoxicity. Generally, cisplatin is administered through a vein (intravenously or IV) as an infusion [22]. Minimization of the systemic toxicity of cisplatin has been demonstrated after intraperitoneal cisplatin treatment with comparable anti-tumor efficacy compared to a systemic dose [23-26]. Cisplatin binds to the DNA in the cell nucleus and leads to apoptosis.
In this study, cisplatin was selected as the cancer drug to encapsulate into NPs since it was used to treat several different cancers. We have selected poly(lactic-co-glycolic acid) (PLGA) 50:50 copolymer as a carrier to deliver cisplatin. PLGA co-polymers have been used extensively in several biomedical applications including drug delivery systems since they are considered biodegradable and biocompatible [27]. The goal of our research is to utilize cisplatin loaded NPs to give a controlled, extended, and local drug therapy for the treatment of cancer cells. In this study, we report the preparation, characterization, and degradation of PLGA NPs, encapsulated with the chemotherapy drug cisplatin. We also studied in vitro release behavior of cisplatin from cisplatin encapsulated PLGA NPs while incubating at 37˚C in phosphate buffered saline (PBS). We used cisplatin sensitive A2780 cells to evaluate the apoptosis capability of cisplatin encapsulated NPs.
2. MATERIAL AND METHODS
2.1. Materials
50:50 PLGA co-polymer Medisorb Alkerenes®, amorphous with glass transition temperature (Tg = 55˚C), was used to prepare NPs. Poly(vinylalcohol) (PVA; Mw 30,000 Da, 88% hydrolyzed) powder and dichloromethane solvent were purchased from Sigma (St. Louis, MO). PVA was chosen as a surface active agent for the second emulsion and purchased from Sigma (St. Louis, MO). Cisplatin powder (cis-Diammineplatinum dichloride, Mw 300 Da) was purchased from Sigma. The chemical structure of cisplatin is shown in Figure 1.
2.2. Preparation of Cisplatin Encapsulated PLGA NPs
PLGA NPs were prepared using the same basic principle of the double emulsion as previously described [16,17]. Approximately 4 mg of cisplatin was dissolved in 1 mL of deionized water by heating at 35˚C and stirring for 5 - 10 min. This solution was then added to a 10 mL solution of PLGA beads dissolved in dimethylene chloride (CH2Cl2). The PLGA/cisplatin solution was mixed for 1 min using a probe sonicator. This mixture was added to 25 mL of 1% PVA (v/v), and mixed 2 times for 1 min,
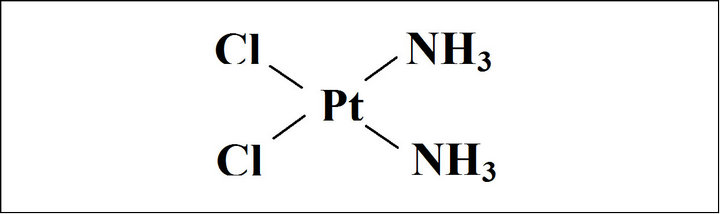
Figure 1. Chemical structure of cisplatin.
again using the probe sonicator. The final solution was then mixed for 3 hrs outside a hood before being placed under a hood overnight to remove the excess CH2Cl2. The next day the NPs mixture was centrifuged for 2 min, at 1000 rpm, and NPs pellet were separated from the solvent. The NP sample (pellet) was resuspended in the solvent and purified by three rounds of centrifuging at 15,000 rpm for 20 min and then dried overnight in a lyophilyzer.
2.3. Morphology of NPs—Light Microscopy and Scanning Electron Microscopy (SEM)
PLGA NPs and cisplatin encapsulated PLGA NPs were characterized by light microscopy (Olympus BH-2 light microscope) and Q Capture Pro software. A drop of NPs suspended in water was placed onto a glass slide and then dried under a hood. The NPs were visualized under the light microscope. SEM was used to quantify the size and morphology of cisplatin loaded NPs. Vacuum dried NPs were fixed on a stub by double-sided sticky tape, NPs were coated with 20 nm gold layer using a sputter coater for SEM.
