Smart Grid and Renewable Energy
Vol.2 No.2(2011), Article ID:4962,6 pages DOI:10.4236/sgre.2011.22017
Is Methanol Using CO2 from the Atmosphere a New Fuel to Replace Gasoline?
![]()
Haile Plantation, Gainesville, USA.
Email: jbockris@cox.net
Received January 31st, 2011; revised March 31st, 2011; accepted April 5th, 2011.
Keywords: Energy, Hydrogen, Methanol, CO2, Costs
ABSTRACT
The recent disaster in the Gulf has drawn attention to the longevity of the oil supply and what alternative to gasoline is the appropriate fuel to which we should turn. The suggestion of Methanol as a substitute for gasoline as been greatly strengthened by George Olah in his publication “Beyond Oil and Gas: The Methanol Economy”. However, there remained the question of burning methanol without special attention to its method of synthesis which would not add to the CO2 content of the atmosphere. Hydrogen has often been suggested as an alternative fuel because it burns clean. A comparison is made of Hydrogen and Methanol synthesized with hydrogen and CO2 from the atmosphere or biomass. The cost of the methanol as prepared would be $28 to $31 per GJ. Development is needed in the method by which to obtain the CO2 from the atmosphere in a stream. Three possible methods are outlined. Only one has been subject to detailed system analysis. However, two independent calculations give highly similar costs. Water, air and wind to produce hydrogen for electrolysis of water, are the only resources necessary to make the methanol required. Changing over to any alternative fuel will impact the Oil companies. However, a change to methanol could be a long term solution for them; whereas a trend towards electricity as the overall medium of energy would not be.
1. Introduction
Gasoline allows us to fill our tanks and drive our cars. Distribution of the resource is very satisfactory. On the other hand, the resource cannot go on forever because it is limited. In addition, it is blamed, by some, for the cause of the gradual warming of the planet.
The trouble in getting oil points to exhaustion of liquid (tar sand free) oil. A replacement should, by now, be at least in the discussion stage [1].
There is evidence that the use of gasoline provides the main source of CO2 pollution [2]. Although the temperature rises are not yet threatening, parts of the planet will become too hot to sustain normal life within the century if gasoline continues to be used.
2. Is There any Doubt It is CO2 Which Forces the Gradual Rise in Temperature?
There is some doubt. The need to replace gasoline would be stronger if there were no doubt [3].
There is no doubt that the earth’s temperatures are rising; however, there are two theories for this. Some look towards the sun which does increase its out put but on an eleven year cycle. This has been going on for a long time and will likely continue. However, global warming seems to be undergoing a continuously slow rise since the nineteenth century and with an enhanced rate since about 1950. This slow rise does not mirror the steady eleven year cycle.
If we stopped putting CO2 into the atmosphere, global warming would stop increasing. This is a fact, and scientists interested in the atmosphere concur that we must eventually eliminate the CO2 buildup which now is occurring and realize we must change our main source of energy.
What stops us from changing is the decision on what energy should replace oil worldwide.
3. Characteristics of a Possible Replacement for Gasoline
One characteristic for the replacement fuel is that it should have the convenience of a liquid. Hydrogen has often been suggested; however, it is difficult to handle and too costly to liquefy making this medium inefficient and expensive.
Other desirable features for the replacement fuel is that it should be clean; e.g. no net CO2.
Finally, the resource should also be used in a fuel cell [4] because to convert gasoline to electricity by conventional means has an efficiency of around 30%. Hydrogen in a fuel cell has the efficiency of conversion to electricity of about 50%.
4. The Contribution of George Olah, Nobel Laureate
Professor George Olah and two coauthors Alain Goeppert, G. K. Surya Prakash wrote a book entitled “Beyond Oil and Gas: The Methanol Economy” (2006) [5]. I strongly advise purchasing this book as it brings out in detail the properties of methanol as a fuel and I believe it to be the most authoritative account of this fuel that there is. A second edition has just been published.
Optimal use of methanol would be attained were it made as a carbon neutral fuel.
