Materials Sciences and Applications
Vol.3 No.6(2012), Article ID:19851,6 pages DOI:10.4236/msa.2012.36058
Production of Ca2AlNbO6 Ceramics and Study of Their Stability in Crude Petroleum for the Conservation of Metallic Sensing Elements Used in Petroleum Extraction
![]()
Departamento de Engenharia Mecânica, Universidade Federal de Pernambuco, Recife, Brazil.
Email: yadava@ufpe.br
Received January 24th, 2012; revised February 27th, 2012; accepted March 26th, 2012
Keywords: Ca2AlNbO6; Production; Microstructure; Mechanical Properties; Stability in Crude Petroleum
ABSTRACT
In the present work a niobium based complex cubic perovskite oxide Ca2AlNbO6 ceramic was produced, characterized and studied its stability in crude petroleum environment for inert ceramic embedding for temperature sensors used in petroleum extraction. Ca2AlNbO6 ceramic powder was prepared through thermo-mechanical processing. Structural characteristics of calcined material was investigated by powder X-ray diffarctometry, which presented a single phase complex cubic perovskite structure with lattice parameter a = 7.6599 Å. Compacted discs of Ca2AlNbO6 ceramics were sintered in the temperature range 1250˚C - 1350˚C during 24 hours in ambient atmosphere. Microstructure of the sintered ceramics was studied by scanning electron microscopy and mechanical behavior was studied by Vicker’s microhardness testing. Ca2AlNbO6 ceramics sintered at 1350˚C presented best results in terms of microstructural homogeneity and mechanical hardness. Therefore these sintered ceramics were submerged in crude petroleum for 60 days. Ceramics were taken out from the petroleum periodically and subjected to structural, microstructural and mechanical characterizations. Results showed that ceramics submerged in crude petroleum did not suffer any change at any stage of submersion. These characteristics make this material suitable for inert ceramic embedding for sensors used in petroleum extraction.
1. Introduction
Nowadays, there is an increasing demand for sustainable materials and systems to operate in hostile environments such as high temperature or chemically aggressive environments of petroleum extraction industry. In petroleum extraction, different types of sensors are required to monitor temperature, pressure and other vital parameters in the petroleum wells. These sensors work in extremely hostile environment. In case of temperature sensors, normally, sensing elements are metals Pt, Nb etc., which are highly sensitive to environmental conditions. Accordingly, these sensors need to be embedded in materials highly inert to petroleum extraction environment. Ceramics are parts of a broad class of materials whose extremely vast characteristics such as: high thermal capacity, resistance to corrosion, fact that they may be insulating, conducting or superconducting, they have magnetic properties or absence of magnetism, and to be hard and resistant but fragile, make them interesting for various technological applications [1-3].
Study of advanced ceramics makes it possible to develop new technologies, which could be inaccessible with conventional materials and also to substitute scarce materials. Their cast of production turns to be more viable than those of metals and metallic alloys, as they are lighter materials and stable at higher temperatures [4,5]. Earlier research works in 1950 and 1960’s [6,7] identified a large group of ceramics, which have the basic ABO3 perovskite structure or a small distortion of that structure. These complex perovskite oxides generally have the formula A2BB’O6 or A3B2B’O9 and result from the ordering of B and B’ cations on the octahedral sites of the basic perovskite unit cell. Due to the increased complexity of the unit cell, a large variety of such materials are possible and hence a more continuous progression of lattice parameter could be produced [7-11]. Due to high stability in hostile environment, many of the new technologies incorporate provskite structure based ceramics in their manufacture activities for such applications. We are working on fabrication of ceramic encapsulated temperature sensor for petroleum extraction industry [12-14] and in the present work we have developed and produced niobium based complex cubic perovskite oxide Ca2AlNbO6 ceramics and studied their stability in crude petroleum environment for the fabrication of inert ceramic embedding for temperature sensors used in petroleum extraction.
