Advances in Bioscience and Biotechnology
Vol.2 No.4(2011), Article ID:6862,8 pages DOI:10.4236/abb.2011.24037
Mutational search for high temperature (60C) tolerantvariant of Rhizobium species CWP G34A
![]()
Department of Microbiology, Federal University of Technology, Akure, Nigeria.
Email: *boboye_b@yahoo.com
Received 28 April 2011; revised 17 June 2011; accepted 20 July 2011.
Keywords: Mutation; High Temperature; Growth and Rhizobium Species CWP G34A
ABSTRACT
This study focused on the development of thermophilic strain/s of a cowpea (Vigna unguiculata) compatible nitrogen fixing bacterium. A preliminary plant screening was carried out using some strains of tropical rhizobia and cowpea. Rhizobium species CWP G34A that formed Fix+ nodules repeatedly was selected for further studies. First, it was tested for growth at high temperatures of 40˚C to 55˚C at 5˚C interval with 28˚C as the control temperature. Mutagenesis was conducted on the bacterium with ethylmethane sulphonate (EMS). The wild-type and mutants generated were tested for high temperature tolerance by growing them individually in nutrient broth at 60˚C for 24 hours. Optical density (670 nm) was read before and after incubation. The mutants were grouped into classes based on percentage difference in OD values obtained before and after exposure to 60˚C. Rhizobium species CWP G34A produced functional pink nodules on the cowpea consistently in three different plant tests. There was no growth at all the temperatures tested except at 28˚C and 40˚C after 24 hours of incubation. It grew better at former (51 × 1010 Cfu/ml) than latter (11 Cfu/ml) temperature. Like the parental strain, all the mutants but one, did not grow after exposure to 60˚C. Sixty degree centigrade caused various reductions in optical density (OD) values of the variants. Eleven classes of the mutants were formed with membership percentage ranging from 1 to 22%. Class 1 contains only one member while class 11 has the highest mutant population of 22% with OD difference of 0 to 10% and −90 to −100% respectively. The high percentage reduction in the OD of variants in class 11 is similar to that of the unmutated cells (−94.56%). The only mutant that survived the 60oC and grew was MU70. An increase of 1.67% in OD was obtained for MU70. Mutant MU70 therefore appeared a promising strain that can be further tested to inoculate cowpea in the dry and warm season for increased nitrogen fixation. This will provide encouraging information for farmers to grow the cowpea throughout the year particularly under high temperatures in summer in order to boost the yield of the legume.
1. INTRODUCTION
Protein that is often deficient in human diets in many parts of the world [1] particularly in the developing tropical countries including Nigeria can be adequately provided by food grain legumes. There is need for optimum production of these legumes which requires provision of sufficient plant’s nutrients. Nitrogen, one of the major elements required by plants is often limiting in many soils in the tropics. In the mutual interaction between rhizobia and legumes, the bacteria generally fix nitrogen leading to boosted yield of the legumes with increased protein supply to the populace.
Currently, rhizobia play an important role in improving soil fertility resulting to reduction in the use of nitrogenous fertilizers in farming systems [2]. Inoculation of cowpea with efficient strains of rhizobia has significant economical and ecological benefits. The presence of natural strains of rhizobia in the soils, usually highly competitive and well adapted to certain environments like high temperature, can reduce the inoculation benefits even with highly efficient strains. In addition, especially in marginal soils of arid and semi-arid regions, survival and effective functioning of natural and inoculated rhizobia are reduced by high soil temperatures, salt and osmotic stress, soil acidity, alkalinity and heavy metals in soils [2]. For legumes to fix nitrogen in symbiosis with rhizobia the bacteria must cope with the above stated abiotic stresses and they must survive as saprophyte and persist in such marginal soils in the absence of host plants [3].
Rhizobia thrive well only in mesophilic temperature. In reality, most rhizobial species grow best between 20 and 28˚C. The highest temperature range for the best growth of most rhizobial cultures is 28˚C - 31˚C, and many cannot grow even at 37˚C [4]. In an experiment carried out by Marsh et al. [1], where 12 Bradyrhizobium species strains were examined on pigeon pea (Cajanus cajan (L.) Millsp) and cowpea (Vigna unguiculata (L.) Walp), only a few strains could grow at the high day/ night temperature of 38˚C/25˚C (100˚F/78˚F) but most grew readily at 30˚C/20˚C (88˚F/69˚F).
In contrast, certain rhizobia like R. meliloti grow optimally at 35˚C to 37˚C [5]. It was shown in a project carried out on rhizobia from extreme soil environments tested for growth performance or tolerance at a wide range of temperatures (30˚C - 55˚C), that growth of five isolates was highest between 30 and 40˚C, while four isolates showed considerable growth at 35˚C. All isolates demonstrated elevated growth at 35˚C. Ninety percents of cowpea Rhizobium strains obtained from the hot, dry environment of the Sahel Savannah grew well at 40˚C [6]. Strain adaptation to high temperature has also been reported by Hartel and Alexander [7] and Karanja and Wood [8].