5. Methanol - Atmosphere
Methanol is an excellent fuel as it is a liquid and can be used in a fuel cell. The aspect needed to make it a perfect fuel is to stop methanol from contributing net CO2 into the atmosphere which leads to global warming.
The solution to this would be to make methanol by combining CO2 with hydrogen made by a means which does not co-produce CO2; e.g. by means of wind, solar or enhanced geothermal energy producing electrolysis.
If it is possible to include CO2 from the atmosphere in the synthesis of methanol (Bockris and Zaromb 2006) [6], then, when this fuel is burned, CO2 is re-injected back into the atmosphere. There would be no build up of CO2. With this solution, global warming caused by CO2 is halted.
There is a way to reduce the CO2 in the atmosphere; however, this is hardly necessary. A certain amount of CO2 in the atmosphere (about 330 ppm) is necessary to maintain a comfortable temperature (of 57˚F). Removing it back to the level where it was in 1900, the earth would be colder and we would spend energy in heating our environments.
Methanol can be made from hydrogen by electrolyzing water. This would give us the necessary stream of hydrogen. Then the CO2 from the atmosphere is combined with the hydrogen using a copper-zinc catalyst. Now one has “methanol from the atmosphere”, or methanolAT.
The materials needed to make methanol in this way are inexhaustible and plentiful.
6. Hydrogen
A Hydrogen Economy [7] has been frequently discussed and explored to replace gasoline. As mentioned earlier, hydrogen is a clean medium of energy. Were we to replace gasoline with hydrogen on a worldwide scale, global warming would be halted. The sun’s radiation cyclically increasing would be the only contender towards warming the earth’s temperature.
The idea of using hydrogen has been around since the 1970s so why have we not used this resource? The primary reason is the cost. A book published in 2007 by Tapan Bose and Pierre Malbrunot, two French Canadians, entitled “Hydrogen” [8] outlines costs of not only producing the hydrogen, but accounts for all costs associated with the use of hydrogen.
The easiest way to get hydrogen without the coproduction of CO2 is to electrolyze water. The problem comes in with hydrogen is that it is a gas, not a liquid. To be stored, hydrogen as a gas has to be compressed around 500 atmospheres.
Then there is the cost of transporting hydrogen. It needs to be transported from the electrolyzer through storage tanks to the end user. Unfortunately, hydrogen cannot be put into ordinary steel pipes because the pipe will decay due to hydrogen embrittlement. The material needed is an alloy of nickel steel which is more expensive than steel.
But when converting hydrogen into usable energy, the most obvious form is electricity. In order to reconvert hydrogen back to electricity, the best source is a fuel cell. Although fuel cells bring an increase in efficiency, the present rate is 50%. Conversion means the cost is doubled compared with the cost of hydrogen directly from an electrolyzer.
The conclusion in the book “Hydrogen” is that the ancillary costs of dealing with hydrogen after it has been produced by the electrolyzer would make the cost of the hydrogen $48 per GJ. From the electrolyzer, depending on the temperature of electrolysis, it can be well below $20 per GJ [9].
7. Methanol Made from CO2 the Atmosphere: Comparison with Hydrogen [10]
To begin, neither fuel will cause global warming.
The availability of methanol is much better than that of hydrogen as seen by the French-Canadians. Admittedly, you have to have hydrogen to make methanol so how could it be cheaper? The reason is that one has to take into account storage, transport or conversion. So the end user pays more for a GJ of energy as seen in practice in the case of the hydrogen compared to methanol.
Hydrogen is a gas whereas methanol is a liquid. It is easier to deal with a liquid than with a low boiling gas, hydrogen.
8. What Methods are Available for Synthesizing Methanol with CO2 from the Atmosphere?
The first reaction of many to this question is one of surprise that it should even be brought up. Is not the CO2 in the atmosphere the principal problem and cause of the global warming? There is certainly plenty of CO2 in our atmosphere (1012 tons). The final step in the synthesis of the methanol advocated is to directly combine hydrogen and CO2, it is necessary to provide a stream of pure CO2 at about one-third the rate of hydrogen. The hydrogen would be coming from an electrolyzer with no problem about the rate of which it could be produced.