2. Experimental Details
Ca2AlNbO6 ceramic powder was prepared through thermo-mechanical solid-state processing route. Stoichiometric ratios of high purity (99.99%) constituent oxides CaO, Al2O3, and Nb2O5 were mixed and homogenized in a high energy ball mill using steel cylinder and alumina balls. Homogenized mixture was compacted as circular discs of 30 mm diameter with a thickness of about 2 - 3 mm. Uniaxial powder compaction was carried out in a hydraulic press and hard steel mould made of abrasion resistant AISI A2 steel (HRC 58) at a pressure of 4 ton/cm2. The compacted discs were calcined in a high temperature muffle furnace (Forno Jung model 0614) at a temperature of 1200˚C for 24 hours in ambient atmosphere. After calcination, samples were furnace cooled to room temperature. Calcined material was subjected powder X-ray powder diffraction (XRD) analysis for structural characterization and phase identification studies. X-ray diffraction patterns were recorded by using a Shimadzu X-ray powder diffractometer equipped with Cu - Kα radiation (λ = 1.5406 Å). After the structural analysis and identification of Ca2AlNbO6 single phase formation, calcined Ca2AlNbO6 material were again subjected to grinding in high energy ball for 24 hours for ceramic fine powder production and homogenization of particle size distribution. Particle size distribution analysis of was carried out using a LASER particle size analyzer Malvem Instruments model MASTERSIZER 2000, through wet process.
For the fabrication of ceramic components and sintering of Ca2AlNbO6 ceramics, circular discs of 30 mm diameter and 3 mm thickness were produced by uniaxial pressing technique using the same above referred mould and a hydraulic press at a pressure load of 12 ton/cm2. For better compression, each fraction of pressure was applied for 5 minutes to stabilize the pressure load distribution in the compact and homogenize the necessary pressure load. The compressed ceramic discs were subjected to normal solid state sintering process in a temperature range of 1250˚C - 1350˚C for 24 hours in ambient atmosphere, using the same above referred high temperature muffle furnace and finally furnace cooled to room temperature.
Microstructural analysis of the sintered Ca2AlNbO6 ceramics was carried out by scanning electron microscopy (model JEOL JSM-5900), using secondary electrons. To observe the microstructure, the samples were placed in a metal coating device to receive a thin layer of gold, since they are not electrically conducting, necessary for secondary electron emission to get SEM images. Mechanical properties of the sintered ceramics were studied through Vicker’s micro-hardness testing. For the indentation in Vicker’s micro-hardness tester, the samples were polished with # 220, # 400, # 600, # 1200, # 1500 grade sand papers, followed by mechanical polishing using diamond paste with 1 micron particle size. For this testing a Vicker’s hardness indenter model HVS-5 N˚ 0021 was used. The Vickers microhardness (MHV) is given by Equation (1) [15]
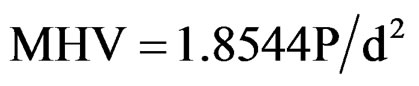 (1)
(1)
where, P is the load and d is the average diagonal of the square indentation produced by the pyramidal indentor in the sample.
For the study of stability of Ca2AlNbO6 ceramics in petroleum extraction environment, sintered ceramics were submerged in crude petroleum for 60 days. Crude petroleum for this study, extracted from the earthen and offshore petroleum wells of the North-east region of Brazil, was provided by PETROBRAS, Brazil. Ca2AlNbO6 ceramics were periodically taken out from the petroleum reservoir every 15 days and subjected to X-ray diffractometry, optical microscopy and mechanical testing to observe if there are any changes in their structural and mechanical characteristics due to crude petroleum environment.
