Journal of Environmental Protection
Vol. 3 No. 8 (2012) , Article ID: 21758 , 6 pages DOI:10.4236/jep.2012.38091
Steviaside Containing Plant as Deconstractive (Degradative) Agent for Persistent Organic Pollutants
![]()
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan.
Email: hamid_khodjaniyazov@yahoo.com
Received May 21st, 2012; revised June 17th, 2012; accepted July 13th, 2012
Keywords: Chlorinated Pesticides; Extracts of Stevia; Steviaside; Degradation
ABSTRACT
Steviaside containing plant extracts have been used for degradation of persistent chloroorganic pesticides. Reactions between DDT and Steviaside or sum of extractive substances isolated from ground up part of plant Stevia were studied to give of less toxic DDE. Herein researches on studying interaction sum of polysaccharides of Stevia with DDT in various ratios resulted also. The GC-MS and GLC methods were used for analyzing degradation degree of pesticides and to determine obtained compounds. Treat HCCH by water extract of Stevia basically formed tetrachlorocyclohexadiene (HCH) with 86.9% yield and in particularly formed of tri-, tetrachlorobenzenes. The HCH formed in 79.7% on treat pesticide by 80% Steviaside. Degradation of HCCH and DDT by water extract of Stevia in a presence of anabasine in a ratio of 2:1:1 occur to degrade of HCCH up to 70% - 80%, and DDT on 25% - 30%.
1. Introduction
Polychlorinated organic pesticides (POP) are well-known recalcitrant environmental pollutants. Although the metabolism of the POPs has been intensively studied, very little is known about their mechanism of toxicity in living organisms or how they are degraded in water and soil. We have examined interaction between POPs and Steviaside, also its analogical compounds.
The widespread use of agricultural pesticides in many countries resulted in a serious pollution of the environment (soil, water and atmosphere). Persistent halogenated pesticides such as DDT, lindane, heptachlor, pentachlorophenol, aldrin, keltan and their several combined derivatives were used in large scale due to their efficiency and non-specificity. Moreover, DDT and hexachlorocyclohexane (HCH) were used as effective pesticides for treatment of plant seeds, soil, and crops, and in livestock production, as well as disinfectants against bugs, mosquitoes, flies and other harmful insects for household use.
Purpose of this work was study of degradation of persistent chloroorganic pesticides by using plant extracts, which contain Steviaside.
Air dried plant material was extracted by various solvents at room temperature by method of standing and boiling water extracts. There were isolated polysaccharides from the plant of Stevia and Steviaside with 80% purity [1].
Possibility of degradation of chloroorganic pesticides by plant extracts, such as extractive compounds of the plant Stevia and alkaloid containing plant extracts were shown as the result [2-5].
2. Extraction of Ground up Part of Plant Stevia
An optimal condition for extraction of the plant Stevia finds by following procedure. It was studied influence from the degree of crushing of plant material, which was dried at room temperature in a shadow. For this purpose there were studied investigations with crushed and none crushed dry materials. All of experiments were done in the analogical conditions: 10 g crushed and none crushed lives extracted with ethanol by the method of insisting. Obtained results given in Table 1.
As shown in Table 1 in the case of extraction of none crushed lives, at first, it needs to use much amount solvent, at second, contact of particles with solvent is low, and so yield of sum of extractive compounds are relative small. Littered filters at a moment of grinding create inconvenience in extraction process. Optimal variant for obtaining extractive compounds were crushing up to 2.0 - 3.0 mm.
Another factor which influence to extraction process is choice of hydromodule. So we have done investigations
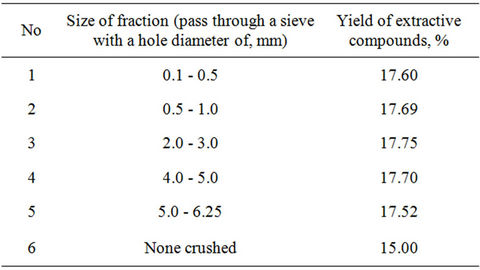
Table 1. Influence of crushing degree of the raw materials (leaves) to the yield of extractive compounds.
on selection of the hydromodule value. For this purpose extraction of 10 g plant material with the value of crushing 2.0 - 3.0 mm have done at room temperature at ratio of plant material: a solvent 1:7, 1:10, 1:15 by method of insisting during 12 hours. In this case yields were 16.2%, 17.75%, and 18.1% correspondently. Obtained results are showed, that in one time extraction by increasing of hydromodule yield increase also. For full isolation we use tree times extraction at hydromodule 1:6, 1:5, 1:4 (summary hydromodule 1:15). Yield of extractive compounds were 18.4%.