The immediate challenge is to obtain a stream of CO2 from the atmosphere. Part of the objective of this paper is to present three possible methods, although only one has been subject to detailed analysis.
Suggestion 1: Obtaining methanol from the atomsphere by absorbing it on magnesium oxide.
One takes a stream of air and combines it at a suitable temperature (about 300˚C) with powdered magnesium oxide. Under these conditions, it will form magnesium carbonate. The flow rate and time of contact with magnesium carbonate have to be carefully determined in a further development of the method. Thereafter, heating the magnesium carbonate to about 700˚C causes it to disassociate and provide the stream of pure CO2.
Although this process has been cost calculated, no laboratory work has been performed. The following is largely the result of discussions between Sol Zaromb [11] and the author.
The first requirement is a strong stream of air. This could be obtained by the use of fans, but more likely connected with wind generators for electricity, particularly when a large scale is needed. Changes to wind energy are readily appearing in Germany, northern Europe and the USA.
Use of the considerable source of air at the back of rotating blades can be cowled and the air taken through pipes which decrease in diameter as they progress until they meet with the powdered magnesium oxide kept in a tube with suitable spaces for it to provide a large area with contact with air at 300˚C.
This could be enhanced by the use of a funnel outside the wind generator [12] which would increase the amount of air taken in.
The final CO2 stream then has to be lead to the chamber in which the CO2 and hydrogen are combined. Here is the place where a copper-zinc catalyst is needed [13].
Suggestion 2: An Electrochemical Approach [14, 15].
One takes a stream of air and passes it through a solution of potassium hydroxide, to form potassium carbonate in solution. This carbonate can be electrolyzed, the anodic reaction producing pure CO2 and the cathodic reaction producing hydrogen. The amount produced would correspond to about one-third the needed amount of hydrogen to form methanol so an auxiliary water electrolysis device would have to couple with the device of electrolyzing the carbonate solution. We need three molecules of hydrogen to one molecule of CO2. The overall reaction is:
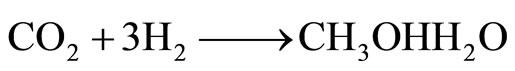 (1)
(1)
Hence two-thirds of the necessary hydrogen could be supplied by an auxiliary water cell. The combination of hydrogen and CO2 to make methanol is a well traveled pathway. It was mainly Japanese workers in the 1990s that made the optimal conditions for producing methanol in this direct way. The essential point is one has to have a copper-zinc catalyst of a certain constitution [16-19].
The reversible potential at pH = 14 and 4 oxygen with 386 ppm of CO2 bubbled through the solution gives –0.63 for the reversible electrode potential of the CO2 oxidation on the standard hydrogen scale.
The standard potential for oxygen evolution at pH = 14 is 0.812. Hence, even the most extreme overpotential, say of 1 volt could not run into this oxygen evolution at this pH. However, if the bubbling of the air was no longer continued, the electrode potential (not potensiostated) would shift up to interact with oxygen evolution.
Suggestion 3: Deep Freeze The amount of CO2 in the air is 384 ppm. The condensation temperature is 177˚K. Thus, if one passed the air through a cold zone at 10˚ to 20˚ below the condensation temperature, the CO2 in the atmosphere would become solid and drop out as flakes. These flakes would then be separated from their surroundings and allowed to vaporize.
9. Cost Estimate of Methanol Made from a Hydrogen-CO2 Combination
Dr. Rey Sidik, of Case-Western University [2], worked under my supervision analyzing the cost of the MgO path. His work is detailed below.
The question is: How does the cost of 1 GJ of CH3OH per Equation (1) compare to the cost of 1 GJ of H2 (including storage + transportation + delivery costs)?
