Advances in Bioscience and Biotechnology
Vol.3 No.3(2012), Article ID:20004,7 pages DOI:10.4236/abb.2012.33034
In vitro activity of cationic peptides against Neisseria gonorrhoeae and vaginal Lactobacillus species: The effect of divalent cations
![]()
1Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh, Pittsburgh, USA
2Magee-Womens Research Institute, Pittsburgh, USA
3Department of Molecular Genetics and Biochemistry, School of Medicine, Biomedical Sciences Tower, University of Pittsburgh, Pittsburgh, USA
Email: *bjm4@pitt.edu, mietzner@pitt.edu, slh6@pitt.edu
Received 21 March 2012; revised 30 April 2012; accepted 5 May 2012
Keywords: Microbicides; HIV; Lactobacillus; Antimicrobial Peptides; Cations; Neisseria gonorrhoeae
ABSTRACT
One of the new strategies for the prevention of HIV acquisition is the use of microbicides such as topical microbicides including antimicrobial and antiviral peptides. Ideally, new drug candidates should kill pathogens without determent to the normal bacterial flora considered important in health; such as hydrogen peroxide producing Lactobacillus species. The antimicrobial peptides LL-37 and LSA-5 were studied to determine their spectrum of activity against bacterial pathogens and normal flora organisms. The effects of divalent cations at biologically relevant concentrations were determined. We show the synthetic lytic peptide LSA-5 and the naturally occurring peptides LL-37 inactivate Neisseria gonorrhoeae but are less active against many normal flora members such as Lactobacillus species. Biologically relevant concentrations of calcium and magnesium prevented killing of sensitive strains. LSA-5 is more potent than LL-37, both are inhibited from killing sensitive strains by calcium and magnesium. Strains of Lactobacillus iners were killed by both microbicides even in the presence of the divalent cations. Antimicrobial peptides, such as LSA-5, have good potential for use in prevention of sexually transmitted disease, if formulated to sequester calcium and magnesium present in biological fluids.
1. INTRODUCTION
Antimicrobial peptides, both host-derived and engineered, have great potential for the treatment and prevention of human diseases [1-3]. The rapid development of antibiotic resistance among many of the important pathogens extends the potential application of peptides to include topical and systemic uses. Internal uses such as intravenous or as a topical vaginal microbicides introduce the possibility of interference or inactivation by endogenous factors present in plasma or vaginal fluid. Microbicides are being developed for intra-vaginal use in the prevention of the acquisition of sexually transmitted infections (STIs) such as HIV, Neisseria gonorrhoeae and Trichomonas vaginalis. A microbicide must prevent infection by either rapidly killing or reducing the number of pathogens to below the infectious dose in a reasonably short time. Microbicides are evaluated by their killing efficacy, which describes the drug concentration required to reduce viability by 99.99% (a four log reduction) in a given time. This differs from conventional antibiotics where inhibition of growth is used to determine activity [4].
Lentivirus Lytic Peptides (LLPs) are highly conserved among the HIV isolates and are structurally similar to the magainins and human cathelicidin [5-8]. These peptides are based on the 28-residues of the C-terminus of the HIV-1 transmembrane protein gp41 [8]. Cellular toxicity results from the perturbation of the cell membranes [9-14]. By making specific amino acid substitutions different antimicrobial peptide derivatives have been devised with increased potency and specificity [6,9,10].
The normal healthy vagina has a predominance of hydrogen peroxide (H2O2) producing Lactobacillus species that are important in the maintenance of health and prevention of the STI acquisition [15,16]. Disease states such as bacterial vaginosis are characterized by a decrease or loss of H2O2 producing lactobacilli [17]. Candidate microbicides are screened for their ability to specifically kill pathogens but not beneficial members of the normal flora such as L. crispatus and L. jensenii.
The vaginal ecosystem is complex and dynamic. Vaginal fluid contains serum transudate, mucins and other proteins. Vaginal fluid has higher concentrations of calcium (8.5 mM) and magnesium (2.5 mM) than are found in plasma or interstitial tissues [18,19]. The pH of the vagina is usually around 4.0 - 4.5 but does increase to around pH 6.0 - 7.0 during menses or following intercourse. Therefore, testing at both low and high pH is advisable. We have examined several antimicrobial peptides to determine their efficacy in killing sexually transmitted pathogens and normal flora using conditions that mimic those found in the vagina.