2.4. Physical Structure of NPs-XRD
A Rigaku Ultima III X-ray diffractometer was used with a Cu-Kα target to determine the physical structure of PLGA NPs, cisplatin, and cisplatin encapsulated PLGA NPs. Approximately 250 mg of sample was used with the XRD. The X-ray generator was operated at voltage 40 kV and current 100 mA. XRD patterns were obtained between 2θ angle range 10˚ - 60˚ with a scan speed of 2 deg/min.
2.5. In Vitro Degradation of PLGA NPs
NP degradation was performed using 125 mg of NPs placed in 125 mL of PBS at 37˚C, with slight shaking for a period of 3 weeks, taking samples every 3 days. At each time point, NPs were removed from the incubation medium and washed thoroughly with deionized water, and subsequently frozen and lyophilized. In order to mimic the human in vivo environment, we have used dynamic conditions for degradation and drug release experiments giving slight motion for the PLGA NPs [28].
2.6. Controlled Release of Cisplatin
For drug release experiments, 45 mg of cisplatin encapsulated NPs were incubated for 3 weeks in 45 mL of PBS at 37˚C while being constantly stirred at 50 rpm. At regular intervals three 1.5 mL samples were removed and replaced with 4.5 mL of PBS sample. Next the samples were centrifuged, separated, and frozen. The supernatants of these samples were later thawed and then analyzed with UV spectroscopy (Perkin Elmer Lambda Bio UV/Vis Spectrometer) at 301 nm [29-31] to determine the amount of cisplatin that had been released compared with a standard curve. Cisplatin encapsulation efficiency (%) was determined by the ratio of the amount of drug encapsulated in the NPs and the initial amount of drug used in the process. The amount of cisplatin encapsulated into the NPs was determined by using a known amount of NPs encapsulated with cisplatin, dissolving them in CH2Cl2, washing with a known amount of PBS, then separating the organic PLGA/CH2Cl2 layer from the aqueous PBS/cisplatin layer. This sample was then analyzed using UV at 301 nm.
2.7. Cell Apoptosis Study
Cells were seeded at 70,000 cells/well in a 24-well plate and allowed attaching the cells for 48 hrs in culture medium containing in DMEM medium with 10% FBS and 100 μg/mL penicillin G and streptomycin at 37˚C in a humidified and 5% CO2 atmosphere. At day 2, the medium was removed and replaced it with either: 1) 300 μL of medium (control); 2) pure cisplatin solution of 50% of the theoretical encapsulation efficiency of a normal PLGA NP sample; 3) pure cisplatin solution of 100% of the theoretical encapsulation efficiency of a normal PLGA NP sample; 4) 300 μL of a 10 mg of blank PLGA NPs in 1 mL of medium solution; 5) 300 μL of a 10 mg of cisplatin NPs in 1 mL of medium solution. Experiments were performed in triplicates. After treating 4 days with above culture conditions, the cells were trypisinized and counted using a hemacytometer. We kindly received cisplatin A2780 cells from Dr. Steve Patrick in the Department of Biochemistry and Cancer Biology at University of Toledo.
2.8. Statistical Analysis
Controlled cisplatin release and normalized cell data were reported as mean ± Standard Deviation. Student’s-t test was used to assess the statistical significance of the data obtained over time for controlled release and normalized cell number in different groups respect to controls.
3. RESULTS
3.1. Morphology of NPs Encapsulating Cisplatin
PLGA NPs and cisplatin encapsulated PLGA NPs were prepared using a double emulsion method as shown in Figure 2. These NPs were characterized for particle size and shape using light microscopy combined with software as shown in Figure 3. The PLGA NPs are approximately spherical in shape and most of them are in the 600 - 1000 nm size range. We also observed similar images for cisplatin encapsulated NPs and did not observe a difference in either size or shape.
3.2. Evidence of Encapsulation of Cisplatin into PLGA NPs—XRD
Since cisplatin contains platinum, the cisplatin encapsulated NPs were analyzed using XRD to confirm cisplatin incorporation in NPs. Figure 4(a) exhibits the typical broad XRD peak of PLGA from 10 - 25 degrees due to the amorphous nature of PLGA. The sharp peaks appeared in Figure 4(b) are due to the platinum in the powder sample of cisplatin. The PLGA/cisplatin sample (Figure 4(c)) shows a broad PLGA peak at 10 - 25 degrees and other peaks at 22.8, 27, 38.7, 46.1, 48.2 and 56 degrees corresponding to the cisplatin. Most of the XRD peaks

Figure 2. Schematic representation of cisplatin encapsulated NPs preparation using double emulsion technique.