3. Degradation of Chloroorganic Pesticides by Extracts of Stevia
It is known, that chlorophyll containing extracts have properties for degradation of persistent chloroorganic pesticides [6]. We studied interaction of hexachlorocyclohexane with 80% Steviaside, sum of polysaccharides and water extracts, isolated from plant Stevia in ratio of pesticide: an extract 1:1, 1:2 at room temperature. Results of carried out searches are given in Table 2.
Data resulted in the Table 2 show that in the cases of polysaccharides, obtained from the plant of Stevia and water extract II, obtained by method of insisting, content of HCCH in reaction mixture is smaller, than in a case of extract I.
It was shown formation of HCH as the main product by investigation of degradation with water extracts of Stevia with the 97.2% yield.
From the given data (Figure 1) it is clear that at interaction of the water extract with pesticide HCCH is observed formation of tetrachlorocyclohexadiene-86.9% (HCH), with small content of tri-, tetrachlorobenzenes. In the case of 80% Steviaside was observed formation of HCH with 79.7% yield, and content of trichlorobenzene was increased up to 20.3%. Obtained results are given in Table 3.
Continuing researches on studying interaction of extract of the plant Stevia with chloroorganic pollutants, it was found, that 96%-ethanol extract is more effective for
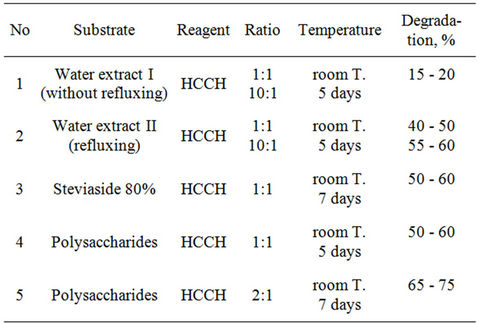
Table 2. Interaction of hexachlorocyclohexane with extracts of plant Stevia in 50%-ethanol.
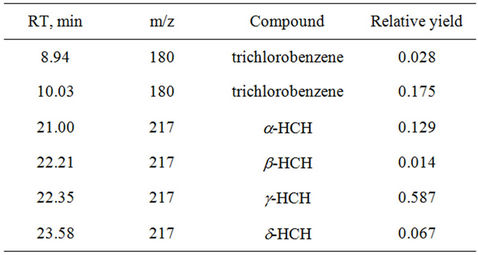
Table 3. Influence of Steviaside to degradation of pesticide hexachlorocyclohexane.
degradation of DDT—65% - 70% (Table 4).
Interaction of water extract of plant Stevia with HCCH and DDT were studied at room temperature at stirring during 5 days in the presence of alkaloid anabasine in ratio 2:1:1. Process was controlled by TLC: significant changes did not observe. The mixture was sent for 7 days, after that reaction mixture checked by TLC. In the case of HCCH degradation was 70% - 80%, and in case of DDT it was only 25% - 30%.
Interactions some of extracts of ground up part of plant Stevia (water, aqueous ethanol, ethanol) with HCCH in the presence of alkaloid Anabasine were studied also. It was determined, that in this case HCCH is degraded to tetrachlorocyclohexadiene with the yield of 60%.