Rhizobia that can grow at and above 28˚C were isolated from hot and dry regions of southern Morocco [9]. In addition, at 28˚C, 32˚C and 36˚C, respectively, 100%, 96.81% and 87.26% of the isolates sampled grew well. However, at 40˚C, only 57.96% of the isolates (including 16 isolates of S. medicae) grew. This data complements similar observations made in cowpea rhizobia in which it was suggested that sampling from hot and dry areas facilitates selection for high temperature tolerance from the natural rhizobia populations [6].
Environmental temperature of 32˚C - 33˚C are harmful to most species [5]. High soil temperature could contribute to the frequency of non-infective isolates in soil; Segovia et al. [10] noted that such isolates actually outnumbered those that were effective in the rhizosphere of bean. The 30˚C/20˚C temperature regime was highest for effective symbiotic association (optimum nitrogen fixation and plant growth) with only three of the rhizobia in pigeon peas and cowpeas and also for Bradyrhizobium survival [1]. High root temperatures strongly affect bacterial infection and N2 fixation in several legume species, including soybean [11], guar [12], peanut [13], cowpea [14] and beans [15,16]. Temperature affects root hair infection, bacteroid differentiation, nodule structure, and the functioning of the legume root nodule [17,18]. High (not extreme) soil temperatures will delay nodulation or restrict it to the subsurface region [4]. Munns et al. [19] found that alfalfa plants grown in desert environments in California maintained few nodules in the top 5 cm of soil but were extensively nodulated below this depth. Piha and Munns [16] reported that bean nodules formed at 35˚C were small and had low specific nitrogenase activity, and Hernandez-Armenta et al. [20] found that transferring nodulated bean plants from a daily temperature of 26˚C to 35˚C markedly inhibited nitrogen fixation.
In Nigeria, cowpea is cheaper than animal meat that is widely consumed as a major source of proteins. The legume is grown in the southern and northern parts, but more in the latter region of Nigeria where the climate is hotter. It is cheaper and apparently healthy during the harvest than dry season. At off season, cowpeas are insufficient, many of the grains are usually spoilt and their prices increase making them expensive and unaffordable by average citizens. It is important to produce more of the legume to satisfy the populace in order to improve their standard of living. In the tropics including Nigeria, the environmental temperature can be high up to 40˚C - 50˚C particularly in the summer. Inoculation of cowpeas with thermophilic strain(s) that can survive and fix nitrogen at high temperatures can encourage the farmers to grow the crop in the dry and hot season in addition to the season currently in use. Therefore, it is pertinent to source for thermophilic, cowpea-compatible-rhizobia. In order to achieve this purpose, we designed a preliminary research to develop thermophilic strains of Rhizobium species CWP G34A by chemical mutation.
2. METHODOLOGY
2.1. Plant Screening of Rhizobial Isolates for Nitrogen Fixation on Cowpea
Some tropical rhizobial isolates (Rhizobium species N1b, R. spp. N2a, R. spp. N2b, R spp. CWP G2, R. spp. CWP G34A and R. spp. CWP G34B) were grown in nutrient broth containing malachite green (0.003% w/v) for 24 hours. Each culture was spun at 12,168 × 103 g for 15 min (MSE Minor 35 Centrifuge). Cells were washed twice and resuspended in sterile B and D plant nutrient [21].
Grains of a cowpea variety “Oloyin” commonly used in Nigeria were surface sterilized with 3.5% (w/v) sodium hypochlorite for 7 min and rinsed 7 times in sterile water. The seeds were transferred onto 1.2 % (w/v) agar and germinated in the dark at 28˚C for 3 days. The seedlings (3) were planted in sterile demineralised riverside sand contained in a Magenta-like plant jar. The washed bacterial culture (0.5 ml) was added to the root system of each seedling. Three replicates of the jar were used for the respective strain. The plants were fed with the B and D plant nutrient and grown in a plant house at 22˚C - 22˚C (10 h during the night) and 24˚C - 30˚C (14 h at every day) with sunlight intensity of 300 - 320 Einstein m–2s–1 and a relative humidity of 70% - 75%. After 42 days of seedling inoculation, cowpea plants were harvested, colours of leaves and stems were observed. The nodules were counted and cut into two parts to examine the inner colour.
2.2. Determination of the Growth Effect of High Temperatures on Rhizobium species CWP G34A
Rhizobium spp. CWP G34A that formed Fix+ nodules repeatedly on the cowpea in three different experiments was used for this study. First, the Rhizobium species CWP G34A was tested for growth at high temperatures of 40˚C to 55˚C at 5˚C interval with 28˚C as the control temperature. Growth was carried out in nutrient broth for 24 hours. Optical density (670 nm) was read at 0 and 24 hours of incubation.
2.3. Mutation Experiment
A young exponentially grown (16 hours/28˚C) culture of the Rhizobium spp. CWP G34A was mutated with ethylmethane sulphonate (EMS) according to the method of Parkinson [22] modified as described by Boboye and Alao [23]. Mutational rate was estimated. The variants were screened for growth at 60˚C.
2.4. Screening for Thermophilic Mutants
Effect of high temperature (60˚C) was tested on 100 variants by growing each of them in nutrient broth for 24 hours. Optical density (670 nm) was read at 0 and 24 hours of incubation. The mutants were grouped into classes based on percentage difference in OD values obtained before and after exposure to 60˚C in a range of –09.99 and below starting with 10.00 - 0.00 for class 1.
