Health
Vol. 4 No. 10 (2012) , Article ID: 24075 , 4 pages DOI:10.4236/health.2012.410129
Associations between muscular fitness and metabolic syndrome: Cross-sectional study of Japanese women and men
![]()
1Fujisawa City Health and Medical Foundation, Kanagawa, Japan; *Corresponding Author: ayumi-t@fhmc.or.jp
2Graduate School of Sport Sciences, Waseda University, Saitama, Japan
3Faculty of Sport Sciences, Waseda University, Saitama, Japan
4Sports Medicine Research Center, Keio University, Kanagawa, Japan
Received 17 August 2012; revised 16 September 2012; accepted 28 September 2012
Keywords: Muscle Strength; Muscle Endurance; Muscular Fitness; Metabolic Syndrome
ABSTRACT
Metabolic syndrome (MetS) is a complex interrelated risk factor for cardiovascular disease and type 2 diabetes mellitus. High cardiorespiratory fitness is known to contribute to prevention of MetS. However, little is known regarding the association between muscular fitness and MetS in Japanese adults. The purpose of this study was to examine the associations between muscular fitness and MetS in Japanese women and men. This cross-sectional study included 335 women and 209 men aged 30 - 79 y. MetS was determined according to the 2009 criteria of the International Diabetes Federation. Muscular fitness was evaluated by muscular fitness composite score (MFS), which was determined using Z scores from grip strength and sit-ups. Participants were classified by MFS tertile into low, middle, and high MFS groups. We used multiple logistic regression analysis to estimate odds ratios for the incidence of MetS in each group. The prevalence of MFS was 27.2% in women and 27.3% in men. Adjusted odds ratios for MetS prevalence in the low, middle, and high MFS groups, after adjusting for age, smoking status, alcohol intake, and exercise habits, were 1.0 (referent), 0.90 (95% confidence interval [CI], 0.50 - 1.62), and 0.49 (95% CI, 0.25 - 0.94; P for trend = 0.03) in women; in men, they were 1.0 (referent), 0.49 (0.23 - 1.04), and 0.42 (0.18 - 0.97; P for trend = 0.04), respectively. Muscular fitness is inversely associated with the prevalence of MetS in Japanese women and men.
1. INTRODUCTION
Metabolic syndrome (MetS) is a clustering of central obesity and cardiovascular disease (CVD) risk factors, including abnormal blood pressure, lipids, and blood glucose [1]. Insulin resistance occurring as a result of visceral fat accumulation is a key factor in MetS and is considered a strong predictor of CVD events [2]. Over the past 2 decades, the prevalence of MetS has increased in Japan as well as in Western countries. Because individuals with MetS have an elevated risk of developing type 2 diabetes [3,4] and CVD [5,6], strategies to prevent an epidemic of this syndrome are urgently required [7].
The primary management approach for MetS is healthy lifestyle promotion such as increased physical activity and diet modification [8]. Because physical fitness (i.e., cardiorespiratory fitness [CRF] and muscular fitness) is primarily determined by physical activity, high physical fitness is thought to be effective for improving MetS. Previous studies have demonstrated an inverse association between CRF and MetS prevalence and suggested that CRF is an independent predictor of MetS incidence [9-11]. Compared with CRF, fewer studies have been conducted on the association between muscular fitness and MetS. While an inverse relationship between muscular strength and MetS has been previously illustrated in American [12,13], Australian [14], and European populations [15], this relationship has not been well studied in populations of Japanese adults [16-18], especially Japanese women. The purpose of this study was to examine the associations between muscular fitness and MetS in Japanese women and men.
2. METHODS
2.1. Subjects
The subjects were 335 women and 209 men, aged 30 79 y, who underwent a baseline preventive medical examination and physical fitness tests between 2006 and 2010 and were recruited to participate in a training program for health promotion at Fujisawa City Health and Medical Center. All subjects provided written informed consent before enrollment in the study. This study was approved by the Ethics Committee of Waseda University and conducted in accordance with the spirit of the Declaration of Helsinki.
2.2. Clinical Examination
All subjects received preventive medical examinations at the medical institution in Fujisawa City The exam included a measurement of height, body weight, waist circumference (WC), blood chemistry analyses (triglycerides, TG; high-density lipoprotein, HDL-c; fasting blood glucose, FPG), and resting blood pressure (BP; systolic blood pressure, SBP; diastolic blood presser, DBP). Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2), and WC was measured at the umbilicus with subjects in the standing position.