We report here that the engineered peptide LSA-5 and the naturally occurring peptide LL-37 demonstrated the appropriate selectivity to kill pathogens such as Neisseria gonorrhoeae and non-beneficial bacteria such as Lactobacillus iners while not killing the beneficial Lactobacillus species. The activity of both peptides against N. gonorrhoeae and sensitive Lactobacillus isolates was inhibited by calcium and magnesium ions. These ions also inhibited other antimicrobial peptides.
2. MATERIAL AND METHODS
2.1. Antimicrobial Peptides
Peptides were synthesized using the previously described FMOC protocols. Synthetic peptides were characterized and purified by reverse-phase HPLC procedures using C18 resin and increasing concentrations of acetonitrile as an eluent in the presence of 0.1% trifluoroacetate [20]. The formula weight of LL-37 is 4492 and LSA-5 is 3817. The sequence of LL-37 is: leu-leu-gly-asp-phe-phe-arglys-ser-lys-glu-lys-ile-gly-lys-glu-phe-lys-arg-ile-val-gln-ile-arg-lys-asp-phe-leu-arg-asn-leu-val-pro-arg-thr-glu-ser [21]. The sequence of LSA-5 is: arg-val-ile-arg-valval-gln-arg-ala-cys-arg-ala-ile-arg-his-ile-val-arg-arg-ile-arg-gln-gly-leu-arg-arg-ile-leu-arg-val-val.
2.2. Microorganisms and Culture Conditions
Reference strains were obtained from the American Type Culture Collection, ATCC (Manassas, VA). Clinical isolates were obtained from swab specimens from women enrolled in clinical studies from Allegheny County Health Department and Magee-Womens Hospital. N. gonorrhoeae was identified using Gonochek IIÒ (PML Microbiologicals) and confirmed using an amplified nucleic acid test with the BDProbe Tec ET instrument (strand displacement amplification assay [SDA]; Becton Dickenson, (Sparks, MD) [22]. Lactobacillus species were identified to the species level based on DNA-DNA homology to the type strains as previously described [17]. Organisms were stored frozen at –70˚C in litmus milk until needed. Stock cultures were revived by inoculation onto either 5% sheep blood agar plates (Columbia blood agar base for Lactobacillus, PML Microbiologicals, Wilsonville, OR.) or chocolate agar (PML Microbiologicals, or prepared in house) for N. gonorrhoeae. Cultures were incubated at 37˚C in air enriched to 6% CO2 overnight and evaluated for growth.
ACES buffer (N-(2-acetamido)-2-aminoethanesulfonic acid, Sigma Chemical Co. St. Louis, MO) was used at a pH of 7.0 and lactate buffer was used at pH 5.0. The isotonic strength of each lot of buffer was determined in a Wescor Vapro Pressure Osmometer 5520 (Logan, UT) and adjusted to 200 - 300 mosm/kg prior to use.
2.3. Antimicrobial Assays
In preliminary experiments, the activity and final peptide concentration of the peptides were compared when solid agents were first dissolved in distilled water or distilled water containing 0.01% glacial acetic acid. For both conditions comparable concentrations and experimental results were observed; therefore, in all subsequent work peptides were dissolved in distilled water to a concentration of 1.1 mM. Peptide concentrations of the working stock solutions were determined using a ninhydrin reaction [23].
Minimum cidal concentrations (MCCs) were determined in lactate (pH 5.0) and ACES (pH 7.0) buffers as previously described [4,24]. Initial concentrations used ranged from 40 mM down to as low as 0.01 mM. In subsequent work, more focused concentrations were used. Assays were performed in Costar (Corning, NY) polypropylene 96 well plates Briefly, isolated colonies were selected from fresh overnight culture plates and suspended in saline to a density of a 0.5 McFarland standard and diluting 1:10 in sterile saline. A 10 µl volume of the bacterial suspension was added to 90 µl of test solution. After incubation at 35˚C for 30 min, 25 ml samples were taken and plated on to the appropriate medium, allowed to absorb and dry for 10 - 15 min then spread over the surface of the agar plate. This allows for an initial separation of organisms from the antimicrobial agent and facilitates the observation of inhibitory activity since organisms that are inhibited, but not killed, are observed as growth away from the point of sample application. Plates were incubated as described above for 24 h and evaluated for killing of the test microorganisms by counting colony forming units. Samples yielding 10 or fewer colony forming units represent a four log kill and were considered sensitive to killing.
The effects of divalent cations were determined by the addition of CaCl2 or MgCl2 prepared as 1 M stock solutions and filter sterilized. The salt solutions were added to the assay buffers prior to the addition of peptides or bacteria.