3. RESULTS AND DISCUSSION
All the rhizobia but strains N2b, G34A and G34B, did neither form nodules nor fix nitrogen in the plant tests (Table 1). Among the Nod+Fix+ strains, only G34A produced nodules in three different plant experiments. The N2b and G34B nodulated the cowpea once in the plant tests. Rhizobium spp. N2b formed many tiny nodules while R. species CWP G34A and G34B produced some big to moderate size nodules with inner pink colour indicating the presence of leghemoglobin. The protein impacts pink to red colour to the inside of the nodules that are functional to fix atmospheric nitrogen. Effective
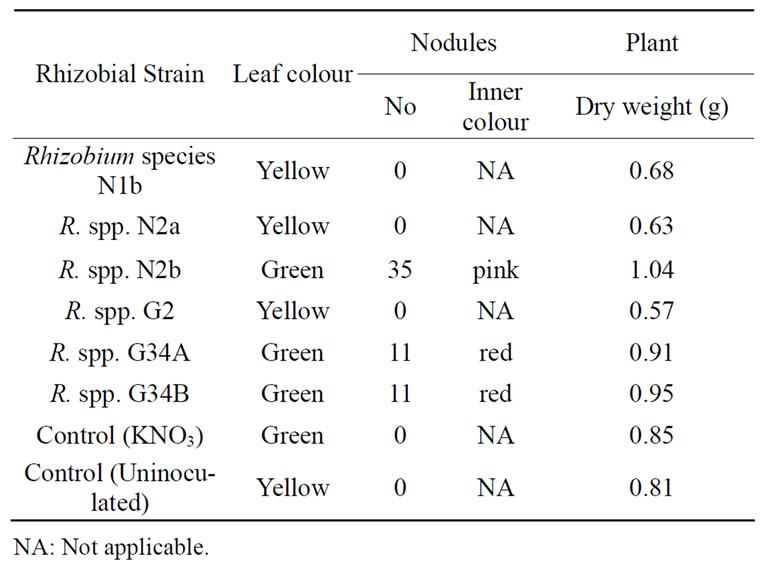
Table 1. Effect of some tropical rhizobia on the nodulation, nitrogen fixation and growth of cowpea.
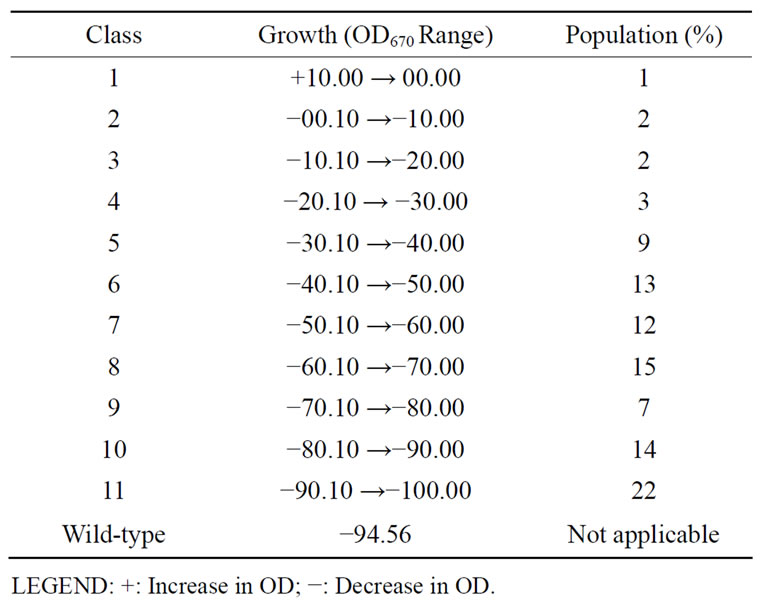
Table 2. Groups of Rhizobium species CWP G34A mutants based on their growth at high temperature (60˚C).
nodules are characterized by pink nodules [24]. Plants lacking nodules on their roots were unable to fix atmospheric nitrogen. This Nod-Fix- phenotype showed that the bacteria are incompatible with the cowpea. Rhizobia are host specific in symbiosis with legumes.
The stems and leaves of plants inoculated with rhizobial strains N2b, G34A and G34B were green at harvest because the nodules fixed nitrogen into the legumes in contrast to the cowpea plants infected with Rhizobium spp. N1b, N2a and G2 which leaves turned yellow but their stems were slightly green. Also, the dry weights of the cowpea plants with Fix+ nodules were higher than those without nodules. The former set of cowpea plants got nitrogen needed for the building of structural components, hence the higher weights obtained for them. Also, the leaves of these Nod+Fix+ were intact while many leaves on unnodulated plants had dropped before harvest, contributing to loss in weights for the latter set of plants. This means that nitrogen encouraged better growth of the legume. Nitrogen fixation is directly related to legume’s plant growth rate [25].