3. Results and Discussion
The XRD pattern of a typical Ca2AlNbO6 ceramics, produced in this work is shown in Figure 1. It consists of strong peaks characteristics of primitive cubic perovskite structure plus few weak reflection lines arising from the superlattice reflections. No evidence for a distortion from the cubic symmetry is observed in the XRD spectrum. The basic perovskite composition is ABO3, where A is a large ion suitable to the 12-coordinated cube-octahedral
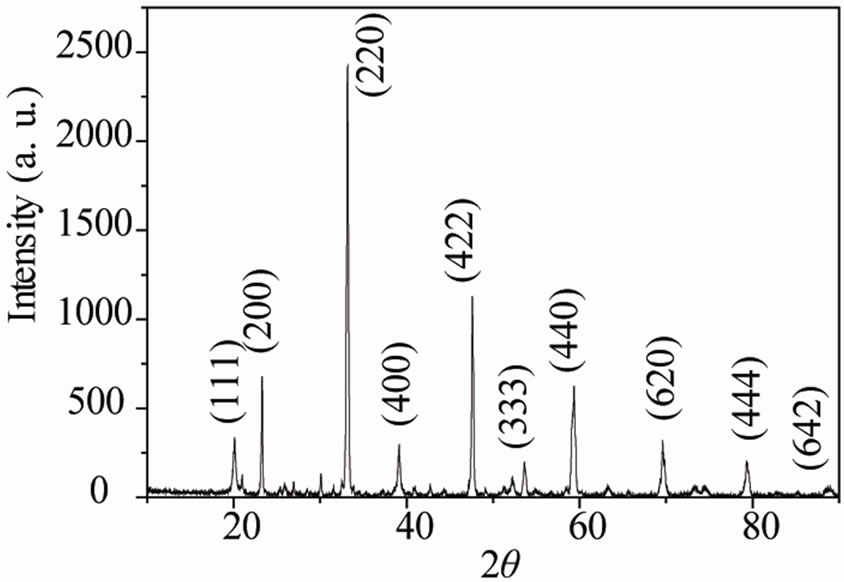
Figure 1. X-ray diffraction pattern of Ca2AlNbO6 ceramic.
sites and B is a smaller ion suitable to the 6-coordinated octahedral site. Complex perovskite with mixed species on a site (particularly the B site) may be represented by multiples of this formula unit and a larger unit cell, e.g. A2BB’O6, A3B2B’O9 etc. Thus, in Ca2AlNbO6 composition, Ca2+, with largest ionic radius (1.05 Å) occupies position A, Al3+ (ionic radius 0.55 Å) and Nb5+ (ionic radius 0.70 Å) cations occupy B and B’ positions on the B site due to their smaller ionic radii compared to that of Ca2+ cation. Due to the ordering of B and B’ on octahedral site of the ABO3 unit cell there is a doubling in the lattice parameter of the basic cubic perovskite unit cell. Thus, the whole XRD pattern of Ca2AlNbO6 can be indexed in an A2BB’O6 cubic cell with the cell edge a = 2ap where ap is the cell lattice of the cubic perovskite. The XRD spectrum of Ca2AlNbO6 is similar to A2BB’O6 type complex cubic perovskite oxides e.g. Ba2YNbO6, Ba2ErSbO6, Ba2DyNbO6 etc. reported in the JCPDS files, as judged by the similarity in d-spacings and intensity ratios. Experimental XRD data of Ca2AlNbO6 ceramics is presented in Table 1 of the superstructure reflection lines (111) and (333) in the XRD spectrum of Ca2AlNbO6 is the signature of an ordered complex cubic perovskite structure. In a substitutional solid solution BB’, there is a random arrangement of B and B’ on equivalent lattice positions in the crystal structure. Upon suitable heat treatment, the random solid solution rearranges into a structure in which B and B’ occupy the same set of positions but in a regular way, such a structure is described as superstructure. In the superstructure, the positions occupied by B and B’ are no longer equivalent and this feature is exhibited in the XRD spectrum of the material by the presence of superstructure reflection lines [16,17].