The thermodynamic data for the chemicals [20]: CO2 + 3H2 = CH3OH (liq.) at standard state: kcal/mol My assumptions are:
1) CO2 capture efficiency is 100% and energy use is also close to 100% efficiency;
2) CO2 conversion to methanol is 100% efficient;
3) Capital cost of the equipment can be recouped within short period time, say 1-2 years.
del.G - 94.25 0 -39.76 - 56.68 del.H - 94.05 0 - 57.04 - 68.31 del.G for the reaction = (–56.68 - 39.76) - (–94.25) = –2.19 Kcal/mol = –9 kJ/mol del.H for the reaction = (–68.31 - 57.04) - (–94.05) = –31.3 Kcal/mol = –131 kJ/mol So the CO2 conversion reaction is exothermic and spontaneous at room temp.
To find out how many moles of CH3OH gives 1 GJ of heat energy:
CH3OH (liq.) + 3/2 (O2) = CO2 + 2 H2O (liq.)
del.H -57.04 0 - 94.05 - 68.31 del.H for reaction = 2(–68.31) - 94.05 + 57.04 = –173.63 Kcal/mol =– -726 kJ/mol 1 GJ/[726 ´ 10-6] = 1377 moles of CH3OH.
But to produce 1 mole of CH3OH, we need 3 moles of H2.
Thus, 1 GJ of methanol needs 3 ´ 1377 = 4132 moles of H2.
Since 1 GJ of H2 is equivalent to 3499 moles of H2 {1 GJ/[285.81 kJ/mol ´ 10-6 = 3499 moles H2} to produce 1 GJ of methanol, we need 4132/3499 = 1.18 GJ of H2.
Thus, 1 GJ of methanol needs 1.2 GJ of H2 and 1377 moles of CO2.
1 GJ of methanol = 1377 moles x 32 g/mol = 44 Kg/density = 56 liter = 15 gallon.
To calculate the air volume and diameter of the cylinder (cowl) just after the wind turbine that are required to CAPTURE 1377 moles of CO2 if the wind blows at 20 mph:
CO2 concentration in the air is 0.037%v, using PV=nRT, n =1.5 ´ 10-5 moles/literAt 100% capture efficiency, we need an air volume of 1377/n ~ = 92000 cubic meter A wind of 20 mph travels 20 ´ 1.6/12 = 2.7 km/5min, which means this wind can form an air column of 2.7 km in 5 min., so the radius of this column is what we need to find out:
Air volume = h ´ pi x r2, where r is the radius of column92000 = 2.7 ´ 1000 ´ 3.14 x r2, r= 10.85 ~ 11 meter.
Hence, the diameter of column or cowl that is needed to supply enough CO2 to produce 1 GJ of methanol in 5 minutes is 22 meter. This seems to be the size of a typical wind generator.
The minimum energy required to capture CO2 with MgO absorption is calculated as:
Cp [cal/K, mole]: 8.9 (CO2), 9.0 (MgO), 18.0 (MgCO3)
del.H = sum of Cp ´ (700 – 300 degree) = (18 + 9) ´ 400 = 400 = 14.36 Kcal/mole = 60 kJ/mole to capture 1377 moles of CO2, we need 1377 ´ 60=83 MJ = 23 kW.hr ~ 1$ worth of electricity @ 4 cents/Kw.hr.
Thus, the CO2 capture at least cost $1 per 1GJ of methanol production, once the capital cost of equipment is paid for.
The final answer to the question of the cost of 1 GJ of methanol obtained as in reaction [1] is cheaper than 1 GJ H2 plus its storage + transportation + delivery cost:
CO2 + 3 H2 = CH3OH (liq.) H2O (liq.) (1)
1$/GJ methanol 20$/GJ 1.2 ´ 20 + 1 = 28$/GJ 4) The cost of H2 storage + transportation + delivery is about 20$/GJ H2
A separate analysis of this method can be seen as having been done by Frank Zeman and David Keith at the University of Calgary [21]. They produced a comprehensive paper on the cost of synthesizing organic compounds using CO2 from the atmosphere. They also have looked at a similar process using CO2 from biomass which would give CO2 from the atmosphere in an indirect way. The biomass production reaction equation could be regarded as
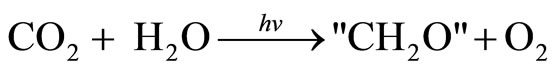 (2)
(2)
The “CH2O” represents a polymer of a biomass produced; e.g. a natural product such as grass.