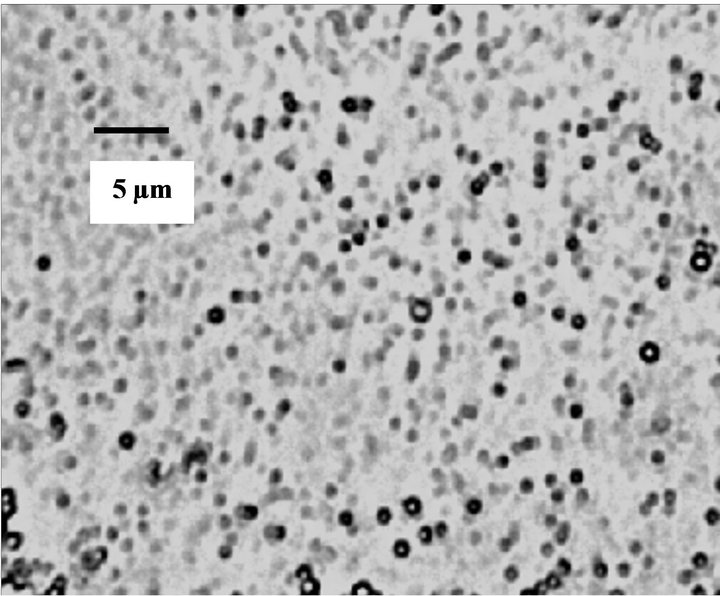
Figure 3. Optical microscopy image of PLGA NPs prepared by double emulsion technique.
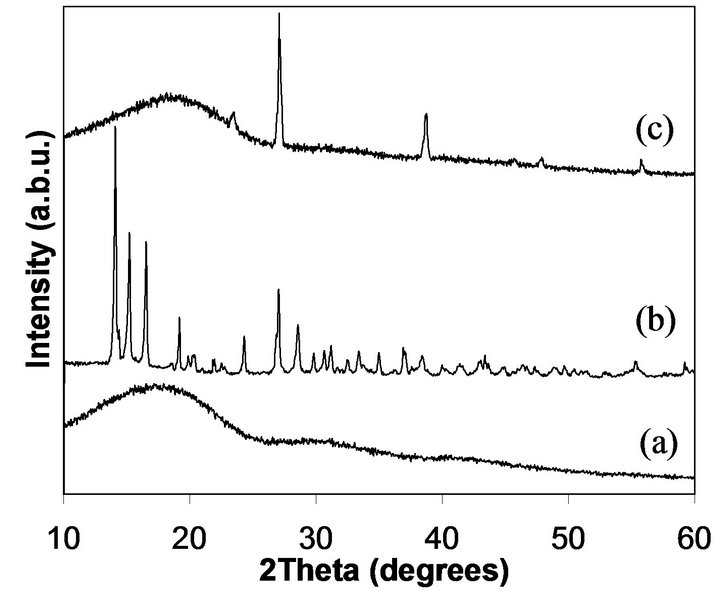
Figure 4. X-ray diffraction spectra of PLGA NPs (a), cisplatin (b), and cisplatin loaded PLGA NPs (c).
that corresponded to cisplatin did not appear with the cisplatin encapsulated NPs due to the interference from the broad peak of PLGA at 2θ = 10 - 25 degrees. The reason for this phenomenon is that the cisplatin to PLGA ratio in NPs is very small. In a similar study, no XRD peaks related to cisplatin appeared for NPs encapsulated cisplain [32]. Therefore, our XRD results revealed strong evidence for successful cisplatin encapsulation into PLGA NPs given both PLGA and cisplatin features of the XRD spectra.
3.3. Degradation of NPs
The degradation rate of the polymer matrix is an important determinant of the in vitro release of the therapeutic agent from PLGA NPs [33,34]. We studied PLGA NPs degradation in a physiological medium as a function of time. A comparison of SEM images of NPs taken after 6 days (Figure 5(a)) and 21 days (Figure 5(b)) of incubation in PBS at 37˚C shows morphological changes evident with the loss of spherical shape. However, NPs were not completely degraded at 21 days. At this point some cisplatin would remain in the polymer matrix.