2.3. Criteria for MetS
MetS was defined as meeting 3 or more of the following criteria [19]: abdominal obesity (WC ≥ 80 cm in women, WC ≥ 90 cm in men); high TG (≥150 mg/dL or taking medicine to lower TG); low HDL-c (<50 mg/dL in women, <40 mg/dL in men); high BP (SBP ≥ 130 mmHg or DBP ≥ 85 mmHg, or taking medicine to lower BP); and high FPG (≥100 mg/dL or taking medicine to lower FPG).
2.4. Muscular Fitness
Grip strength test, used as a proxy for overall strength [20], was assessed using a handgrip dynamometer (EDD100PNR, Yagami, Nagoya, Japan) [21]. The subject stood with the arm completely extended and squeezed the dynamometer with maximum isometric effort. Grip strength was measured twice on each side. The best of the 4 grip measurements was use to characterize maximum muscle strength. To account for differences in body size, total handgrip was adjusted for body weight (kg).
Abdominal muscle endurance was evaluated by a situp test [21]. The subject started in a lying position with hands crossed over the chest, knees bent at a 90˚ angle, and heels and feet flat on the floor. The subject had to rise to a position with the elbows pointed forward until they touched the thighs. The total number of correctly performed and completed sit-ups within 30 s was counted.
Muscular fitness was evaluated by muscular fitness composite score (MFS), which was determined using Z scores from grip strength and sit-ups.
2.5. Confounding Variables
Several confounding variables were included in the analyses: age (y), smoking status (current, former, never), daily alcohol intake (g/day), and exercise habits (never, once/wk, 2 - 3 times/wk, 4 - 5 times/wk, 6 - 7 times/wk). These variables were assessed by means of a questionnaire.
2.6. Statistical Analysis
Measured and calculated values are presented as mean ± SD or number (%). Participants were classified by MFS tertile into low, middle, and high MFS groups. Analysis of variance was used for continuous variables with a normal distribution, the Kruskal-Wallis test was used for continuous variables with a non-normal distribution, and the chi-square test was used for categorical variables. The association of muscular fitness with the risk of having MetS was estimated using multiple logistic regression analysis adjusted for age (Model 1), and further adjusted for smoking status, alcohol intake, and exercise habits (Model 2). The data were analyzed with SPSS 19.0 for Windows (IBM Japan, Tokyo, Japan). The statistical significance level was set at P < 0.05.
3. RESULTS
Table 1 shows the characteristics of individuals according to MFS level. Women with the highest MFS demonstrated a significantly lower body weight, BMI, WC, SBP, DBP, and TG level (P < 0.05) and a higher Grip strength, Sit-ups, and HDL-c level (P < 0.01). Men with the highest MFS were significantly younger and had a lower body weight, BMI, WC, and TG level (P < 0.05) and higher Grip strength, Sit-ups, and HDL-c level (P < 0.05). Women in the highest MFS tertile, but not men, had a lower prevalence of MetS.
Adjusted odds ratios (ORs) for MetS prevalence in the low, middle, and high MFS groups, after adjusting for age, were 1.0 (referent), 0.92 (95% confidence interval [CI]: 0.51 - 1.63), and 0.53 (95% CI, 0.28 - 0.99) (P for trend = 0.04) in women; in men, they were 1.0 (referent), 0.48 (95% CI, 0.23 - 1.01), and 0.42 (95% CI, 0.19 - 0.90) (P for trend = 0.02), respectively (Figure 1, Model 1). In addition, after further adjusting for smoking status, alcohol intake, and exercise habits, adjusted ORs were 1.0 (referent), 0.90 (95% CI, 0.50 - 1.62), and 0.49 (95% CI, 0.25 - 0.94) (P for trend = 0.03) in women; in men, they were 1.0 (referent), 0.49 (95% CI, 0.23 - 1.04), and 0.42 (95% CI, 0.18 - 0.97) (P for trend = 0.04), respectively (Figure 1, Model 2).