The mutation rate was 95.4% showing that the impact of the EMS on the genome of the bacterium is high. There was no growth of the R. spp. CWP G34A at all the temperatures tested except at 28˚C and 40˚C. The bacterium, grew better at former (51 × 1010 Cfu/ml) than latter (11 Cfu/ml) temperature after 24 hours of incubation. This showed that this bacterium is not a thermophile but mesophile. Mesophiles grow best within a temperature range of 25˚C - 40˚C [26]. Thermophiles grow at above 45˚C - 50˚C [27]. Thermophilic trait is genetically based. It then means that the tropical Rhizobium species CWP G34A that could not grow at 60˚C does not possess the characteristic genetically. The membrane of rhizobia generally lacks lipids rich in saturated fatty acids that are present in thermophilic microbes. These lipids allow the membrane to remain stable and function at high temperatures [28].
Similar to the parental strain, all the mutants except one did not grow after exposure to 60˚C. Sixty degree centigrade caused various reductions in optical density (OD) values of the variants. Eleven classes of the mutants were formed with membership percentage ranging from 1% to 22%. Class 1 contains only one member while class 11 has the highest mutant population. The high reduction in the OD of variants in class 11 is similar to that of the unmutated cells (−94.56) (Table 2). The increase in optical density (or growth) of MU70 in contrast to the wild type and other variants means that the mutant’s growth encoding gene(s) has been modified to endow the bacterium to grow at high temperature. The reduction in the optical densities of the other mutants on exposure to the 60˚C suggests that cells of the mutants were unable to grow at the test temperature; thus they died and their cells were lysed. The existence of the highest percentage of mutants in class 11 (22%) is an indication that most of the rhizobial mutants could not withstand the high temperature (60˚C). The cells died, hence the high negative O.D. observed when the mutants were incubated at 60˚C.
There were some variations in the percent growth reduction of mutants between the classes (Figure 1). Among the groups, only class 1 (with a single member, MU70) grew (1.67%) when exposed to the high temperature. The average percentage growth reduction produced by mutant classes 2 to 11 ranged between 4.25 and 95.17 (Figure 1). Growth reduction showed by some mutants in the different classes varied. These variations were very close to each other in classes 2 to 9
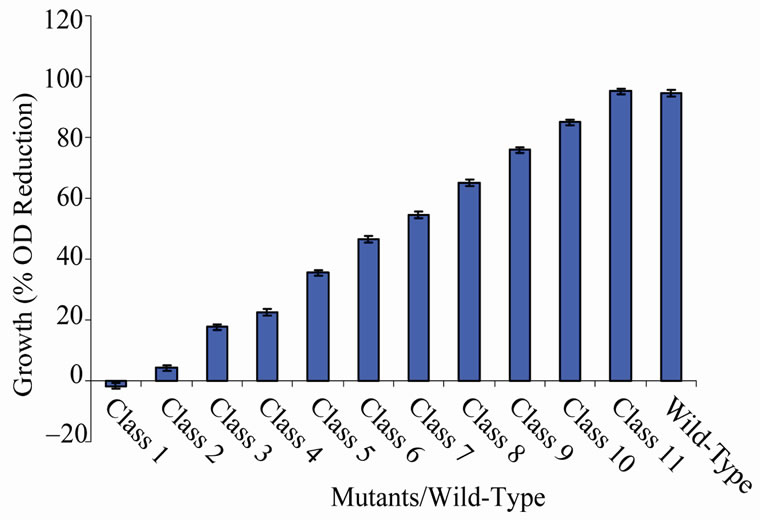
Figure 1. Growth variations between classes of mutants relative to the wild-type based on percentage reduction in cell density.

Figure 2. High temperature growth variation in class 1 mutant.
Figure 3. High temperature growth variation among class 2 mutants.
(Figures 2-9). Members of classes 10 and 11 however, had high variation among their members particularly class 11 (Figures 10 and 11). Mutant MU70 was the only member of class 1 with percent growth reduction of −1.67. This reduction is highly different from that of the parental strain that produced growth reduction of 94.56% (Figure 12). The growth variation among the
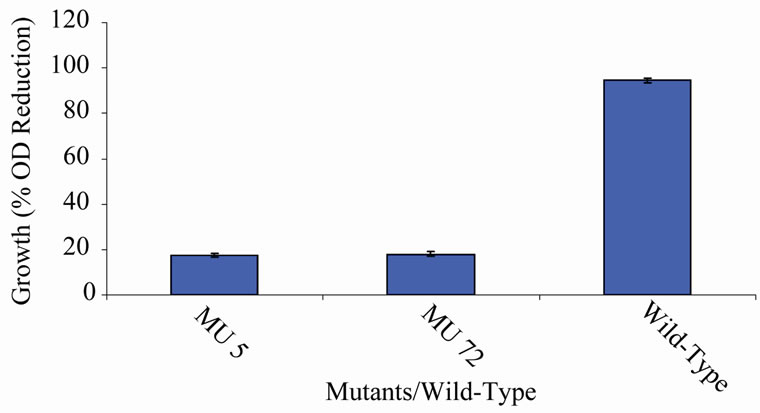
Figure 4. High temperature growth variation among class 3 mutants.