3. RESULTS
The minimum cidal concentrations (MCC) of LSA-5 and LL-37 for N. gonorrhoeae, L. crispatus, L. jensenii, L. iners and P. aeruginosa are presented in Tables 1 and 2. For the strains where killing was observed, the general trend was that LSA-5 was more active as evidenced by the lower MCC values compared with LL-37.
The differences in the MCCs were five-fold higher for LL-37 at pH 7.0 than at pH 5.0. Differences in MCCs for the two peptides for L. iners and P. aeruginosa ranged from 2 to greater than 20 fold, respectively. The other microorganisms were consistently more sensitive to LSA-5 than LL-37. N. gonorrhoeae strains DOD 431 and UPS 2015 required higher concentrations than the other isolates tested >40 µM and 20 µM respectively.
Calcium and magnesium effects on antimicrobial activity. In early experiments we attempted to determine bacterial killing in rich media such as minimal essential medium (MEM) and trypticase soy broth, but killing was never observed. Because the concentration of divalent cations in MEM is about 26 mM, an equal concentration of EDTA was incorporated into MEM. EDTA binds calcium and magnesium ions effectively removing them from solution and therefore unavailable. This restored antimicrobial activity for sensitive bacteria; therefore, we systematically investigated the role of calcium and magnesium ions on killing by the panel of antimicrobial peptides in buffers appropriate for use with these ions. The results are presented in Tables 1 and 2. N. gonorrhoeae were protected by both ions, but calcium and magnesium afforded protection to more strains at pH 5.0 than 7.0, and calcium was more effective than magnesium.