3.4. Controlled Release of Cisplatin
We studied the trend of cisplatin release using cisplatin encapsulated NPs exposed to physiological conditions such as PBS at 37˚C. In this study, cisplatin encapsulation efficiency was approximately 30%. Cisplatin release showed an initial burst release (<20%) for the first three days after which it maintained a controlled release for 21 days (Figure 6). It is estimated that the incubated NPs released more than 50% of their encapsulated cisplatin after 3 weeks of incubation.
3.5. Apoptosis of Cells Presence of Cisplatin
We examined the apoptosis of cells exposed 4 days in different treatment groups by normalizing to the cells in
 (a)
(a) (b)
(b)
Figure 5. SEM images of PLGA/cisplatin NPs in PBS at 37˚C for (a) 5 days and (b) 21 days.
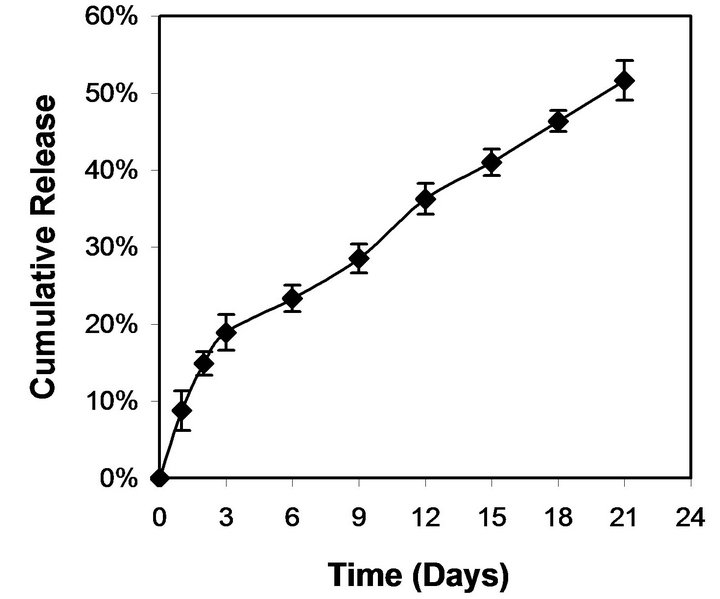
Figure 6. Cumulative release of cisplatin from PLGA/cisplatin NPs as a function of incubation time in PBS at 37˚C.
the control wells (Figure 7). We used four different treatment groups: 1) pure cisplatin solution with 50% of the theoretical encapsulation efficiency of a PLGA NP sample; 2) pure cisplatin solution with 100% of the theoretical encapsulation efficiency of a PLGA NP sample; 3) PLGA NPs (no cisplatin); 4) cisplatin encapsulated PLGA NPs. Each of the four groups decreased the cell count when compared to the control wells. The maximum apoptosis occurred with the cells treated with pure cisplatin solution with 100% of the theoretical encapsulation efficiency of a PLGA NP sample. Cisplatin encapsulated PLGA NPs had the same level of apoptosis as the pure cisplatin solution with 50% of the theoretical encapsulation efficiency of a PLGA NP sample. PLGA only NPs, not encapsulated any cisplatin, displayed the minimum apoptosis among all the groups. Cell attachment and proliferation may not occur when NPs presence in the wells compared to the tissue culture treated well surfaces.