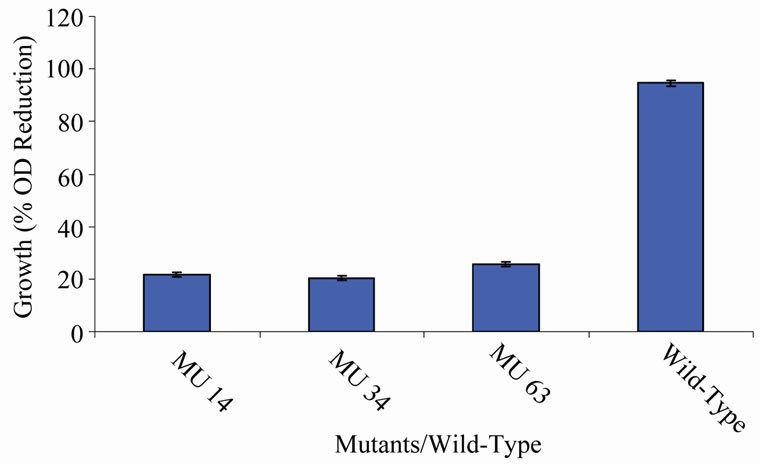
Figure 5. High temperature growth variation among class 4 mutants.
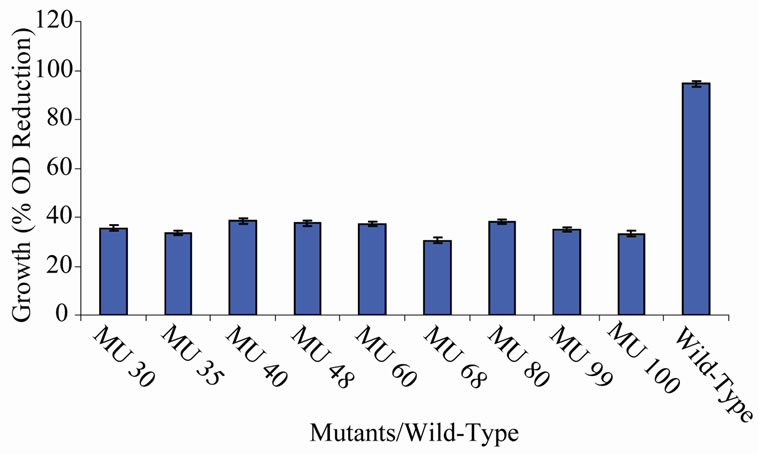
Figure 6. High temperature growth variation among class 5 mutants.
members of class 6 was less than or equal to 9.73%, which was a marginal value when related to the percent reduction in the growth of the wild-type (Figure 6). There was still a wide gap of differences between members of class 7 and the wild-type bacterium (Figure 7). The growth reduction values of members of group 8 drew more closely to that of the wild-type (Figure 8). The difference between the percent growth reduction values among members of class 9 was less as it was for the other classes (Figure 9). There was not much varia-
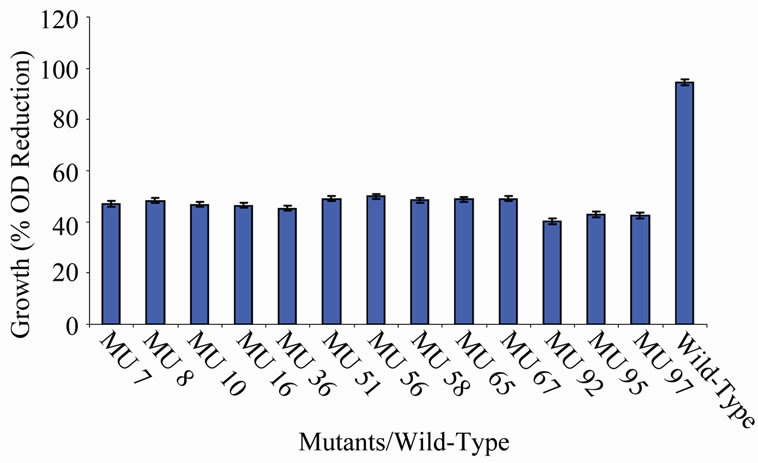
Figure 7. High temperature growth variation among class 6 mutants.
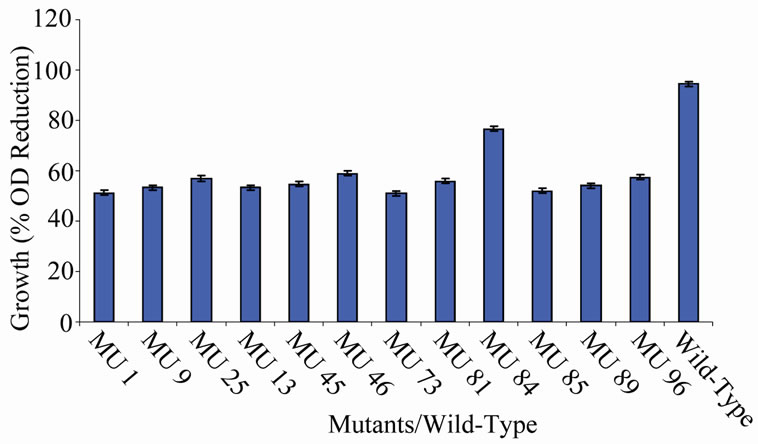
Figure 8. High temperature growth variation among class 7 mutants.