Interaction of dry crushed of ground up part of plant Stevia, water extract Stevia and 80% Steviaside with preparative form, which contains 10% of DDT in ratio of reagents 2:1 in the presence of toluene and aqueous ethanol were studied. Degradation products of DDT were analyzed by TLC and chromato-mass spectra. In this case are formed complex mixture, which contained several products of DDT degradation, such as 1-chloro-2(2- chloro-1(4-chlorophenyl)ethylenebenzene, 2,4-dichloro- 1-(2-chloroethylene)benzene, 1,4-dichloro-2-(2-chloroethyl) benzene, 1,2,2-trichloro-1,4-(chlorophenyl)ethane
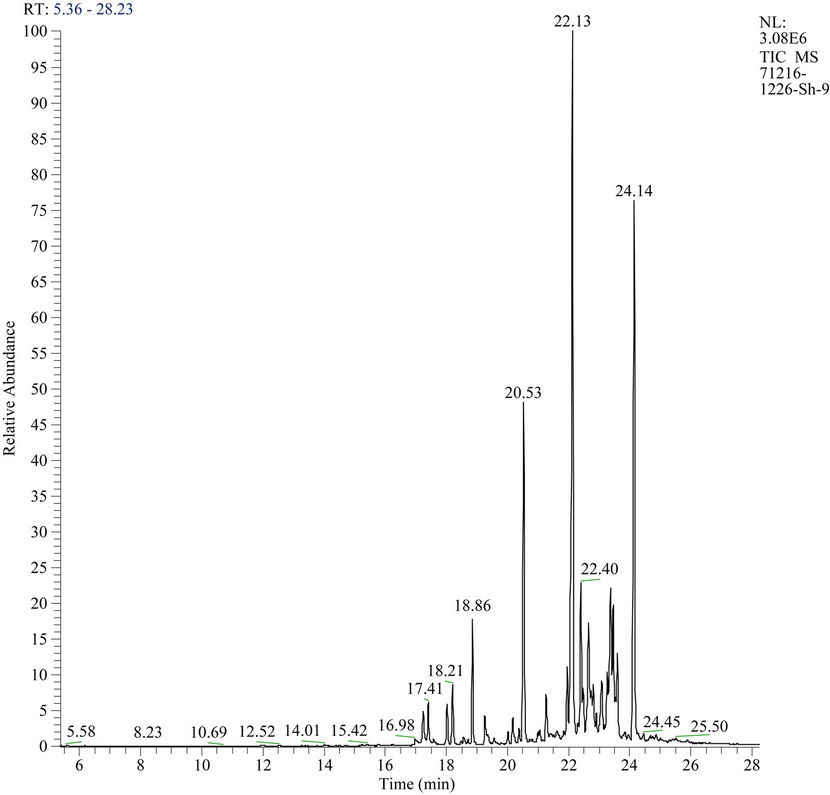
Figure 1. Mass-chromatogram of degradation products of HCCH by water extract of Stevia.
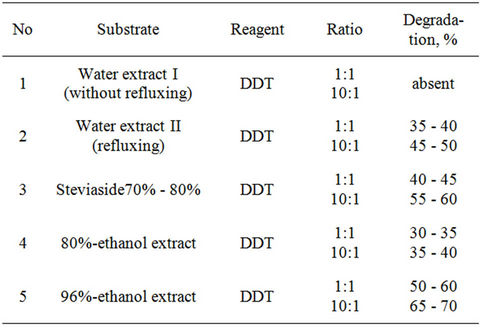
Table 4. Interaction of DDT with extracts of plant Stevia in 50%-ethanol at room temperature.
and others.
There were studied interaction tetrachlorobenzene (TTCB) with extracts of plant Stevia. It was determined that degradation goes faster then in a case of HCCH during of 5 days and more effective is water-ethanol extract where degradation was 85% - 90% (Table 5).
Interaction of ground up part and sum of extracted compounds, isolated from plant of Stevia with TTCB in a ratio of 2:1 were studied at room temperature in the presence of toluene, benzene, ethanol and aqueous ethanol. Formation of insignificant amount of triand dichlorobenzenes were also determined.
4. Experiments
4.1. GC-MS Analysis
The analysis was performed on gas chromatograph trace GC with ion trap mass-spectrometric detector Polaris Q (Thermo Finnigan). Analysis conditions of: silica capillary column 7 m × 0.32 mm (DB-5 ms, film thickness 0.25 micron), temperature programming from 60˚C (2 min) to 200˚C with a rate 6˚C/min then to 280˚C with a rate 8˚C/min, injector temperature 220˚C, interface- 220˚C, electron ionization at energy 70 eV, full scan 41 -
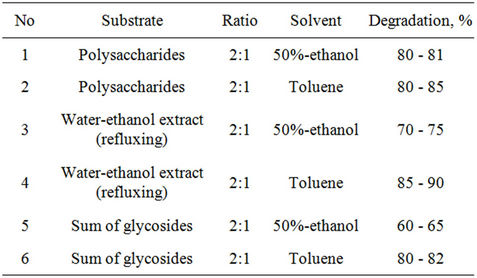
Table 5. Interaction of tetrachlorobenzene (TTCB) with extracts of plant Stevia at room temperature.
450 Da. Splitless injection (1:25).
4.2. GLC Analysis
GLC analysis carried out on chromatograph “Chromotec Crystal 5000”, detector EZD-2 temperature 250˚C, a column: 1500 mm glass, diameter 3 mm, a nozzle: SE-30, the evaporator temperature is 260˚C, VN2 = 30 ml/min; volume of test 2 ml.
4.2.1. Isolation of HCH from Preparation “Hexachlorane”
10 g 12% p.s. pesticide “Hexachlorane” heated up with 100 ml heptane and filtered in a hot kind. A filtrate cooled and a dropped out deposit have filtered. Received hexachlorocyclohexane recrystallized from ethanol. Yield was 80%.