L. crispatus was not killed by the LSA-5 at any concentration tested (up to 40 µM) and the divalent cations had no influence. L. jensenii demonstrated protection by calcium and magnesium ions among the strains with MCCs less than 10 µM (Table 1). L. iners was uniformly sensitive to killing by LSA-5 and was not protected from killing by the addition of the ions (Table 2). Experiments using LL-37 demonstrated the same trends as observed with the LSA-5, except 5 of 11 L. iners strains demonstrated MCCs to LL-37 greater than 20 mM, Table 2. Unlike LSA-5 there were no differences in MCCs for the individual L. iners strains with the LL-37 at the two different pHs (Table 2). Experiments with the structurally similar synthetic lytic peptides LBU-2, WLSA-5 and WLBU-2, that are under development for other therapies demonstrated protection by calcium and magnesium (data not shown) [6,9,12].
4. DISCUSSION
Condom use by males significantly reduces the risk of STD acquisition [25] but often women are coerced into unsafe sex practices by the unwillingness of their male partners to use condoms. The use of topical microbicides would allow protection in the absence of condom use. Antimicrobial peptides occur naturally, and are believed to play an important role in the host defenses [26,27].
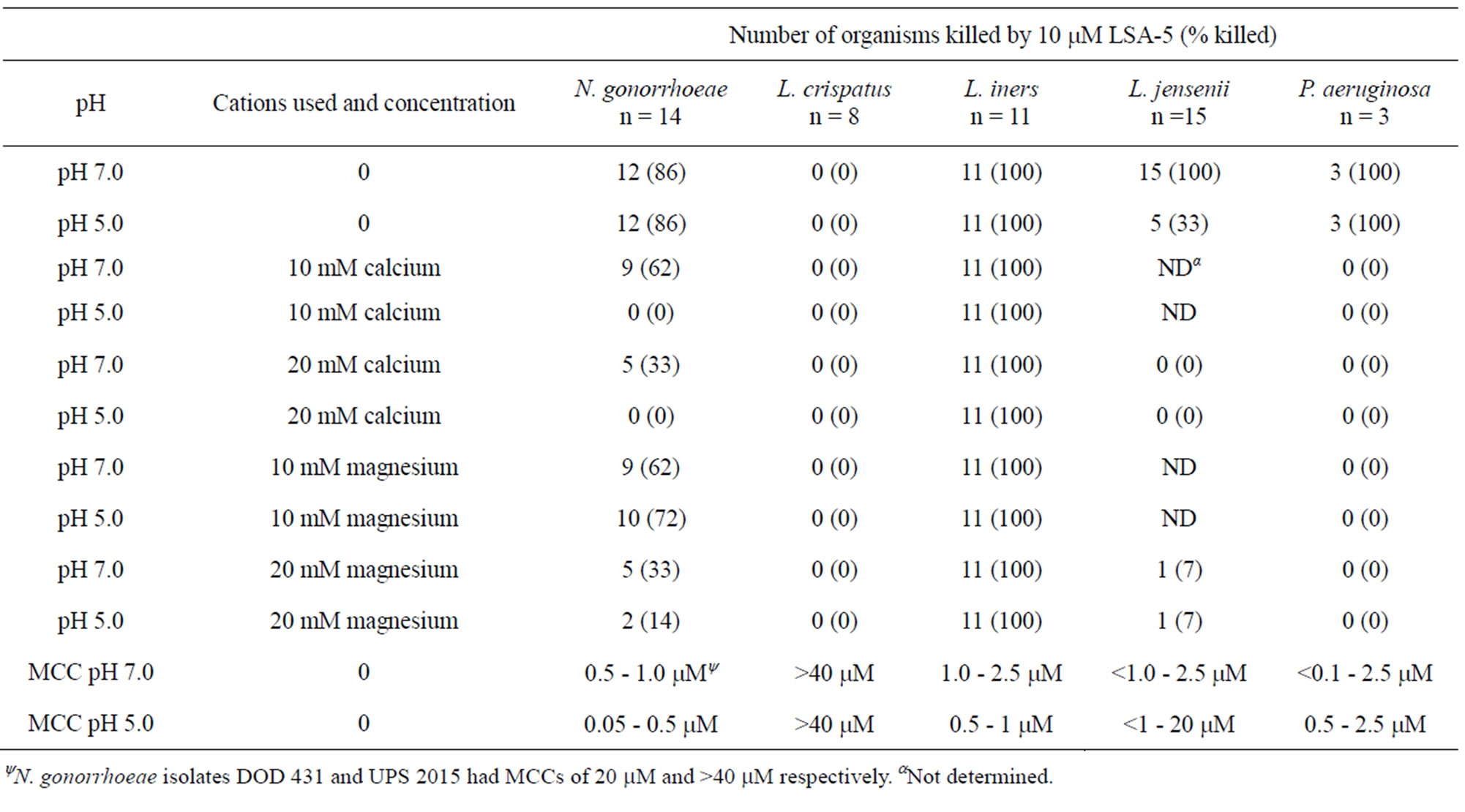
Table 1. Minimal cidalconcentrations, and the effects of calcium and magnesium ions and pH on killing of test organism by the antimicrobial peptide LSA-5.
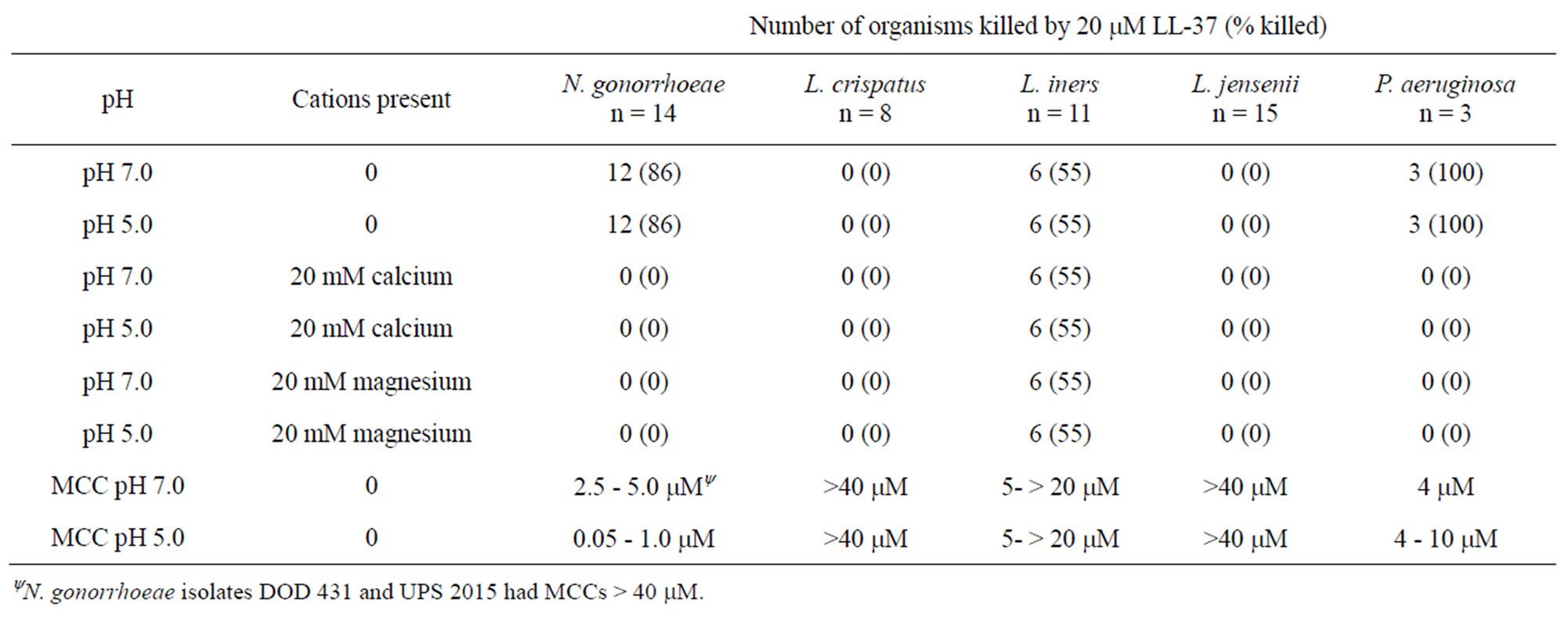
Table 2. Minimal cidal concentrations and the effects of calcium and magnesium ions and pH on killing of test organism by the antimicrobial peptide LL-37.