4. DISCUSSION
In this study, the entire procedure for cisplatin encapsulated PLGA NP preparation was carried out in a sim- ple way. The bioactivity of cisplatin will be protected since it is encapsulated in the PLGA NPs without using high temperature, pressure or voltage. Typically cisplatin
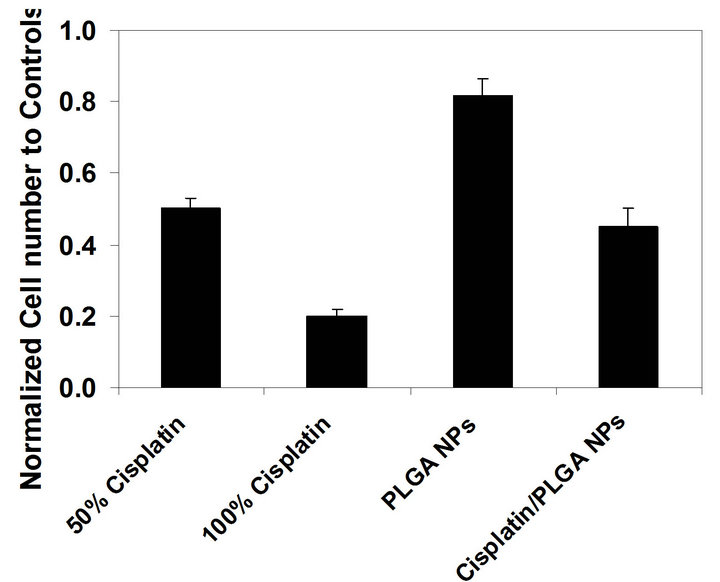
Figure 7. Normalized cell count of differently treated cell groups to cells in control wells: pure cisplatin solution of 50% of the theoretical encapsulation efficiency of a normal NP sample; pure cisplatin solution of 100% of the theoretical encapsulation efficiency of a normal NP sample; PLGA NPs (no cisplatin); cisplatin encapsulated PLGA NPs.
solubility is approximately 2.5 mg in 1 mL of water at room temperature [35]. We were able to increase the solubility of cisplatin to 4 mg in 1 mL of water by increasing the temperature up to 35˚C. Therefore, we were able to encapsulate a higher loading density of cisplatin (approximately 30%) into PLGA NPs using the double emulsification method. Recent studies have shown that cisplatin was encapsulated into the PLGA (50:50) NPs and microparticles (MPs) [36,37]. However, their encapsulation efficiency was only 11.23% and 10.33% (w/w) for NP and MP, respectively. Compared to our study, this low encapsulation efficiency seems to be attributed to the lower solubility of cisplatin (2.5 mg/mL) in the solvent.
In our study, around 20% cisplatin was released during a three day period followed by more than 50% of cisplatin release during a three week period (Figure 6). The cisplatin release appeared to be biphasic, a rapid initial (burst) followed by a slower exponential phase. The rapid initial release may be attributed to the fraction of cisplatin content, which was adsorbed or close to the surface of the NPs. A few studies have shown the cisplatin encapsulation into PLGA NPs or MPs and their release kinetics [36-38]. However, these studies have reported a more rapid release of cisplatin compared with our study. The release kinetics of cisplatin from NPs showed a biphasic profile, characterized by a rapid initial burst effect followed by an exponential phase and reaching a plateau after 30 days for both NPs and MPs. Almost 50% of cisplatin was released from PLGA NPs within 3-day period compared to total 40-day period. In another study, 60% of cisplatin released from PLGA MPs which are in the size range of 5 - 30 µm, within initial 3 days during 28 day total released period [38].
In our study, more than 40% of cells exposed to cisplatin encapsulated NPs went through apoptosis compared to the cells exposed to only PLGA NPs (Figure 7). Therefore, this result suggests that cisplatin encapsulated PLGA NPs induced the cell apoptosis upon releasing the ciaplatin. Bioactivity of the encapsulated cisplatin in NPs was remained protectively. Long-term incubation of cancer cells with cisplatin encapsulated NPs may enhance the apoptosis of the cancer cells. Even though we encapsulate cisplatin into these NPs, we expect that we can encapsulate other drugs, proteins, and genes into these PLGA NPs.
Cisplatin release was governed by diffusion and degradation of NPs without using any external stimuli. The degradation of PLGA in aqueous media is a hydrolytic process and occurs in two stages [33]. The first stage consists of water diffusion into the amorphous regions with random hydrolytic scission of ester bonds. The second stage starts when most of the amorphous regions are degraded. During the early phases the release of the entrapped therapeutic agent occurs mainly through its diffusion in the polymer matrix, while during the later phases the release is mediated through both diffusion of the therapeutic agent and the degradation of the polymer matrix itself [33,34]. In this study, the PLGA NPs were not completely degraded within 3 weeks as shown in Figure 6. Therefore, even after 3 weeks of incubation of cisplatin encapsulated NPs in PBS, some cisplatin would remain in the NPs. PLGA (50:50) NPs degradation seems to be biphasic with correlation to the molecular weight loss over degradation time: initial rapid degradation occurs over the first 20 - 30 days followed by a slower rate of degradation over the next 60 - 70 days [28].