Zeman and Keith [21] limit the range of their compounds to include methanolAT. The cost of carbon neutral compounds which would include methanol, according to these workers, would be between $23.5 to $30 per GJ.
There is another possibility which has been suggested by Sol Zaromb and that is the effluent from burning coal, natural or even gasoline. However, there may be some doubt about this source being truly “from the atomphere” so I prefer to limit the recommendation here to biomass and directly from the air.
10. A Possible Practical Arrangement for the Supply of MethanolAT
There are several ways in which one might extract CO2 from the atmosphere. The least costly of the three mentioned here would be optimized.
All gas stations have water and electricity available. It may be optimal in cost and cause minimal disruption to the country if the creation of the methanolAT takes place underneath the gas station.
MethanolAT can also be made in central plants and transported where needed. Transportation would be carried out the same as gasoline – in trucks.
If necessary, water can be extracted from the air which contains around 2% water.
11. Positive Consequences of a Transfer from Gasoline to MethanolAT
One considers the consequences of the use of methanolAT.
One would be the transfer from gasoline could be carried out easily, without massive changes of infrastructure. The sole would be the building in the electrolysis plant to make hydrogen. All the paraphernalia necessary for a conversion to a Hydrogen Economy is not needed for methanolAT.
The manufacture of electric cars, which is now just beginning for massive consumption, could be maintained if desired because of favorable properties, less maintenance, and could be fueled by methanolAT available as a substitute for gasoline and used as a fuel for fuel cells So methanolAT is not only easy to transfer, but also a quick way to halt the increase in CO2 in the atmosphere which is now occurring by about 1/2% per year.
Another advantage is inexhaustibility of water, air and wind which are available everywhere.
12. Oil Company Attitudes
It is too early to forecast the oil companies attitude to the challenge of methanolAT as their successor as being put forward in this article. In favor of the methanolAT, the conversion could be made “easily” as sections of the country could be converted without any stress upon the consumer.
There is a group of people in Europe who think that all sources of energy should be converted to electricity. Electricity need not produce CO2 upon its manufacture by using renewables. It may well be that for small countries the storage problem is less pressing than that of larger countries. It may be necessary to produce hydrogen or wind energy overnight or at times which are not matched to the use times so that storage of the energy from the sporadic source is necessary. This could not be done with electricity where the storage possibilities are limited.
A MethanolAT Economy would be attractive to gas companies because methanolAT is similar to gasoline and would be handled in a similar way. It could be transported in trucks. MethanolAT would save the Gas Companies from what they would have to do if electricity became the only practical medium. Substituting an organic liquid for gasoline, the very large oil industry, particularly in the United States, would be confronted with far less challenges than it would have to face were the conversion largely to electricity.
Introducing the methods of making methanolAT from the atmosphere around the world would be beneficial to everyone. Moreover, it could be regarded as a permanent fuel. There is nothing to exhaust. Nothing would cause problems in the atmosphere.
13. Acknowledgements
The author has presented and supported A Hydrogen Economy. However, the publication by Tapan Bose and Pierre Malbrunot [8] containing an analysis of the expensive ancillary costs of using hydrogen has made clear the cost problem of hydrogen. The French Canadian authors are to be thanked for this contribution.
Professor George Olah has played a major part in making consideration of conversion to methanol a practical possibility. The fact that he is not only a famous organic chemist but also has been honored by the award of a Nobel Prize implies a weighty influence on his opinion.
Lastly, I must extend acknowledgments to Sol Zaromb, a long time colleague, for taking part in discussions with me in which he was the first to suggest magnesium oxide as a primary substance on which to absorb CO2 from the atmosphere.