Table 2 shows age-adjusted and multivariate-adjusted ORs. In women, MFS was inversely associated with HDL-c and WC. Age-adjusted ORs for the highest versus lowest tertiles were 0.17 (95% CI, 0.05 - 0.60; P for

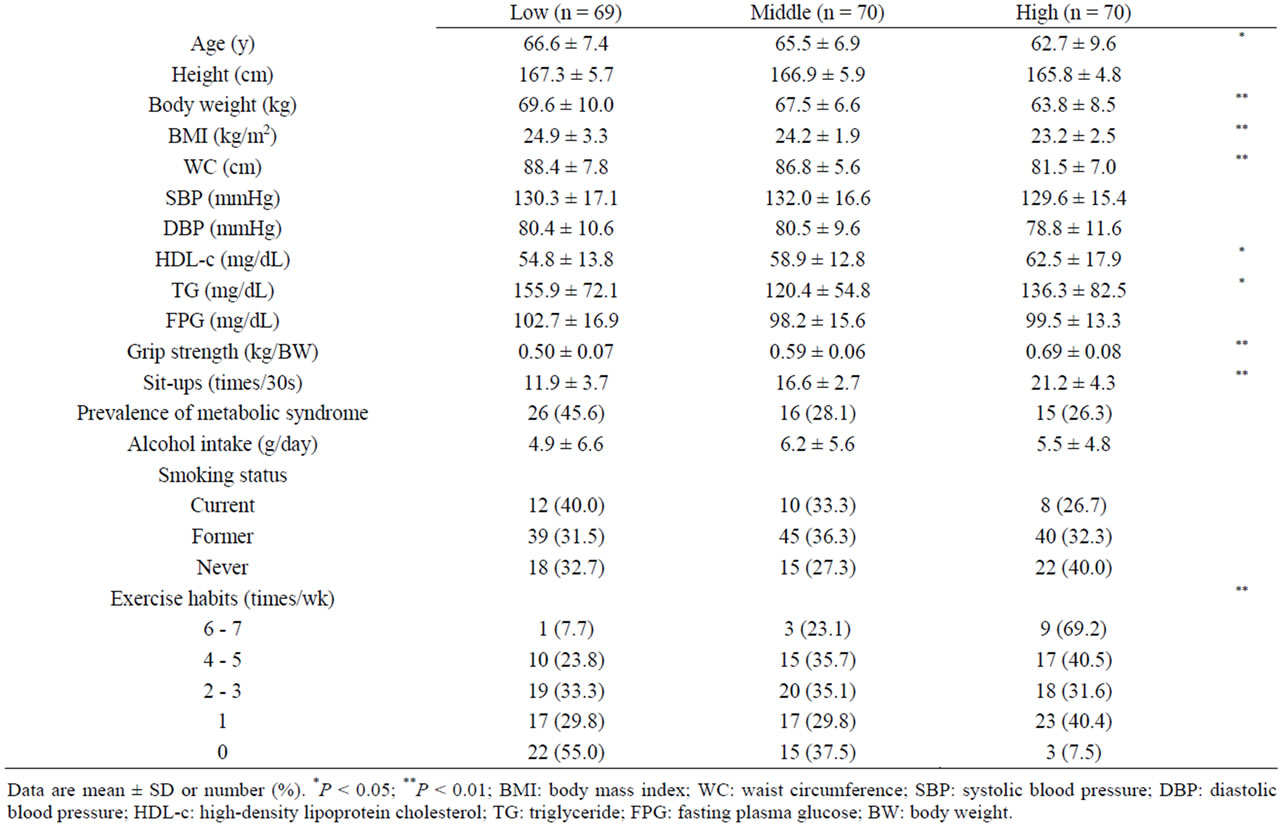
Table 1. The characteristics of individuals across MFS tertiles.