In systemic chemotherapy drug delivery, toxicity is a major issue due to the large dose of drugs needed. A large dose is needed in order to reach the target tumor tissue. Therefore, chemotherapy treatment severely damages the normal cells surrounding the cancer tissue. In order to minimize the toxicity produced by large drug doses, it is necessary to target only the cancer cells. If NPs targeted over expressed growth factors in the cancer cells, normal cells would not be affected by NPs. Since cisplatin release is sustained from these PLGA NPs, cisplatin can be retained longer in the cancer cells to deliver enough platinum to the nucleus to damage the DNA compared with other delivery methods.
Another issue with systemic drug delivery of PLGA NPs, as all conventional colloidal carriers, is that they are rapidly removed from systemic blood circulation following IV administration by the macrophages of the mononuclear phagocyte system, mainly the Kupffer cells in the liver and the spleen macrophages [39]. This issue may potentially be avoided by intertumoral injection of NPs directly into the local tumor site.
5. CONCLUSION
In this study, we have successfully encapsulated cisplatin into PLGA NPs. We were also able to increase the solubility and encapsulation of cisplatin using slightly elevated temperatures while stirring the solution for several minutes. Encapsulated cisplatin should be released completely upon complete degradation of PLGA NPs. Our results have shown that cisplatin released from cisplatin encapsulated PLGA NPs was in a controlled manner during a 21-day period. We observed that cancer cell apoptosis occurred when they were exposed to cisplatin encapsulated PLGA NPs. Collectively, these results suggest that the NPs can be used to deliver cisplatin into local cancer cells in a sustained manner for a long term, avoiding multiple administrations of cisplatin.
6. ACKNOWLEDGEMENTS
One of author (ACJ) greatly acknowledges the College of Medicine at the University of Toledo for Startup funding to accomplish this work.
REFERENCES
- Bagi, C.M. (2005) Targeting of therapeutic agents to bone to treat metastatic cancer. Advanced Drug Delivery Reviews, 57, 995-1010. doi:10.1016/j.addr.2004.12.014
- Hunter, W., Burt, H.M. and Machan, L. (1997) Local delivery of chemotherapy: A supplement to existing cancer treatments. A case for surgical pastes and coated stents. Advanced Drug Delivery Reviews, 26, 199-207. doi:10.1016/S0169-409X(97)00035-5
- Santini, J.T., Richards, A.C., Scheidt, R.A., Cima, M.J. and Langer, R.S. (2000) Microchip technology in drug delivery. Annals of Medicine, 32, 377-379. doi:10.3109/07853890008995941
- Frank, A., Rath, S.K. and Venkatraman, S.S. (2005) Controlled release from bioerodible polymers: Effect of drug type and polymer composition. Journal of Controlled Release, 102, 333-344. doi:10.1016/j.jconrel.2004.10.019
- Vogelhuber, W., Rotunno, P., Magni, E., Gazzaniga, A., Sprub, T., Bernhardt, G., Buschauer, A. and Gopferich, A. (2001) Programmable biodegradable implants. Journal of Controlled Release, 73, 75-88. doi:10.1016/S0168-3659(01)00282-6
- Langer, R. (1990) New methods of drug delivery. Science, 249, 1527-1533. doi:10.1126/science.2218494
- Vogelhuber, W., Spruss, T., Bernhardt, G., Buschaue, A. and Gopferich, A. (2002) Efficacy of BCNU and paclitaxel loaded subcutaneous implants in the interstitial chemotherapy of U-87 MG human glioblastoma xenografts. International Journal of Pharmaceutics, 238, 111-121. doi:10.1016/S0378-5173(02)00061-3
- Attwood, D. (1994) Microemulsions. In: Kreuter, J., Ed., Colloidal Drug Delivery Systems, Drugs and the Pharmaceutical Sciences, Marcel Dekker, New York, 31-71.