For double cubic perovskite of the formula A2BB’O6 the intensity, in particular of the (111) and/or (311)
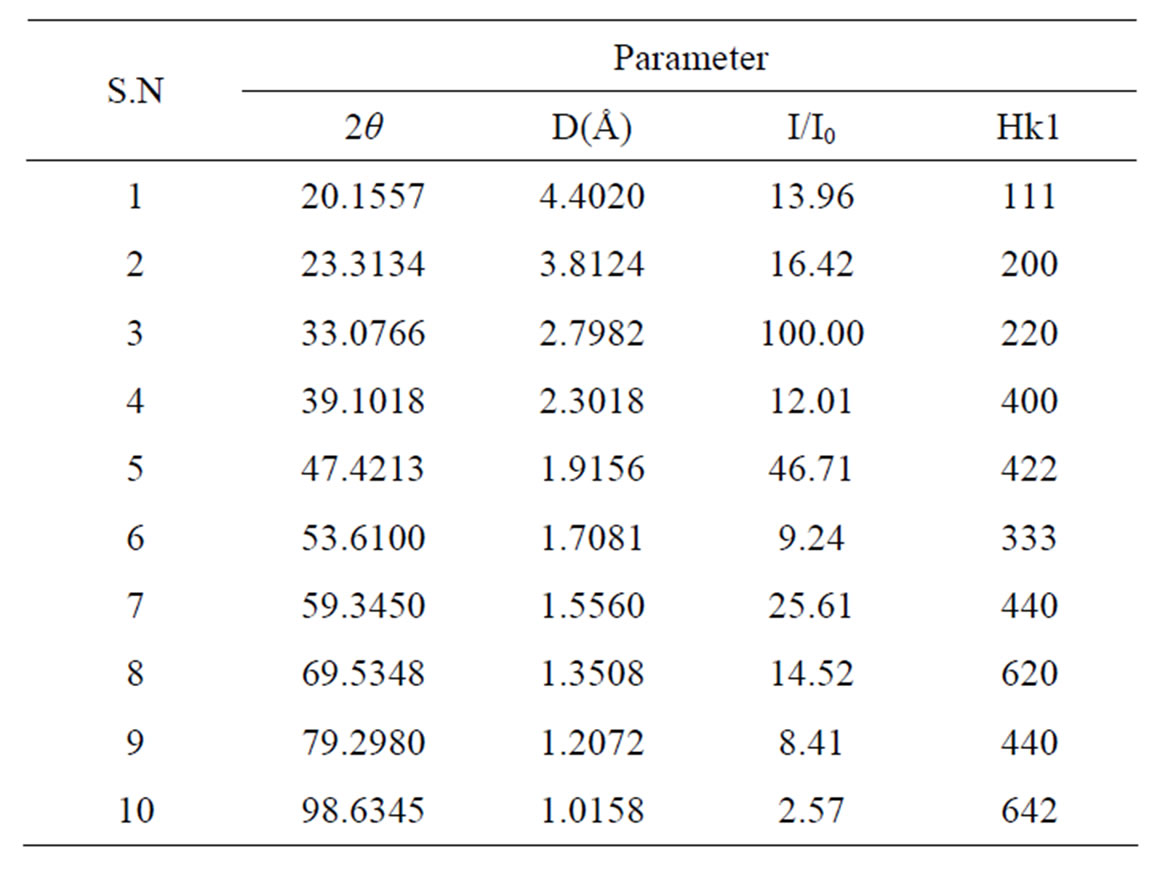
Table 1. XRD data of Ca2AlNbO6.
superstructure reflection, is proportional to the difference in scattering power of the B and B’ atoms, when all the atoms are situated in the ideal position. A disordered arrangement of B and B’ should result in zero intensity. Therefore Al3+ and Nb5+ cation ordering in Ca2AlNbO6 in B and B’ positions is clearly distinguished by presence of the significant intensity of (111) and (333) superstructural reflection lines. Based on above discussion we have now indexed the XRD peaks of Ca2AlNbO6 as an ordered complex cubic perovskite with A2BB’O6 crystal structure. The lattice parameter of Ca2AlNbO6, calculated from the experimental XRD data is aexp = 7.6599 Å.
For the A2BB’O6, type complex cubic perovskite oxides, theoretical value of the unit cell parameter can be calculated using following Shannon and Prewit model Equations (2), (3) and (4) [18]:
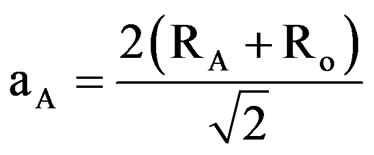 (2)
(2)
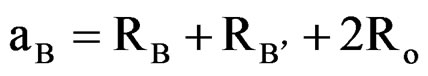 (3)
(3)
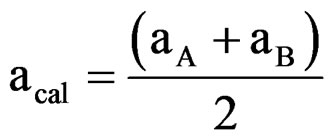 (4)
(4)
where, 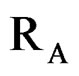 ,
, 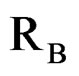 ,
, 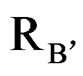 and
and 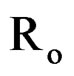 are ionic radius of A, B, B’ cations and oxygen, respectively. Using these equations theoretical value of unit cell parameter of Ca2AlNbO6 is acal = 7.5148Å. Experimental value of unit cell parameter of Ca2AlNbO6 (aexp = 7.6599 Å) is 1.93% higher than its theoretical value (acal = 7.5148 Å), which can be justified because Shannon and Prewit model21 is based on hard sphere approximation.
are ionic radius of A, B, B’ cations and oxygen, respectively. Using these equations theoretical value of unit cell parameter of Ca2AlNbO6 is acal = 7.5148Å. Experimental value of unit cell parameter of Ca2AlNbO6 (aexp = 7.6599 Å) is 1.93% higher than its theoretical value (acal = 7.5148 Å), which can be justified because Shannon and Prewit model21 is based on hard sphere approximation.