4.2.2. Isolation of 80% Purity Steviaside
From 1000 g of ground up part of plant Stevia 80 g dry extract (brown resinous weight) was isolated from it by 96%-ethanol and filtration fraction which consists of 80% Steviaside in amount of 4 g was isolated.
4.2.3. Preparation of a Standard Steviaside
In analytical weights weigh 0.05 g Steviaside and dissolve in 100 ml 96%-ethanol, from this solution take 5 ml aliquot and lead up 96%-ethanol up to 50 ml.
4.3. Determining of Steviaside in Ground up Part of Plant Stevia
4.3.1. First Method
On 1.2476 g crushed raw material I, II till the size of particles 2 - 3 ml place in a flask capacity 100 ml with a return refrigerator, add 50 ml distilled water and maintain on a boiling water bath 2 hr. Cool, filter and wash out water and repeat this operation of 2 more times on 20 min. Extracts unite and drive away under vacuum dry. Extracted with 96% ethanol of 5 times on 20 ml on a flask capacity 100 ml place the rest on 5 ml from this solution and dilute up to 50 ml with ethanol in a measured flask and measure optical density on SP-46 at λ = 330 nm.
Single 96% ethanol. For preparation of the standard Steviaside weigh 0.05 g Steviaside and dissolve in 100 ml of 96%-ethanol, from this solution take 5 ml aliquot and lead up 96%-ethanol up to 50 ml. The contents of Steviaside determine under the formula.
4.3.2. Second Method
On 1.0 g crushed raw material I, II till the size of particles 2 - 3 mm place in a flask capacity of 100 ml supplied with a return refrigerator, add 50 ml distilled water and maintain on a boiling water bath 2 hours. The cooled mix filter and wash out water and repeat this operation of 2 more times on 20 min. Extracts unite and drive away under vacuum up to 5 - 10 ml. Extracted 96% ethanol of 5 times on 20 ml and from this solution take the rest on 5 ml and dilute up to 50 ml with spirit in a measured flask. Quantity of Steviaside determine by a method TLC with photodensitometer. On a plate silica gel without the fluorescent indicator, the size 20 × 20 cm rendered on three layers of researched solutions of samples and four tests of the standard Steviaside. System (eluent)—chloroform:methanol:water in the ratio 3:2:0.4, dried up in an exhaust case, spray 10% a solution of a sulfuric acid and maintain in a drying case at temperature 110˚C during 5 minutes. Rf for Steviaside was 0.80.
4.4. Extracts of Stevia
4.4.1. Fractional Extraction with Ethanol and Aqueous Ethanol of Stevia
50 g a plant material (an elevated part of plant Stevia) with a degree of crushing 2.0 - 3.0 mm extracted at room temperature three times at a ratio plant raw material:ethanol 1:6, 1:5, 1:5 insisting within 12 hours. Ethanol extracts united all and have received 550 ml of an extract.
200 ml of extract I diluted equal volume of water and extracted consistently with gasoline, benzene (neutral substances), ethyl acetate (low-molecular phenolic compounds) and n-buthanole-olygomer phenolic compounds (on 100 ml three times). Every of petrol, benzene, ethyl acetate and n-buthanol extracts united and condensed up to 2/3 volumes and have left in a dark place for the further researches with the purpose of studying degradation chloroorganic pesticides under their action.
4.4.2. Extraction Ground up Part Plant Stevia
10 g a plant material with a degree of crushing 2.0 - 3.0 mm extracted at room temperature at a ratio plant raw material:ethanol 1:7, 1:10, 1:15 insisting within 12 hours. Thus yield of the sum extracted substances have made 16.2%, 17.75%, 18.1% accordingly.
4.4.3. Isolation of Water Extract from of Ground up Part of Plant Stevia
25 g of plant material with a degree of crushing of 3.0 - 5.0 mm extracted by water at a ratio 1:6 within 6 hrs, three times. Water extracts unite and evaporate before reception of the waterless rest. The rest brownish—the yellow color, looking like dense syrup; at cooling hardens. The yield was 5.4 g (21.6% I).
4.4.4. Isolation of Water Extract from of Ground up Part of Plant Stevia
25 g plant material with a degree of crushing 3.0 - 5.0 mm extracted by hot water at a ratio 1:6 within 3 hrs, three times. Water extracts unite and evaporate before reception of the waterless rest. The rest brownish—the yellow color, looking like dense syrup; at cooling hardens. The yield was 8.2 g (32.8% II).
4.4.5. Isolation of Polysaccharides
For isolation water-soluble polysaccharides free from lipophylic compounds plant material extracted all over again 300 ml cool, then heated water; water extracts summarized and evaporate before reception of the waterless rest. The rest of brown yellow color also looks like dense syrup. Yield was 2.4 g.