- Maincent, P., Thouvenot, P., Amicabile, C., Hoffman, M., Kreuter, J., Couvreur, P. and Devissaguet, J.P. (1992) Lymphatic targeting of polymeric nanoparticles after intraperitoneal administration in rats. Pharmaceutical Research, 9, 1534-1539. doi:10.1023/A:1015895804597
- Nakada, Y., Fattal, E., Foulquier, M. and Couvreur, P. (1996) Pharmacokinetics and biodistribution of oligonucleotide adsorbed onto poly(isobutylcyanoacrylate) nanoparticles after intravenous administration in mice. Pharmaceutical Research, 13, 38-43. doi:10.1023/A:1016017014573
- Sabate, R., Barnadas-Rodriguez, R., Callejas-Fernandez, J., Hidalgo-Alvarez, R. and Estelrich, J. (2008) Preparation and characterization of extruded magnetoliposomes. International Journal of Pharmaceutics, 347, 156-162. doi:10.1016/j.ijpharm.2007.06.047
- Sahoo, S.K., Ma, W. and Labhasetwar, V. (2004) Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. International Journal of Cancer, 112, 335-340. doi:10.1002/ijc.20405
- Fukui, H., Koike, T., Nakagawa, T., Saheki, A., Sonoke, S., Tomii, Y. and Seki, J. (2003) Comparison of LNSAmB, a novel low-dose formulation of amphotericin B with lipid nano-sphere (LNS), with commercial lipidbased formulations. International Journal of Pharmaceutics, 267, 101-112. doi:10.1016/j.ijpharm.2003.08.002
- Ai, H., Jones, S. A., de Villiers, M.M. and Lvov, Y.M. (2003) Nano-encapsulation of furosemide microcrystals for controlled drug release. Journal of Controlled Release, 86, 59-68. doi:10.1016/S0168-3659(02)00322-X
- Astete, C.E. and Sabliov, C.M. (2006) Synthesis and characterization of PLGA nanoparticles. Journal of Biomaterials Science, Polymer Edition, 17, 247-289.
- Lamprecht, A., Ubrich, N., Hombreiro Pérez, M., Lehr, C.-M., Hoffman, M. and Maincent P. (1999) Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. International Journal of Pharmaceutics, 184, 97-105. doi:10.1016/S0378-5173(99)00107-6
- Avgoustakis, K. (2004) Pegylated poly(lactide) and poly- (lactide-co-glycolide) nanoparticles: Preparation, properties and possible applications in drug delivery. Current Drug Delivery, 1, 321-333. doi:10.2174/1567201043334605
- Lamprecht, A., Yamamoto, H., Takeuchi, H. and Kawashima, Y. (2005) Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. Journal of Pharmacology and Experimental Therapeutics, 315, 196-202. doi:10.1124/jpet.105.088146
- Barratt, G. (2003) Colloidal drug carriers: Achievements and perspectives. Cellular and Molecular Life Sciences, 60, 21-37. doi:10.1007/s000180300002
- Alyautdin, R.N., Petrov, V.E., Langer, K., Berthold, A., Kharkevich, D.A. and Kreuter, J. (1997) Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharmaceutical Research, 14, 325-328. doi:10.1023/A:1012098005098
- Bourel, D., Rolland, A., Le Verge, R. and Genetet, B. (1988) A new immunoreagent for cell labeling. CD3 monoclonal antibody covalently coupled to fluorescent polymethacrylic nanoparticles. Journal of Immunological Methods, 106, 161-167. doi:10.1016/0022-1759(88)90192-5
- O’Dwyer, P.J., Stevenson, J.P. and Johnson, S.W. (1999) Clinical status of cisplatin, carboplatin, and other platinum-based antitumor drugs. In: Lippert, B., Ed., Cisplatin, Chemistry and Biochemistry of a Leading Anticancer Drug, Wiley-VCH, Zürich, 31-72.