As stated earlier, the objective of this work is to produce Ca2AlNbO6 ceramics with good mechanical strength in order to guarantee the required qualities that a final ceramic product must have for structural applications. Production and functional ability of polycrystalline ceramic products are highly dependent on their microstructural features, which in turn are highly influenced by sintering kinetics. Microstructural features define the final product quality of the ceramic products and their mechanical strength.
Microstructural features of Ca2AlNbO6 ceramics sintered at different temperatures (1250˚C - 1350˚C) for 24 hours, was studied by scanning electron microscopy. Figure 2(a)-(c) present typical microstructures of these ceramics. As seen from these figures, sintering temperature has a significant role in sintering behavior of these ceramics. Ca2AlNbO6 ceramics sintered at 1350˚C presented better microstructures with uniform surface morphology, grain homogeneity and particle size distribution.
Mechanical properties of Ca2AlNbO6 ceramics sintered at different temperatures were evaluated by Vicker’s mi-
 (a)
(a) (b)
(b) (c)
(c)
Figure 2. SEM micrographs of Ca2AlNbO6 ceramics sintered at different temperatures: (a) 1250˚C; (b) 1300˚C and (c) 1350˚C.
crohardness tests. These tests were performed on 10 polished discs of Ca2AlNbO6 ceramics, sintered at each temperature (1250˚C - 1350˚C), with 10 indentations on each discs to obtain the arithmetical mean value. Results of these tests are presented in Table 2. Ceramics sintered at 1350˚C presented much higher MHV values than ceramics sintered at lower temperatures, which is closely related to better microstructure of these ceramics observed in SEM studies.
For the study of stability of these ceramics in petroleum extraction environment, we used Ca2AlNbO6 discs sintered at 1350˚C as these discs presented better microstructural features and mechanical hardness. These discs were firstly polished with mirror like polished surface and subjected to optical microscopy. After observation in optical microscopy these discs were partitioned in two equal parts, using diamond disc cutter, to be submersed each one part in the crude petroleum extracted from earthen and offshore petroleum wells, respectively. This was carried out purposely to ensure that ceramics submersed in crude petroleum extracted from earthen and offshore petroleum wells have the same microstructure and mechanical hardness. Ceramics were submerged in crude petroleum for 60 days. Ceramics were taken out from the petroleum reservoir periodically, every 15 days and tested to observe if there are any changes in their structural, microstructural and mechanical characteristics due to crude petroleum environment.
Typical results of X-ray diffractometry carried out on Ca2AlNbO6 ceramics after 60 days of submersion in earthen and offshore crude petroleum are shown in Figure 3 along with the XRD spectra of Ca2AlNbO6 ceramics before submersion in crude petroleum. As seen from Figure 3 both the XRD spectrum of Ca2AlNbO6 ceramics submersed in earthen and offshore petroleum are
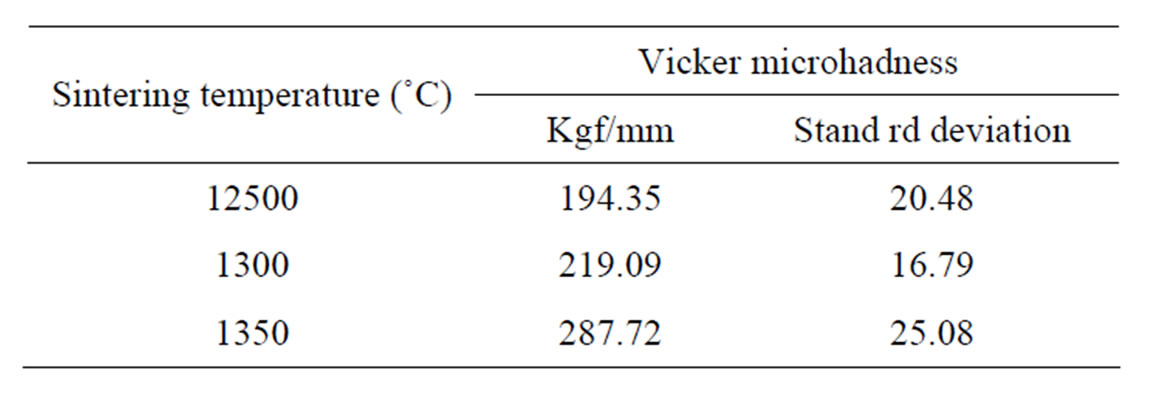
Table 2. Vicker’s microhardness of Ca2AlNbO6 ceramics sintered at different temperatures.