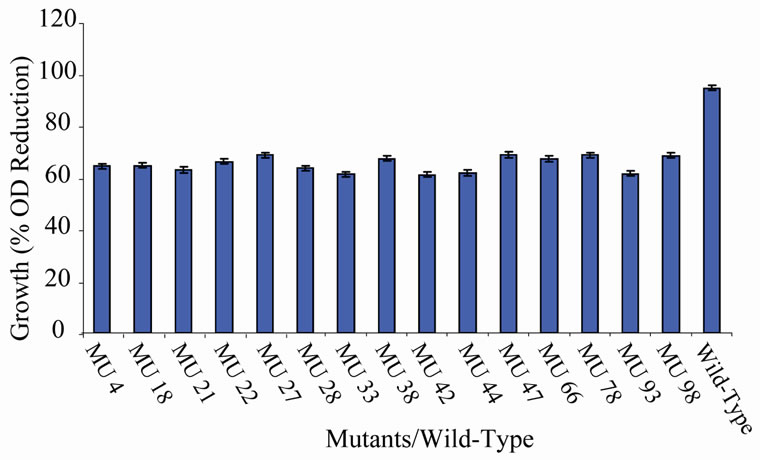
Figure 9. High temperature growth variation among class 8 mutants.
tion in the growth between members of the group 10 in relation to the wild-type (94.56%) (Figure 10). In class 11, MU53 had the highest drop in growth (99.69%). It was followed by MU12 (99.52%), while MU75 showed the lowest growth reduction rate (91.10%). Members of this class however, had high variation among its members. The percentage reduction in growth of each mutant in this class is closer to that of the wild-type strain (Figure 11). Growth variation among the mutants means that
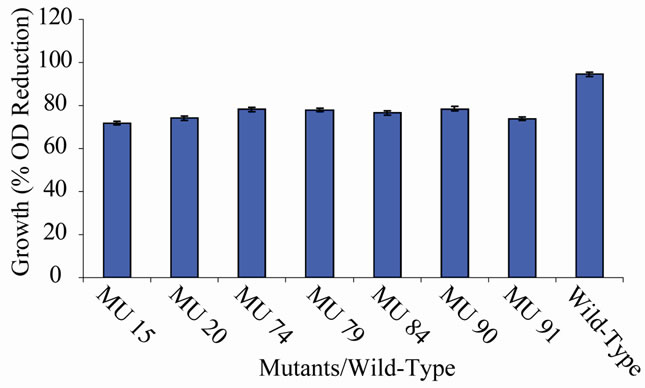
Figure 10. High temperature growth variation among class 9 mutants.
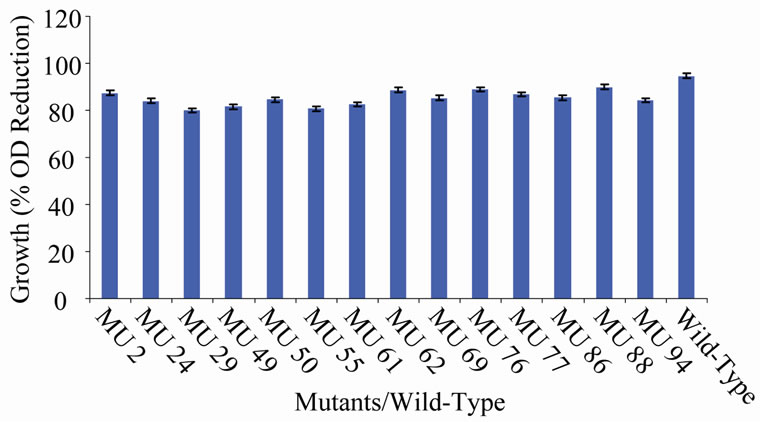
Figure 11. Growth variation among class 10 mutants.
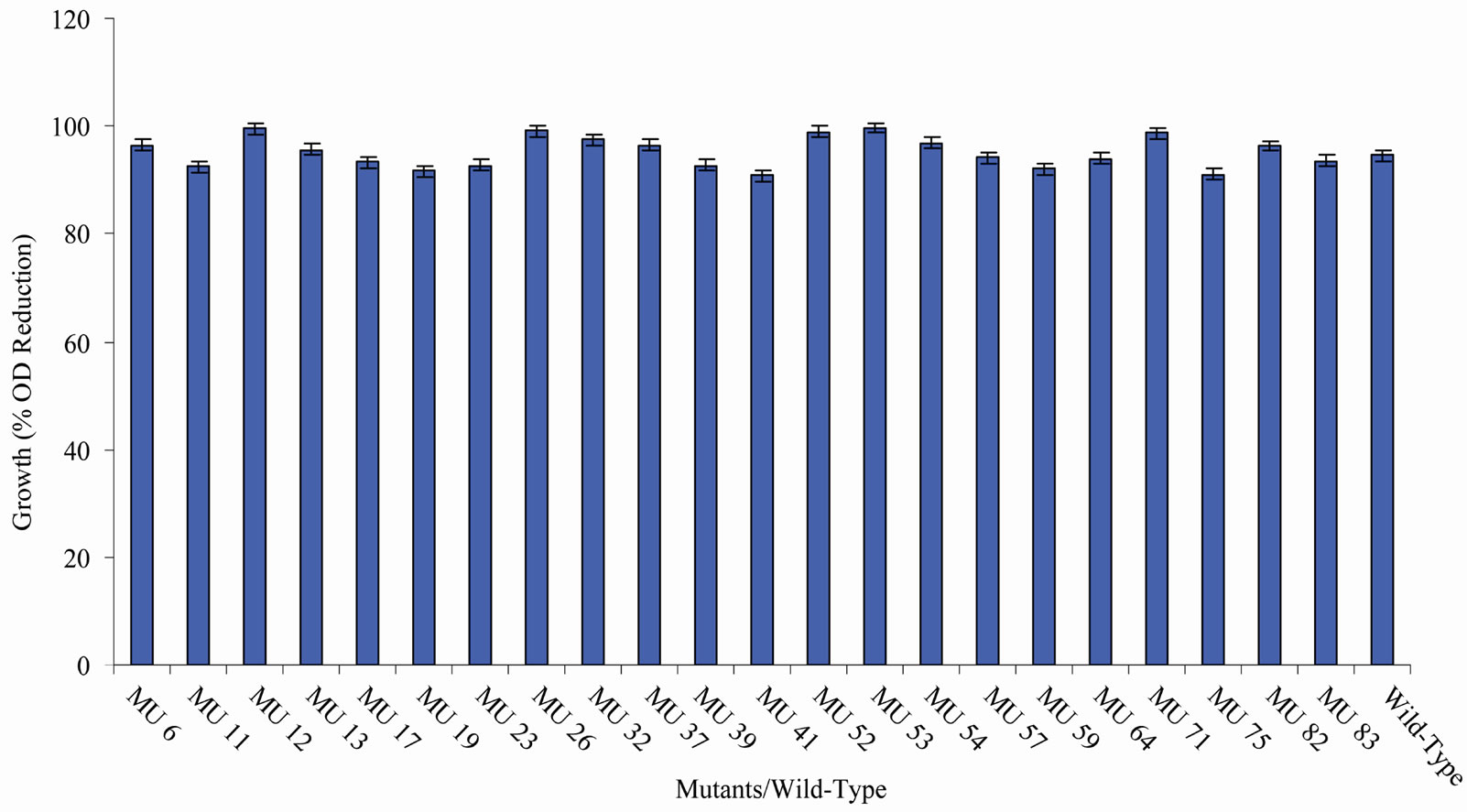
Figure 12. High temperature growth variation among class 11 mutants.
the EMS randomly hit the rhizobial genome. The EMS hit the genome to various extents, hence, the differences in the level of expression of the growth encoding gene/s in each mutant. Those mutants that showed 90% to 100% reduction have their genes encoding cell multiplication inhibited from reproduction. On the other hand, the mutagen changed the gene(s) in the variant MU70 to support its survival and growth at 60˚C. The change appeared to have endowed the mutant with ability to synthesize into its membrane, certain substances, possibly lipids rich in saturated fatty acids that enable thermophiles to live at high temperatures [28]. Ethylmethane sulphonate (EMS), used for the mutation in this experiment is a mono-functional akylating agent that acts by adding methyl group to guanine causing faulty pairing with thymine in nucleotides, resulting in the transition of G-C to A-T [27-29].
4. CONCLUSIONS