We have surveyed a number of antimicrobial peptides whose structures are based on the lytic portion of the HIV gp41 transmembrane glycoprotein, and found that LSA-5 appears to meet our requirements of killing pathogens such as N. gonorrhoeae at low concentrations, and not active against the protective/healthy microbiota of the vagina such as the hydrogen peroxide producing lactobacilli L. crispatus and L. jensenii.
The coating and duration of the epithelial and vaginal surface by products will vary with product formulation [28]. The standard for microbicides is a four log reduction in the viability within 30 min. Another useful measure a bactericidal activity is the decimal reduction value (D-value) which describes the time required by a given agent to reduce the viability of the test cultures by 90% or one log. The MCC simply describes the concentration at which organisms have a decimal reduction value (D value) of 6 minutes or less. Decimal reduction times for the test organisms in the buffers used (at or above the MCC) ranged from 1 - 1.5 min for N. gonorrhoeae and from 2 - 3 min for the lactobacillus (data not shown). Therefore the viability of the test organisms should have been reduced to less than 1/ml–1 in 5 minutes. In theory, after 30 minutes a 20 log reduction in viability could be expected but the cultures were still viable in the presence of calcium or magnesium and peptide. This and the fact that all experiments used controls that omitted peptide for comparison, suggests the protection from the divalent cations is not an artifact of the system.
Comparing the activity of LL-37 (cathelicidin) with that of LSA-5 for the lactobacilli, the LSA-5 was much more active against the L. iners than was LL-37, ranging from 5 to greater than 20 times, at both pH 5.0 and 7.0. Most of the L. jensenii were more resistant to killing by LSA-5 at pH 5.0 than pH 7.0 which may reflect the natural habitat of this species; the vagina where the pH is about 4.0. Interestingly, the hydrogen peroxide producing species (L. jensenii and L. crispatus) were more resistant to the antimicrobial peptides than L. iners, which does not produce H2O2. The presence of L. iners has been associated with increased risk of HIV acquisition [29], suggesting decreasing its’ numbers would be beneficial.
Caution should be used in the interpretation of the data obtained with N. gonorrhoeae at pH 5.0, since the organisms are sensitive to acidic pH. However, we have compared viability of strains of N. gonorrhoeae in the buffers used and GC broth buffer to pH 5.0 with lactate buffer, pH 7.0 with ACES buffer or phosphate buffer. At pH 7.0 there was no difference in viability; however, at pH 5.0 cells suspended in lactate buffer had a loss in viability which was about 20% after 30 minutes of incubation. The presence of added calcium to the lactate buffer did not increase survival. Therefore, the differences observed in the MCCs at pH 5.0 and pH 7.0 may reflect increased sensitivity to killing brought on by the acidic conditions. The same does not apply to Lactobacillus species tested whose natural habitat is the vagina where the pH is usually low (pH 4 - 5), suggesting the observations at acidic pH are not an artifact of the system.