4.4.6. Isolation of Sum of Glycosides
For increase in completeness of extraction 25 g a plant material with a degree of crushing 3.0 - 5.0 mm extracted by boiled water in a ratio of 1:6 during 3 hr., three times. Water extracts united and evaporate before reception of the waterless dry rest. The rest brownо-yellow colors, looking like dense syrup, at cooling hardens. Yield was 8.2 g (32.8% III). Further for reception sum of glycosides extract III three times solve by 96%-ethanol. Ethanol extracts summarized and solvent drove away on potophom the evaporator. Have received 4.0 g (yield 16% from air-dried weight) IV-sum of glycosides. Remains water-soluble part (V), yield 4.0 g (16% from air-dried weight).
4.5. Reactions with DDT
In a conic flask were placed 100 mg extracts and 50 mg DDT. Stirred at room temperature during 3 days, controlled by TLC, significant change was not, leave still for 7 days, results of TLC showed that content of DDT in reactionary mixture has decreased for 45% - 50% in a case of extract II, in case of 96%-ethanol extract on 50% - 60%.
5. Conclusions
Influence of a degree of crushing of plant raw material, value of the hydromodule and temperatures on process of extraction of ground up part of the plant Stevia was investigated. It was isolated Steviaside of 80% purity from plant of Stevia.
Interactions of individual water and ethanol extracts of Steviaside from ground up part of Stevia with chloroorganic pesticides (DDT, HCCH and ТТCB) were studied. It was identified products of reforming of chloroorganic pesticides degradation by plant extract.
Interaction of water extracts of ground up part of plant Stevia with pesticide HCCH basically formed tetrachlorocyclohexadiene (HCH) with the yield of 86.9% and in particularly formed of tri-, tetrachlorobenzenes. In a case of Steviaside 80% purity also observed formation of the HCH with the yield of 79.7%. It was determined, that at interaction of water extract of plant Stevia with HCCH and DDT at presence of alkaloid Anabasine in a ratio of 2:1:1 occurs degradation HCCH up to 70% - 80%, and in case DDT on 25% - 30%. On the basis of research interaction of extracts isolated from of ground up part of plant Stevia with pesticides DDT, HCCH and TTBC it is possible to conclude, that most effective reagents for degradation of persistent chloroorganic pesticides are ethanol, aqueous ethanol, water extracts of Stevia and Steviaside of 80% purity isolated from the plant Stevia.
6. Acknowledgements
The research was made by financial support of the INTAS M3-7271.
REFERENCES
- T. A. Henry, “Chemistry of Plant Alkaloids (in Russian),” Translation from English, under Edition Acad. Rodionov, Goskhimizdat, Moscow, 1956, pp. 59-71.
- K. M. Shakhidoyatov, M. M. Khakimov, N. I. Mukarramov, N. K. Khidyrova, K. U. Khodjaniyazov and B. A. Urakov, “The Ways of Degradation of Proof Chlororganic Pollutants by Plant Extracts,” 7th International Symposium on the Chemistry of Natural Compounds, Tashkent, 16-18 October 2007, p. 147.
- N. K. Khidirova, K. M. Shakhidoyatov and N. I. Mukarramov, “Detoxification of Chloroorganic Pesticides by Extracts of the Elevated Part of Plant Stevia,” 11th International Symposium on Natural Product Chemistry, Karachi, 29 October-1 November 2008, p. 131.
- K. M. Shakhidoyatov, M. M. Khakimov, N. I. Mukarramov and B. A. Urakov, “The Use of Alkaloid Containing Plant Extracts for Detoxification of DDT,” Materials of Mendeleev Congress, Moscow, 23-29 September 2007, p. 131.
- K. U. Khodjaniyazov, N. I. Mukarramov, N. K. Khidirova, M. M. Khakimov, B. A. Urakov, E. S. Brodsky and K. M. Shakhidoyatov, “Degradation and Detoxification of Persistent Organic Pollutants in Soils by Plant Alkaloid Anabasine,” Journal of Environmental Protection, Vol. 3, No. 1, 2012, pp. 97-106. doi:10.4236/jep.2012.31012
- K. Morita, M. Ogata and T. Hasegawa, “Chlorophyll Derived from Chlorella Inhibits Dioxin Absorption from the Gastrointestinal Tract and Accelerates Dioxin Excretion in Rats,” Environmental Health Perspectives, Vol. 1091, No. 3, 2001, pp. 289-294. doi:10.1289/ehp.01109289