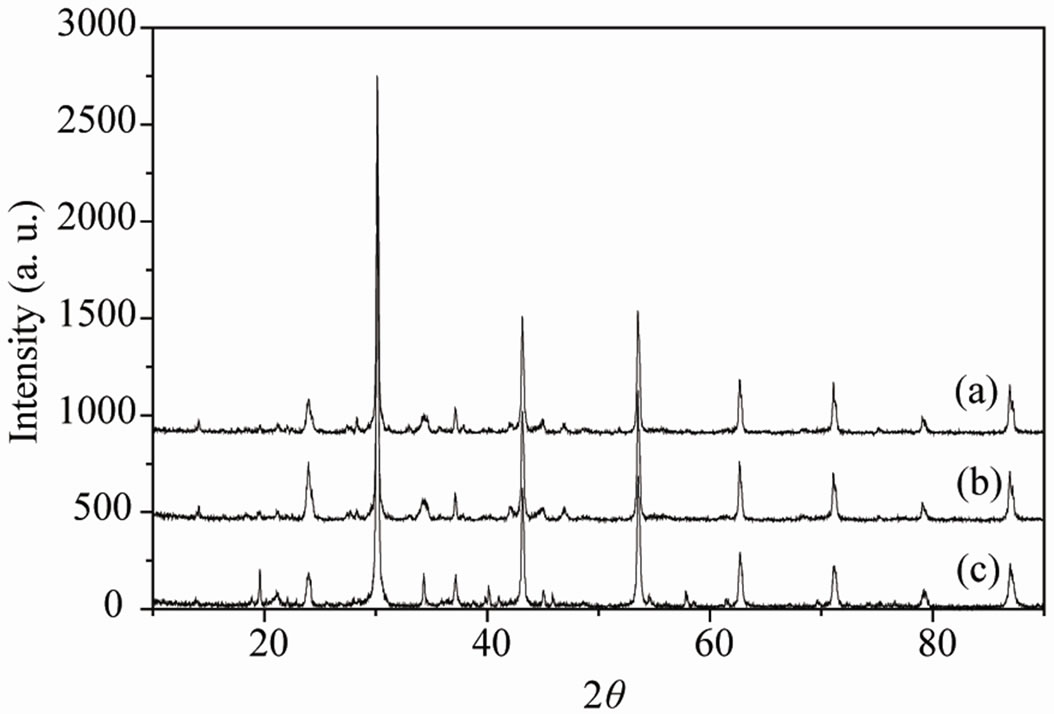
Figure 3. X-ray diffraction patterns of Ca2AlNbO6 ceramics: (a) Before submersion in crude petroleum; (b) after 60 days submersion in earthen crude petroleum and (c) after 60 days submersion in off shore crude petroleum.
identical to the XRD spectrum of XRD spectrum of Ca2AlNbO6 ceramics before submersion in crude petroleum. They did not present any impurity peaks or other phases. This shows that Ca2AlNbO6 ceramics did not suffered any structural changes due to crude petroleum environment.
Typical Vickers microhardness tests results carried out on Ca2AlNbO6 ceramics before and after 60 days of submersion in crude petroleum are presented in Table 3. As we can see from these results Ca2AlNbO6 ceramics did not suffer practically any change in their mechanical hardness due to due to crude petroleum environment.