Most of the N. gonorrhoeae isolates tested, 12 of 14, had low MCCs for LSA-5 (<1.0 µM) and LL-37 (5 µM). Isolates UPS 2015 and DOD 431 have proven to be resistant to killing by all the lytic peptides we have tested to date as well as nonoxynol-9 (29). We have observed no other phenotypic difference with these isolates. Strain DOD 431 demonstrated intermediate sensitivity to LSA- 5, with an MCC some forty fold greater than most other strains tested (at pH 5.0). There are reports in the literature to indicate that Neisseria resistance may develop through modification of the structure of the cell wall lipooligosaccharide and from acquisition of an efflux pump [30-32]. From these data, it is not possible to discriminate the underlying cause for the resistance. Perhaps of more significance is the observation of protection from killing by calcium and magnesium ions. Turner et al. reported difficulty in determining MICs for LL-37 when microbiological culture media was used [26]. When the medium was passed over an ion-exchange column they were able to observe activity. They also noted that the addition of 1 mM calcium resulted in an increase in the MIC for Escherichia coli but not Listeria monocytogenes. Their observations suggest a difference in the other most portions of the cells since E. coli is gram negative while L. monocytogenes is gram positive. The authors suggested that there are peptide fragments which may bind to or possibly precipitate the LL-37 to explain the reduced activity in complex microbiological media. Although we cannot dismiss this possibility, our data suggest that divalent cations are primarily responsible for the reduced cidal activity of these lytic peptides since the addition of EDTA resulted in higher antimicrobial activity. Further, their observations with the addition of 1 mM calcium to the assays, is consistent with ours and supports our hypothesis that the ions have their effects on the bacteria, not the peptide.
The concentration of calcium and magnesium ions in vaginal fluid is about 8 mM and 2.5 mM respectively [18,19]. Many of the N. gonorrhoeae isolates were protected by as little as 1 mM calcium. Turner et al. using an MIC assay reported the protection from LL-37 of Escherichia coli but not Listeria monocytogenes by 1.25 mM calcium [26]. The mechanism for ion induced resistance probably relates to the lipophilic and cationic nature of the peptides. It is believed that the cationic portions of the peptide interact with the negative charge on the surface of the bacteria facilitating the lipophilic region of the peptide’s interaction with the membrane leading to disruption, and ultimately cell death. It is unlikely calcium and magnesium act to alter the structure of these peptides since all these antimicrobial peptides studied demonstrated the same effect. It is well established that the lytic effects of such peptides are directly related to the propensity of a peptide to form an α-helical structure when they interact with a membrane [6]. This property is influenced by the concentration of the peptide and the anions present [5,11]. Finally, if the peptides conformation was changed by the addition of cations the L. iners strains examined should have been protected as well, but were not. Calcium and magnesium cations are known to interact with the bacterial membranes, cytoplasmic and or outer membranes and prevent the binding of the peptide to target or stabilize the membrane [5,10,12-14]. It is significant that the inclusion of EDTA to complex media containing calcium and magnesium was adequate to bind sufficient divalent cations to allow the microbiocidal properties of the peptides to function since many over the counter vaginal products contain EDTA, it would seem reasonable to assume that the topical administration of peptides in vivo would be effective. The inclusion of EDTA in the final formulated product may offer many additional benefits such as preservation, antioxidation and it may destabilize the membranes of target bacteria especially gram negative organisms.
The existence of such a resistant strain of N. gonorrhoeae as UPS 2015 would seem more problematical as it demonstrates the existence of microbicide resistance prior to any in vivo use of such synthetic lytic peptides as LSA-5.
It is difficult to describe or predict the availability of free calcium and magnesium ions in vivo. Large proportions are bound to protein or are complexed in other forms. The presence of similar naturally occurring lytic peptides such as LL-37, suggest they have some role and are active in vivo, even if these ions are present. However, when heat inactivated human or horse serum was added to assays in concentration of 30% or greater; inhibition of cidal activity was observed. These results confirm those of Johansson et al. with LL-37 who reported increases in MICs when serum was added. They also noted that there was complete inactivation of the LL-37 when it was first dissolved in serum rather than buffer [26]. The peptides were active when ETDA was added to the serum or when the serum was dialyzed against EDTA (data not shown). There was still a small inhibition of killing following dialysis of the sera. Additional studies are required to define other inhibitory components of serum. Preliminary data suggest that human serum albumin as well as a and b globulins bind the peptides and are therefore protective. If the observation is confirmed it would demonstrate that microbes have two separate means of using host factors to prevent killing by the microbicides. One is the presence of divalent cations present in secretions binds the bacteria resulting in their resistance to the drug. The other mechanism is the binding of the drugs to serum proteins making them ineffective. In conclusion lytic peptides were found to have the appropriate specificity of killing pathogenic organisms while not the species considered healthy members of the vaginal ecosystem. Calcium and magnesium ions inhibited the killing of susceptible species but the chelation or removal of the ions restored the bactericidal activity of the peptides, suggesting they may be formulated for use intravaginally as a topical microbicide by the inclusion of EDTA. All of the peptides demonstrated inhibition of cidal activity by divalent cations suggesting a common mechanism of action. Our study indicates the peptide warrant further studies and product development for the use in the prevention of acquisition of sexually transmitted infections.