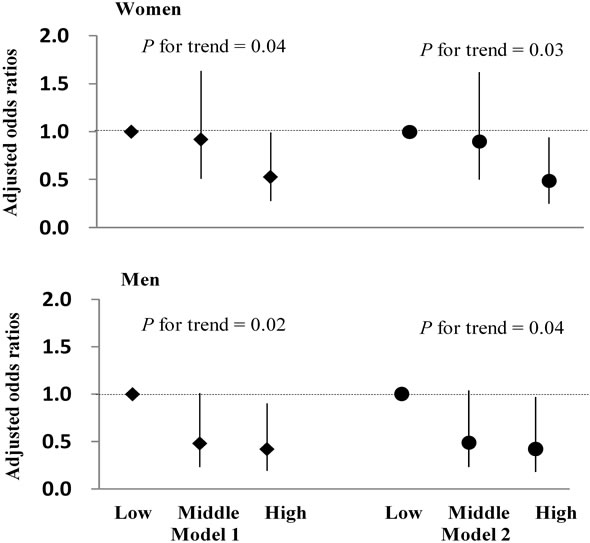
Figure 1. Adjusted odds ratios (ORs) for MetS prevalence in the low, middle, and high muscular fitness composite score (MFS) groups. Results are adjusted for age (Model 1), and additionally adjusted for smoking status, alcohol intake, and exercise habits (Model 2). Vertical bars indicate 95% CIs. Low: Low MFS; Middle: Middle MFS; High: High MFS.
trend < 0.01) for HDL-c and 0.18 (95% CI, 0.10 - 0.32; P for trend < 0.001) for WC; multivariate-adjusted ORs for the highest versus lowest tertiles were 0.15 (95% CI, 0.04 - 0.55; P for trend < 0.01) for HDL-c and 0.17 (95% CI, 0.09 - 0.32; P for trend < 0.001) for WC. In men, MFS was inversely associated with TG and WC: ageadjusted ORs for the highest versus lowest tertiles were 0.39 (95% CI, 0.19 - 0.79; P for trend < 0.01) for TG and 0.06 (95% CI, 0.02 - 0.22; P for trend < 0.001) for WC. However, the MFS-TG relationship was attenuated after further adjustment for smoking status, alcohol intake, and exercise habits; multivariate-adjusted ORs for the highest versus lowest tertiles were 0.54 (95% CI, 0.25 - 1.14; P for trend < 0.09) for TG and 0.07 (95% CI, 0.02 - 0.25; P for trend < 0.001) for WC.
4. DISCUSSION
In this cross-sectional study, we examined the association between MFS levels and the prevalence of MetS and MetS risk factors in Japanese women and men. The primary findings of this study were that 1) low MFS levels were associated with greater risk of incident MetS

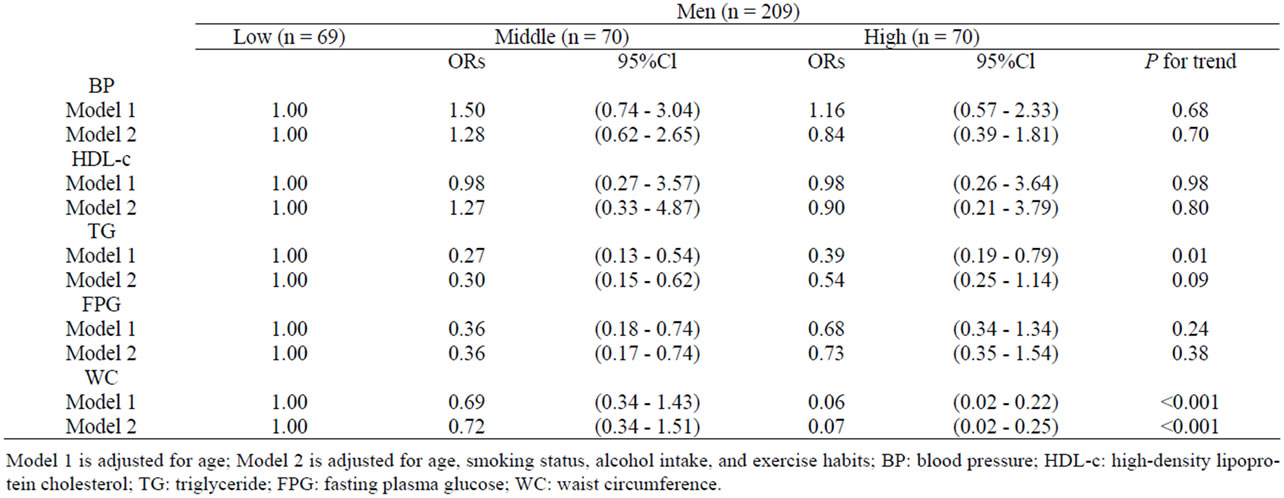
Table 2. Adjusted odds ratios (95% CIs) for MetS risk factors across MFS tertiles.
in women and men; and 2) low MFS levels were associated with higher prevalence of several MetS risk factors after adjustment for age, smoking status, alcohol intake, and exercise habits.