Microstructural analysis of Ca2AlNbO6 ceramics after submersion in crude petroleum was carried out using optical microscopy because after submersion in crude petroleum it was extremely difficult to use scanning electron microscopy because of vacuum necessities. Figure 4 presents the optical micrographs of Ca2AlNbO6 ceramics before and after submersion in crude petroleum for 45 and 60 days respectively. As seen from Figure 4, microstructural features of Ca2AlNbO6 ceramics remain identical before and after submersion in crude petroleum without any degradation in microstructural features due to crude petroleum environment.
From these promising results we conclude that Ca2AlNbO6 ceramics are stable in crude petroleum environment and thus could be potential candidate ceramic encapsulation in the fabrication of temperature sensors for temperature monitoring in petroleum wells.
4. Conclusion
In the present work we have produced a niobium based complex cubic perovskite oxide Ca2AlNbO6 ceramics and studied their stability in crude petroleum environment for the manufacture of inert ceramic embedding for temperature sensors used in petroleum extraction. Ca2AlNbO6 ceramic powder was prepared through thermomechanical solid-state processing route. Structural characteristics and phase identification of calcined material was investigated by powder X-ray diffarctometry, which presented a single phase complex cubic perovskite structure with lattice parameter a = 7.6599 Å. Microstructure of the Ca2AlNbO6 ceramics, sintered at 1350˚C presented a high homogenous morphology and particle size distribution. Mechanical behavior of the sintered ceramics was studied by Vickers micro-hardness testing, which gave a reasonable value of MHV = 287.72, for ceramics sintered at 1350˚C, which is reasonable for encapsulation application of these ceramics. Results of X-ray diffractometry, microstructural and mechanical tests showed that Ca2AlNbO6 ceramics submerged in crude petroleum did not suffer any change at any stage of submersion. From these promising results we conclude that Ca2AlNbO6 ceramics are stable in the aggressive crude petroleum environment
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e)
Figure 4. Optical micrograph (200×) of Ca2AlNbO6 ceramics before and after submersion in crude petroleum: (a) before submersion in crude petroleum; (b) and (c) after 45 and 60 days submersion, respectively, in earthen crude petroleum and ((d) and (e)) after 45 and 60 days submersion, respectively, in offshore crude petroleum.
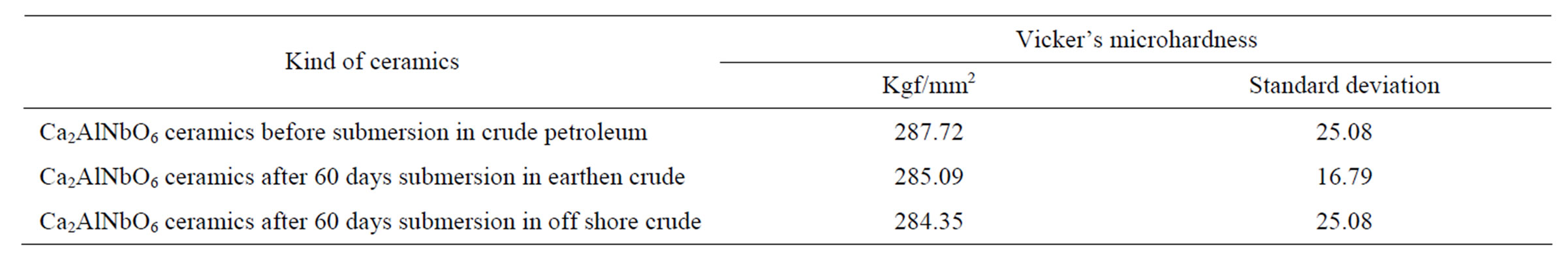
Table 3. Vicker’s microharness of Ca2AlNbO6 ceramics before and after submersion in crude petroleum for 60 days.
and thus could be potential candidate ceramic encapsulation in the fabrication of temperature sensors for temperature monitoring in petroleum wells.
5. Acknowledgements
Authors acknowledge CNPq for the financial support for this research project.
REFERENCES
- M. Chandler, “Ceramics in the Modern World,” Double Day & Co. Inc., New York, 1967.
- J. J. Reed, “Principles of Ceramic Processing,” John Wiley and Sons, New York, 1988.