5. ACKNOWLEDGEMENTS
This work was supported by grant NIAID 6PO1 AI39061 from the National Institutes of Health.
REFERENCES
- Ballweber, L., Jaynes, J., Stamm, W. and Lampe, M. (2002) In vitro microbicidal activities of Cecropin peptides D2A21 and D4E1 and gel formulation containing 0.1% to 2% D2A21 against Chlamydia trachomatis. Antimicrobial Agents and Chemotherapy, 46, 34-41.
- Johansson, J., Gudmundsson, G., Rottenberg, M., Berndt, K. and Agerberth, B. (1998) Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. The Journal of Biological Chemistry, 273, 3718-3724. doi:10.1074/jbc.273.6.3718
- Tencza, S., Douglass, J., Jr, D.C., Montelaro, R. and Mietzner, T. (1997) Novel antimicrobial peptides derived from human immunodeficiency virus type 1 and other lentivirus transmembrane proteins. Antimicrobial Agents and Chemotherapy, 41, 2394-2398.
- Moncla, B. and Hillier, S. (2005) Why nonoxynol-9 may have failed to prevent acquisition of Neisseria gonorrhoeae in clinical trials. Sexually Transmitted Diseases, 32, 491-494. doi:10.1097/01.olq.0000170444.13666.e9
- Oh, J., Hong, S. and Lee, K. (1999) Structure-activity relationship study: Short antimicrobial peptides. The Journal of Peptide Research, 53, 41-46. doi:10.1111/j.1399-3011.1999.tb01615.x
- Phadke, S., Islam, K., Deslouches, B., Kapoor, S., Stolz, D.B., Watkins, S., Montelaro, R., Pilewski, J.M. and Mietzner, T. (2003) Selective toxicity of engineered lentivirus lytic peptides in a CF airway cell model. Peptides, 24, 1099-1107. doi:10.1016/j.peptides.2003.07.001
- Tencza, S., Miller, M., Kslam, I., Mietzner, T. and Montelaro, R. (1995) Effect of amino acid substitutions on calmodulin binding and cytolytic properties of the LLP-1 peptide segment of human immunodeficiency virus type 1 transmembrane protein. Journal of Virology, 69, 5199- 5202.
- Tencza, S., Creighton, D., Yuan, T., Vogel, H., Montelaro, R. and Mietzner, T. (1999) Lentivirus-derived antimicrobial peptides: Increased potency by sequence engineering and dimerization. Journal of Antimicrobial Chemotherapy, 44, 33-41. doi:10.1093/jac/44.1.33
- Deslouches, B., Phadke, S., Cascio, M., Islam, K., Montelaro, R. and Mietzner, T. (2005) De novo generation of cationic antimicrobial peptides: Influence of length and tryptophan substitution on antimicrobial activity. Antimicrobial Agent and Chemotherapy, 49, 316-322. doi:10.1128/AAC.49.1.316-322.2005
- Miller, M., Cloyd, M., Liebmann, J., et al. (1993) Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology, 196, 89-100. doi:10.1006/viro.1993.1457
- Oren, Z., Lerman, J., Gudmundsson, G., Agerberth, B. and Shai, Y. (1999) Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its nocell-selective activity. Biochemical Journal, 341, 501-513. doi:10.1042/0264-6021:3410501
- Phadke, S., Lazarevi, V., Bahr, C., Islam, K., Stolz, D.B., Watkins, S., Tencza, S., Vogel, H., Montelaro, R. and Mietzner, T. (2002) Lentivirus lytic peptide 1 perturbs both outer and inner membranes of Serratia marcescens. Antimicrobial Agent and Chemotherapy, 46, 2041-2045. doi:10.1128/AAC.46.6.2041-2045.2002
- Travis, S., Anderson, N.N., Forsyth, W.R., Espiritu, C., Conway, B.D., Greenberg, E.P., Lehrer, R.I., Welsh, M.J. and Tack, B.F. (2000) Bactericidal activity of mammalian cathelicidin-derived peptides. Infection and Immunity, 68, 2748-2755. doi:10.1128/IAI.68.5.2748-2755.2000
- Zhang, L., Dhillon, P., Yan, H., Farmer, S. and Hancock, R. (2000) Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrobial Agent and Chemotherapy, 44, 3317-3321.