This study has revealed that most of the Rhizobium spp. CWP G34A mutants (including the wild-type) were unable to grow at high temperature of 60˚C. The only mutant that survived the 60˚C and grew is MU70. An increase of 1.67% in OD was obtained for MU70. Mutant MU70 thus appeared a promising strain that can be used to inoculate cowpea particularly in the dry and warm season under high temperature weather conditions that are common in the tropics in summer period. This will increase nitrogen fixation with concomitant improved yield for provision of the legume at affordable, stable price throughout the year especially to low income populace.
5. ACKNOWLEDGEMENTS
We appreciate Federal University of Technology Akure, Ondo State, Nigeria for provision of facilities, materials and reagents used for this study. We thank Daniel Ipinsanmi for the little assistance he rendered in the laboratory.
REFERENCES
- Marsh, L.E., Baptiste, R., Marsh, D.B., Trinklein, D. and Kremer, R.J. (2006) Temperature effects on Bradyrhizobium spp. growth and symbiotic effectiveness with pigeon pea and cowpea. Journal of Plant Nutrition, 29, 331-346. doi:10.1080/01904160500476921
- Zahran, H.H. (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in arid climate. Microbiology Molecular Biology Review, 63, 968- 989.
- Jensen, J.B., Peters, N.K. and Bhuvaneswari, T.V. (2002) Redundancy in periplasmic binding protein-dependent transport systems for trehalose, sucrose, and maltose in Sinorhizobium meliloti. Journal of Bacteriology, 184, 2978-2986. doi:10.1128/JB.184.11.2978-2986.2002
- Graham, P.H. (1992) Stress tolerance in Rhizobium and Bradyrhizobium, and nodulation under adverse soil conditions. Canadian Journal of Microbiology, 38, 475-484. doi:10.1139/m92-079
- Allison, F.E. and Minor, F.W. (2010) The effects of temperature on growth rates of Rhizobia. www.ncbi.nlm.nih.gov/pmc/articles/pmc3745/78.
- Eaglesham, A.R.J. and Ayanaba, A. (1984) Tropical stress ecology of rhizobia, root-nodulation and legume fixaton. In: Subba R.N.S. Ed., Current Developments in Biological Nitrogen Fixation, Edward Arnold Publishers, London, 1-35.