The inverse association between muscle strength and the prevalence of MetS found in the present study is consistent with the results of previous investigations [12- 14]. Jurca et al. reported this association in adult men using a study of cross-sectional design. They found that muscle strength (measured by one repetition maximal leg press and bench press) was associated with a significantly lower risk of developing MetS in men [12]. Further longitudinal analyses in adult men obtained comparable results [13]. In addition, Atlantis et al. reported an inverse association between muscle strength (handgrip strength per lean mass of the arm) and the prevalence of MetS in men [14]. The present study has extended the previous results by revealing inverse associations between MFS and the prevalence of MetS in both women and men.
By contrast, other previous studies have reported a relationship between muscle strength and MetS risk factors [15,22]. Wijndaele et al. reported that muscular strength assessed by measuring isometric knee extension and flexion peak torque was associated with the MetS risk factors of TG, HDL-c, and clustered MetS risk factors in women, and associated with clustered MetS risk factors in men [15]. Aoyama et al. examined the relationship between grip strength and individual and clustered MetS risk factors in Japanese men and women and found that grip strength was inversely associated with plasma glucose levels and clustered MetS risk factors in women [18]. Similar to previous studies, MFS was also associated with lipid profiles and WC in this study.
Although their results are not directly comparable with ours, Katzmarzyk et al. found a significant inverse relationship between musculoskeletal fitness composite score (calculated from the scores for sit-ups, push-ups, grip strength, and trunk flexibility) and all-cause mortality [22] and the incidence of type 2 diabetes among a Canadian population [23]. In addition, Sawada et al. observed a significant inverse relationship between muscular and performance fitness index composite scores (summed Z scores of sit-ups, side step, and functional reach) and the incidence of type 2 diabetes in Japanese men [24]. In their previous study, a significant inverse relationship was observed between muscular fitness and all-cause mortality and type 2 diabetes. These results suggest that maintaining a high level of MFS may prevent the development of MetS and reduce the risk of type 2 diabetes and mortality.
Strength training may lower MetS risk, including improvement in TG and HDL-c [25], BP [26], central adiposity and body composition [27], and whole-body insulin action and glucose uptake [28,29]. The metabolic effects of reduced muscle mass secondary to aging, decreased physical activity, or both contribute to the presence of obesity, insulin resistance, type 2 diabetes, dyslipidemia, and hypertension [30]. Skeletal muscle, the primary tissue for glucose and triglyceride metabolism, is a determinant of resting metabolic rate, and changes in muscle mass may reduce multiple CVD risk factors [31]. Therefore, with the maintenance of high muscle strength, such as that achieved by resistance training, may prove effective for the prevention and treatment of MetS.
This study has some limitations. First, the causality of relationships cannot be determined due to its cross-sectional design. Longitudinal or interventional studies are required to demonstrate this association further. Second, our sample size is small. In order to better clarify these relationships, future research should be done to increase the sample size.
In conclusion, this study suggests that muscular fitness is inversely associated with MetS in Japanese women and men aged 30 - 79 y. This finding may indicate a protective effect of muscular fitness on MetS. Furthermore, muscle fitness was associated with a better profile for several risk factors of MetS.