- D. W. Richardson, “Modern Ceramic Engineering,” Marcel Dekker Inc., New York, 1982.
- L. G. Tejuca and J. L. G. Fierrro, “Properties and Applications of Perovskite Type Oxides,” Marcel Decker Inc., New York, 1993.
- M. H. Lewis, “Engineering ceramics: Twentieth Century Developments and prospects for the New Millennium,” Proceedings of International Symposium on Materials for the Third Millennium, Kanpur, 2001, pp. 47-73.
- F. Galasso, L. Katz and R. Ward, “Substitution in the Octahedrally Coordinated Cation Positions in Compounds of the Perovskite Type,” Journal of the American Ceramic Society, Vol. 81, 1959, p. 820.
- F. Galaso, J. R. Barrante and L. Katz, “Alkaline Earth Tantalum-Oxygen Phases including Crystal Structure of an Ordered Perovskite Compound Ba3SrTa2O9,” Journal of the American Chemical Society, Vol. 83, No. 13, 1961, pp. 2830-2832. doi:10.1021/ja01474a010
- C. D. Brandle and V. J. Fratello, “Preparation of Perovskite Oxides for High Tc Superconductor Substrates,” Journal of Materials Research, Vol. 5, No. 10, 1990, pp. 2160- 2164. doi:10.1557/JMR.1990.2160
- V. J. Fratello, C. W. Berkstresser, C. D. Brandle and A. J. Van Graitis, “Nickel containing Perovskites,” Journal of Crystal Growth, Vol. 166, No. 1-4, 1996, pp. 878-882. doi:10.1016/0022-0248(95)00474-2
- J. E. Hove and W. C. Riley, “Modern Ceramics,” John Wiley and Sons, New York, 1965.
- S. Y. Istomin, G. Svensson and J. Kohler, “Structures and properties of the Perovskite-Type Compounds Na1-xSrx- NbO3 (0.1 ≤ x ≤ 0.9)—From insulating to Metallic Conductivity,” Journal of Solid State Chemistry, Vol. 167, No. 1, 2002, pp. 7-16. doi:10.1006/jssc.2002.9603
- C. M. Lapa, R. A. S. Ferreira, J. A. Aguiar, C. L. Silva, D. P. F. Souza and Y. P. Yadava, “Sintering, microstructure and Mechanical Properties of Ba2HoWO5.5 ceramics,” Materials Science Forum, Vol. 498-499, 2005, pp. 529- 534. doi:10.4028/www.scientific.net/MSF.498-499.529
- Y. P. Yadava and R. A. S. Ferreira, “Study of Sintering Behavior of Ba2AlSn5.5 Ceramic Powder Compacts as substrate for Temperature Sensing Devices,” Materials Science Forum, Vol. 591-593, 2008, pp. 661-666. doi:10.4028/www.scientific.net/MSF.591-593.661
- Y. P. Yadava and R. A. S. Ferreira, “High density Ba2- AlWO5.5 Ceramic Components Produced through Liquid Phase Sintering Route for Temperature Sensors,” Materials Science Forum, Vol. 591-593, 2008, pp. 448-453. doi:10.4028/www.scientific.net/MSF.591-593.448
- A. Iost and R. Bigot, “Identation size Effect: Reality or Artefact,” Journal of Materials Science, Vol. 31, No. 13, 1996, pp. 3573-3577.
- J. Koshy, K. S. Kumar, J. Kurian, Y. P. Yadava and A. D. Damodaran, “Rare-earth Barium Stannates Synthesis, Characterization and Potential use as Substrate for YBa2- Cu3O7-δ Superconductor,” Journal of the American Ceramic Society, Vol. 78, No. 11, 1995, pp. 3088-3092. doi:10.1111/j.1151-2916.1995.tb09087.x
- P. Jha and A. K. Ganguli, “Complex Rare-Earth Titanates with the Perovskite Structure: Rietveld Studies and dielectric properties,” Solid State Sciences, Vol. 6, No. 7, 2004, pp. 663-671. doi:10.1016/j.solidstatesciences.2004.03.028
- R. D. Shannon and C. T. Prewit, “Effective Ionic Radii in Oxides and luorides,” Acta Crystallographica Section B, Vol. 25, 1969, pp. 925-946. doi:10.1107/S0567740869003220