- Goldberg, E.P., Hadba, A.R., Almond, B.A. and Marotta, J.S. (2002) Intratumoral cancer chemotherapy and immunotherapy: Opportunities for nonsystemic preoperative drug delivery. Journal of Pharmacy and Pharmacology, 54, 159-180. doi:10.1211/0022357021778268
- Tahara, Y. and Ishii, Y. (2001) Apatite cement containing cis-diamminedichloroplatinum implanted in rabbit femur for sustained release of the anticancer drug and bone formation. Journal of Orthopaedic Science, 6, 556-565. doi:10.1007/s007760100012
- Fujiwara, K., Armstrong, D., Morgan, M. and Markman, M. (2007) Principles and practice of intraperitoneal chemotherapy for ovarian cancer. International Journal of Gynecological Cancer, 17, 1-20. doi:10.1111/j.1525-1438.2007.00809.x
- Wenzel, L.B., Huang, H.Q., Armstrong, D.K., Walker, J.L. and Cella, D. (2007) Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: A gynecologic oncology group study. Journal of Clinical Oncology, 25, 437-443. doi:10.1200/JCO.2006.07.3494
- Anderson, J.M. and Shive, M.S. (1997) Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews, 28, 5-24. doi:10.1016/S0169-409X(97)00048-3
- Panyam, J., Dali, M.M., Sahoo, S.K., Ma, W., Chakravarthi, S.S., Amidon, G.L., Levy, R.J. and Labhasetwar, V. (2003) Polymer degradation and in vitro release of a model protein from poly(d,l-lactide-co-glycolide) nanoand microparticles. Journal of Controlled Release, 19, 173-187. doi:10.1016/S0168-3659(03)00328-6
- Riley, C.M. and Sternson, L.A. (1985). Cisplatin. In: Florey, K., Ed., Analytical Profiles of Drug Substances, Academic Press, New York, 78-105.
- Czarnobaj, K. and Lukasiak, J. (2004) In vitro release of cisplatin from sol-gel processed porous silica xerogels. Drug Delivery, 11, 341-344. doi:10.1080/10717540490507451
- De Spiegeleer, B., Slegers, G., Vandecasteele, C., Van den Bossche, W., Schelstraete, K., Claeys, A. and De Moerloose, P. (1986) Microscale synthesis of nitrogen- 13-labeled cisplatin. Journal of Nuclear Medicine, 27, 399-403.
- Jeong, Y.I., Kim, S.T., Jin, S.G., Ryu, H.H., Jin, Y.H., Jung, T.Y., Kim, I.Y. and Jung, S. (2008) Cisplatin-Incorporated Hyaluronic Acid Nanoparticles Based on IonComplex Formation,” Journal of Pharmaceutical Sciences, 97, 1268-1276. doi:10.1002/jps.21103
- Li, S., Girod, H.S. and Vert, M. (1994) Crystalline oligomeric stereocomplex as intermediate compound in racemic poly(DL-lactic acid) degradation. Polymer International, 33, 37-41. doi:10.1002/pi.1994.210330105
- Li, S. (1999) Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. Journal of Biomedical Materials Research, 48, 342-353. doi:10.1002/(SICI)1097-4636(1999)48:3<342::AID-JBM20>3.0.CO;2-7
- “The Merck Index,” 12th Edition, Merck & Co., Whitehouse Station, 1996.
- Moreno, D., Zalba, S., Colom, H., Trocóniz, I.F., Tros de Ilarduya, C. and Garrido, M.J. (2009) Biopharmaceutic and pharmacodynamic modeling of the in vitro antiproliferative effect of new controlled delivery systems of cisplatin. European Journal of Pharmaceutical Sciences, 37, 341-350. doi:10.1016/j.ejps.2009.03.005
- Moreno, D., de Ilarduya, C.T., Bandrés, E., Buñuales, M., Azcona, M., García-Foncillas, J. and Garrido, M.J. (2008) Characterization of cisplatin cytotoxicity delivered from PLGA-systems. European Journal of Pharmaceutics and Biopharmaceutics, 68, 503-512. doi:10.1016/j.ejpb.2007.08.006
- Huo, D., Deng, S., Li, L. and Ji, J. (2005) Studies on the poly(lactic-co-glycolic) acid microspheres of cisplatin for lung-targeting. International Journal of Pharmaceutics, 289, 63-67. doi:10.1016/j.ijpharm.2004.10.017
- Owens III, D.E. and Peppas, N.A. (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International Journal of Pharmaceutics, 307, 93-102. doi:10.1016/j.ijpharm.2005.10.010
NOTES
*Corresponding author.