5. ACKNOWLEDGEMENTS
We thank the staff of the Fujisawa City Health and Medical Center and Community Health Division of Fujisawa City Hall. This study was supported by a Grant-in-Aid for the Global COE, Waseda University “Sport Sciences for the Promotion of Active Life”, from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
REFERENCES
- Grundy, S.M., Cleeman, J.I., Daniels, S.R., Donato, K.A., Eckel, R.H., Franklin, B.A., Gordon, D.J., Krauss, R.M., Savage, P.J., Smith, S.C. Jr., Spertus, J.A. and Costa, F. (2005) Diagnosis and management of the metabolic syndrome: An American heart association/national heart, lung, and blood institute scientific statement. Circulation, 112, 2735-2752. doi:10.1161/CIRCULATIONAHA.105.169404
- DeFronzo, R.A. (2010) Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia, 53, 1270-1287. doi:10.1007/s00125-010-1684-1
- Laaksonen, D.E., Lakka, H.M., Niskanen, L.K., Kaplan, G.A., Salonen, J.T. and Lakka, T.A. (2002) Metabolic syndrome and development of diabetes mellitus: Application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. American Journal of Epidemiology, 156, 1070-1077. doi:10.1093/aje/kwf145
- Lorenzo, C., Okoloise, M., Williams, K., Stern, M.P. and Haffner, S.M. (2003) The metabolic syndrome as predictor of type 2 diabetes: The San Antonio heart study. Diabetes Care, 26, 3153-3159. doi:10.2337/diacare.26.11.3153
- Lakka, H.M., Laaksonen, D.E., Lakka, T.A., Niskanen, L.K., Kumpusalo, E., Tuomilehto, J. and Salonen, J.T. (2002) The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Journal of the American Medical Association, 288, 2709-2716. doi:10.1001/jama.288.21.2709
- Saito, I., Iso, H., Kokubo, Y., Inoue, M. and Tsugane, S. (2009) Metabolic syndrome and all-cause and cardiovascular disease mortality: Japan public health center-based prospective (JPHC) study. Circulation Journal, 73, 878-884. doi:10.1253/circj.CJ-08-1025
- Hara, K., Matsushita, Y., Horikoshi, M., Yoshiike, N., Yokoyama, T., Tanaka H. and Kadowaki, T. (2006) A proposal for the cutoff point of waist circumference for the diagnosis of metabolic syndrome in the Japanese population. Diabetes Care, 29, 1123-1114. doi:10.2337/dc05-2540
- Alberti, K.G., Zimmet, P. and Shaw, J. (2006) Metabolic syndrome—A new worldwide definition. A consensus statement from the International Diabetes Federation. Diabetic Medicine, 23, 469-480. doi:10.1111/j.1464-5491.2006.01858.x
- Lakka, T.A., Laaksonen, D.E., Lakka, H.M., Männikkö, N., Niskanen, L.K., Rauramaa, R. and Salonen, J.T. (2003) Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Medicine and Science in Sports and Exercise, 35, 1279-1286. doi:10.1249/01.MSS.0000079076.74931.9A
- LaMonte, M.J., Barlow, C.E., Jurca, R., Kampert, J.B., Church, T.S. and Blair, S.N. (2005) Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: A prospective study of men and women. Circulation, 112, 505-512. doi:10.1161/CIRCULATIONAHA.104.503805
- Hassinen, M., Lakka, T.A., Savonen, K., Litmanen, H., Kiviaho, L., Laaksonen, D.E., Komulainen, P. and Rauramaa, R. (2008) Cardiorespiratory fitness as a feature of metabolic syndrome in older men and women: The doseresponses to exercise training study (DR’s EXTRA). Diabetes Care, 31, 1242-1247. doi:10.2337/dc07-2298
- Jurca, R., Lamonte, M.J., Church, T.S., Earnest, C.P., Fitzgerald, S.J., Barlow, C.E., Jordan, A.N., Kampert, J.B. and Blair, S.N. (2004) Associations of muscle strength and fitness with metabolic syndrome in men. Medicine and Science in Sports and Exercise, 36, 1301-1307. doi:10.1249/01.MSS.0000135780.88930.A9
- Jurca, R., Lamonte, M.J., Barlow, C.E., Kampert, J.B., Church, T.S. and Blair, S.N. (2005) Association of muscular strength with incidence of metabolic syndrome in men. Medicine and Science in Sports and Exercise, 37, 1849-1855. doi:10.1249/01.mss.0000175865.17614.74
- Atlantis, E., Martin, S.A., Haren, M.T., Taylor, A.W. and Wittert, G.A. (2009) Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism, 58, 1013-1022. doi:10.1016/j.metabol.2009.02.027
- Wijndaele, K., Duvigneaud, N., Matton, L., Duquet, W., Thomis, M., Beunen, G., Lefevre, J. and Philippaerts, R.M. (2007) Muscular strength, aerobic fitness, and MetS risk in Flemish adults. Medicine and Science in Sports and Exercise, 39, 233-240. doi:10.1249/01.mss.0000247003.32589.a6
- Miyatake, N., Wada, J., Saito, T., Nishikawa, H., Matsumoto, S., Miyachi, M., Makino, H. and Numata, T. (2007) Comparison of muscle strength between Japanese men with and without metabolic syndrome. Acta Medica Okayama, 61, 99-102.