REFERENCES
- J. O’M. Bockris, “Energy, Global Warming and the Future,” Pending Publication Nova Publishers, New York, 2011.
- J. O’M. Bockris, “Global Warming,” In: S. A. Harris, Ed., Global Warming, SCIYO Croatia, 2010, pp. 159-220.
- G. A. Florides, P. Christodoulides and V. Messaritis, “Global Warming vs. Sun,” In: S. A. Harris, Ed., Global Warming, SCIYO Croatia, 2010, Chapter 3, pp. 23-62.
- S. Srinivasan, “Fuel Cells: From Fundamentals to Applications,” Springer, Berlin, 2006.
- G. A. Olah, A. Goeppert and G. K. S. Prakash, “Beyond Oil and Gas: The Methanol Economy,” Wiley-VCH, Weinheim, 2006.
- J. O’M. Bockris and S. Zaromb, “Effect of Including CO2 in the Synthesis of Methanol,” International Journal of Hydrogen Energy, Vol. 33, No. 9, 2008, p. 2129.
- J. O’M. Bockris, “Energy: The Solar Hydrogen Alternative,” Wiley, New York, 1975.
- T. Bose and P. Malbrunot, “Hydrogen,” John Libby Eurotext, Esher, United Kingdom, 2007.
- J. O’M. Bockris and T. N. Veziroglu, “Estimates of the Price of Hydrogen as a Medium for Wind and Solar Sources in the Production of Hydrogen,” International Journal of Hydrogen Energy, Vol. 32, No. 12, 2007, pp. 1605-1610. doi:10.1016/j.ijhydene.2007.04.037
- National Research Council and National Academy of Engineering of the National Academies, “The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs,” The National Academies Press, Washington, D.C., 2006.
- S. Zaromb and J.O’M. Bockris, “Discussion Concerning the Effect of Adding Co2 into the Structure of Synthetic Methanol,” 2008.
- B. Sorensen, “Renewable Energy,” Elsevier, Amsterdam, 2005.
- Z. Jiang, T. Xiao and V. L. Kuznetsov, “Turning CO2 into a Fuel,” Philosophical Transactions of Royal Society, Vol. A368, 2010, p. 3363.
- S. Stucki, A. Schulor and M. Constantinescu, “Coupled CO2 Recovery from the Atmosphere and Water Electrolysis: Feasibility of a New Process for Hydrogen Storage,” International Journal of Hydrogen Energy, Vol. 20, No. 8, 1995, pp. 653-663. doi:10.1016/0360-3199(95)00007-Z
- J. O’M. Bockris, A. K. Reddy and M. Gamboe-Aldeco, “Modern Electrochemistry, IIA,” Chapter 7, Plenum Press, New York, 2000.
- D. B. Skoropinski, “Corrosion of Aluminum Fuel System Components by Reaction with EGME Icing Inhibitor,” Energy and Fuels, Vol. 10, No. 1, 1996, p. 103. doi:10.1021/ef950164e
- W. Goehna and P. Koenig, “Producing Methanol from CO2,” Chem Tech, Vol. 69, 1994, p. 30.
- Y. Kita and K. Kishino, “New process for manufacturing maleimides,” Catalysis Survey from Japan, Vol. 2, No. 2, 1998, p. 195. doi:10.1023/A:1019030509156
- M. Saito and K. Mura, “Development of High Performance Cu/Zno-Based Catalysts for Methanol Synthesis and the Water-Gas Shift Reaction,” Catalysis Survey from Asia, Vol. 8, No. 4, 2004, p. 285. doi:10.1007/s10563-004-9119-y
- David R. Lide, “Standard Potential for Electrochemical Reaction,” In: David R. Lide, Ed., CRC Handbook Chemistry and Physics, 85th Edition, CRC Press, New York, 2004, JANAF Thermochemical Tables 1974 Supplement.
- F. Zeman and D. Keith, “Carbon Neutral Hydrocarbons,” Philosophical Transactions of Royal Society, Vol. A366, 2008, pp. 3901-3918. doi:10.1098/rsta.2008.0143