- Hartel, P.G. and Alexander, M. (1984) Temperature and desiccation tolerance of cowpea rhizobia. Canadian Journal of Microbiology, 30, 820-823. doi:10.1139/m84-125
- Karanja, N.K. and Wood, M. (1988) Selecting Rhizobium phaseoli strains for use with beans (Phaseolus vulgaris L.) in Kenya. Tolerance of high temperature and antibiotic resistance. Plant Soil, 112, 15-22. doi:10.1007/BF02181747
- Elboutahiri, N., Thami-Alami, I. and Udupa, S.M. (2010) Phenotypic and genetic diversity in Sinorhizobium meliloti and S. medicae from drought and salt affected regions of Morocco. BMC Microbiology, 10, 15. doi:10.1186/1471-2180-10-15
- Segovia, L., Pinero, D., Palacios, R. and Martinez-Romero, E. (1991) Genetic structure of a soil population of non-symbiotic Rhizobium leguminosarum. Applied and Environmental Microbiology, 57, 426-433.
- Munevar, F. and Wollum, A.G. (1982) Response of soybean plants to high root temperature as affected by plant cultivar and Rhizobium strain. Agronomy Journal, 74, 138-142. doi:10.2134/agronj1982.00021962007400010036x
- Arayankoon, T., Schomberg, H.H. and Weaver, R.W. (1990) Nodulation and N2 fixation of guar at high root temperature. Plant Soil, 126, 209-213. doi:10.1007/BF00012824
- Kishinevsky, B.D., Sen, D. and Weaver, R.W. (1992) Effect of high root temperature on Bradyrhizobiumpeanut symbiosis. Plant Soil, 143, 275-282. doi:10.1007/BF00007883
- Rainbird, R.M., Akins, C.A. and Pate, J.J.S. (1983) Effect of temperature on nitrogenase functioning in cowpea nodules. Plant Physiology, 73, 392-394. doi:10.1104/pp.73.2.392
- Hungria, M. and Franco, A.A. (1993) Effects of high temperature on nodulation and nitrogen fixation by Phaseolus vulgaris L. Plant Soil, 149, 95-102. doi:10.1007/BF00010766
- Piha, M.I. and Munnus, D.N. (1987) Sensitivity of the common bean (Phaseolus vulgaris L.) symbiosis to high soil temperature. Plant Soil, 98, 183-194. doi:10.1007/BF02374822
- Roughley, R.J. (1970) The influence of root temperature, Rhizobium strain and host selection on the structure and nitrogen-fixing efficiency of the root nodules of Trifolium subterraneum. Annals of Botany, 34, 631-646.
- Roughley, R.J. and Dart, P.J. (1970) Root temperature and root-hair infection of Trifolium subterraneum L. cv. Cranmore. Plant Soil, 32, 518-520. doi:10.1007/BF01372887
- Munns, D.N., Keyser, H.H., Fogle, V.W., Hohenberg, J.S., Righetti, T.L., Lauter, D.L., Zaruog, M.G., Clarkin, K.L. and Whitacre, K.W. (1979) Tolerance of soil acidity in symbiosis of mung bean with rhizobia. Agronomy Journal, 71, 256-260. doi:10.2134/agronj1979.00021962007100020010x
- Hernandez-Armenta, R., Wien, H.C. and Eaglesham, A.R.J. (1989) Maximum temperature for nitrogen fixation in common bean. Crop Science, 29, 1260-1265. doi:10.2135/cropsci1989.0011183X002900050034x
- Broughton, W.J. and Dilworth, M.J. (1971) Control of leghemoglobin in Snake beans. Biochemical Journal, 125, 1075-1080.
- Parkinson, J.S. (1976) Che A, Che B and Che C genes of Escherichia coli and their role in chemotaxis. Journal of Bacteriology, 126, 758-770.
- Boboye, B. and Alao, A. (2008) Effect of mutation on Trehalose-catabolic-enzyme synthesized by a tropical Rhizobium species F1. Research Journal of Microbiology, 3, 269-275. doi:10.3923/jm.2008.269.275
- Powar, C.B. and Diganawala, H.F. (1992) General microbiology. 8th Edition, Himalay Publishing House, Delhi, 598-600.
- Hirsch. A.M., Lum, M.R. and Downie, J.A. (2001) What makes the rhizobia-legume symbiosis so special? Plant Physiology, 127, 1484-1492. doi:10.1104/pp.010866
- Pelczar, M.J., Reid, R.D. and Chan, E.C.S. (1983) Microbiology. 4th Edition, Tata McGraw-Hill Publishing Company, Delhi, 111.
- Brock, T.D., Smith, D.W. and Madigan, M.T. (1984) Biology of microorganisms. 4th Edition, Prentice-Hall International, Inc., London, 244, 310.
- Prescott, L.M., Harley, J.P. and Klein, D.A. (2005) Microbiology. 6th International Edition, Mcgraw-Hill Publishing Company, London, 652-668.
- Suzuki, D.T., Griffin, J.E., Miller, H.J. and Lewontin, C.R. (2001) An introduction to genetic analysis. 7th Edition, Freeman Publishers, Beaumont, 101-105.