- Cherpes, T., Meyn, L., Krohn, M., Lurie, J. and Hillier, S. (2003) Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clinical Infectious Diseases, 37, 319-325. doi:10.1086/375819
- Martin, H., Richardson, B., Nyange, P., Lavreys, L., Hillier, S., Chohan, B., Mandaliya, K., Ndinya-Achola, J., Bwayo, J. and Kreiss, J. (1999) Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. Journal of Infectious Diseases, 180, 1863-1868. doi:10.1086/315127
- Antonio, M., Hawes, L. and Hillier, S.L. (1999) The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. Journal of Infectious Diseases, 180, 1950-1956. doi:10.1086/315109
- Mande, H., Spitzbart, H., Sieke, V. and Vogel, C. (1990) Sodium, potassium, magnesium and calcium in vaginal content. Zentralbl Gynakol, 112, 1175-1180.
- Owen, D. and Katz, D. (1999) A vaginal fluid simulant. Contraception, 59, 91-95.
- Fontenot, J., Ball, J., Miller, M., David, C. and Montelaro, R. (1991) A survey of potential problems and quality control in peptide synthesis by the fluorenylmethoxycarbonyl procedure. Journal of Peptide Research, 4, 19-25.
- Gudmundsson, G.H.B., Odeberg, J., Bergman, T., Olsson, B. and Salcedo, R. (1996) The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. European Journal of Biochemistry, 238, 325-332. doi:10.1111/j.1432-1033.1996.0325z.x
- Cosentino, L., Landers, D. and Hillier, S. (2003) Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by strand displacement amplification and relevance of the amplification control for use with vaginal swab specimens. Journal of Clinical Microbiology, 41, 3592-3596. doi:10.1128/JCM.41.8.3592-3596.2003
- Sarin, V., Kent, S. and Tam, J.B.M. (1981) Quantitative monitoring of solid-phase peptide syntheses by the ninhydrin reaction. Analytical Biochemistry, 117, 147-157. doi:10.1016/0003-2697(81)90704-1
- Rabe, L. and Hillier, S. (2000) Effect of chlorhexidine on genital microflora, Neisseria gonorrhoeae, and Trichomonas vaginalis in vitro. Sexually Transmitted Diseases, 27, 74-78. doi:10.1097/00007435-200002000-00004
- Holmes, K., Levine, R. and Weaver, M. (2004) Effectiveness of condoms in preventing sexually transmitted infections. Bulletin WHO, 82, 454-461. doi:10.1016/S0010-7824(99)00010-4
- Turner, J., Cho, Y., Dinh, N., Waring, A. and Lehrer, R. (1998) Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrobial Agent and Chemotherapy, 42, 2206-2214.
- Yang, D., Chen, Q., Schmid, A., Anderson, G., Wang, J., Wooters, J., Oppenheim, J. and Chertov, O. (2000) LL-37, the neutrophil granule-and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. The Journal of Experimental Medicine, 192, 1069-1074. doi:10.1084/jem.192.7.1069
- Witter, F., Barditch-Crovo, P., Rocco, L. and Trapnell, C. (1999) Duration of vaginal retention and potential duration of antiviral activity for five nonoxynol-9 containing intravaginal contraceptives. International Journal of Gynecology & Obstetrics, 65, 165-170.
- Antonio, M.A.D., Rabe, L.K. and Hillier, S.L. (2005) Colonization of the rectum by lactobacillus species and decreased risk of bacterial vaginosis. Journal of Infectious Diseases, 192, 394-398. doi:10.1086/430926
- Hagman, K., Pan, W., Spratt, B., Balthazar, J., Judd, R. and Shafer, W. (1995) Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtr RCDE efflux system. Microbiology, 141, 611-622. doi:10.1099/13500872-141-3-611
- Lucas, C., Hagman, K., Levin, J., Stein, D. and Shafer, W. (1995) Importance of lipooligosaccharide structure in determining gonococcal resistance to hydrophobic antimicrobial agents resulting from the mtr efflux system. Molecular Microbiology, 16, 1001-1009. doi:10.1111/j.1365-2958.1995.tb02325.x
- Shafer, W., Wu, X.-D., Waring, A. and Lehrer, R. (1998) Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides to a member of resistance/nodulation/division efflux pump family. Proceedings of National Academic Science USA, 95, 1829-1833.
NOTES
*Corresponding author.