- Minamishima, D., Niu, K., Momma, H., Kobayashi, Y., Guan, L., Sato, M., Guo, H., Ishii, K. and Nagatomi, R. (2010) The relation between isotonic leg extension strength and the prevalence of metabolic syndrome in male adults. Japanese Journal of Physical Fitness and Sports Medicine, 59, 349-356.
- Aoyama, T., Asaka, M., Ishijima, T., Kawano, H., Cao, Z.B., Sakamoto, S., Tabata, I. and Higuchi, M. (2011) Association between muscular strength and metabolic risk in Japanese women, but not in men. Journal of Physiological Anthropology, 30, 133-139. doi:10.2114/jpa2.30.133
- Alberti, K.G., Eckel, R.H., Grundy, S.M., Zimmet, P.Z., Cleeman, J.I., Donato, K.A., Fruchart, J.C., James, W.P., Loria, C.M. and Smith, S.C. Jr. (2009) Harmonizing the MetS: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 120, 1640-1645. doi:10.1161/CIRCULATIONAHA.109.192644
- Rantanen, T., Era, P. and Heikkinen, E. (1994) Maximal isometric strength and mobility among 75-year-old men and women. Age and Ageing, 23,132-137. doi:10.1093/ageing/23.2.132
- Ministry of Education, Culture, Sports, Science and Technology (2010) Physical fitness test (in Japanese). http://www.mext.go.jp/component/a_menu/sports/detail/_icsFiles/afieldfile/2010/07/30/1295079_03.pdf
- Katzmarzyk, P.T. and Craig, C.L. (2002) Musculoskeletal fitness and risk of mortality. Medicine and Science in Sports and Exercise, 34, 740-744. doi:10.1093/ageing/23.2.132
- Katzmarzyk, P.T., Craig, C.L. and Gauvin, L. (2007) Adiposity, physical fitness and incident diabetes: The physical activity longitudinal study. Diabetologia, 50, 538-544. doi:10.1007/s00125-006-0554-3
- Sawada, S.S., Lee, I.M., Naito, H., Tsukamoto, K., Muto, T. and Blair, S.N. (2010) Muscular and performance fitness and the incidence of type 2 diabetes: Prospective study of Japanese men. Journal of Physical Activity & Health, 7, 627-632.
- Fahlman, M.M., Boardley, D., Lambert, C.P. and Flynn, M.G. (2002) Effects of endurance training and resistance training on plasma lipoprotein profiles in elderly women. The Journals of Gerontology Series A Biological Sciences and Medical Sciences, 57, 54-60. doi:10.1093/gerona/57.2.B54
- Carter, J.R., Ray, C.A., Downs, E.M. and Cooke, W.H. (2003) Strength training reduces arterial blood pressure but not sympathetic neural activity in young normotensive subjects. Journal of Applied Physiology, 94, 2212-2216.
- Banz, W.J., Maher, M.A., Thompson, W.G., Bassett, D.R., Moore, W., Ashraf, M., Keefer, D.J. and Zemel, M.B. (2003) Effects of resistance versus aerobic training on coronary artery disease risk factors. Experimental Biology and Medicine, 228, 434-440.
- Andersen, J.L., Schjerling, P., Andersen, L.L. and Dela, F. (2003) Resistance training and insulin action in humans: Effects of de-training. Journal of Physiology, 551, 1049-1058. doi:10.1113/jphysiol.2003.043554
- Holten, M.K., Zacho, M., Gaster, M., Juel, C., Wojtaszewski, J.F. and Dela, F. (2004) Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes, 53, 294-305. doi:10.2337/diabetes.53.2.294
- Klein, S., Burke, L.E., Bray, G.A., Blair, S., Allison, D.B., Pi-Sunyer, X., Hong, Y. and Eckel, R.H. (2004) Clinical implications of obesity with specific focus on cardiovascular disease: A statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation, 110, 2952-2967. doi:10.1161/01.CIR.0000145546.97738.1E
- Braith, R.W. and Stewart, K.J. (2006) Resistance exercise training: Its role in the prevention of cardiovascular disease. Circulation, 113, 2642-2650. doi:10.1161/CIRCULATIONAHA.105.584060

