Food and Nutrition Sciences
Vol.08 No.01(2017), Article ID:73750,22 pages
10.4236/fns.2017.81010
Discovery of New Stilbene Antioxidants of the Bio-Elicited Peanut Sprout Powder (BPSP) and Longevity Extension of Mice Fed with BPSP-Supplemented Diets
Robin Y.-Y. Chiou1*, Po-Chang Chiu2, Ju-Chun Chang1, Yu-Jang Li2, Chia-Wen Hsieh3, Jin-Yi Wu3, Shu-Mei Lin1, Yun-Lian Lin4, Brian B.-C. Weng3
1Department of Food Science, National Chiayi University, Taiwan
2Department of Applied Chemistry, National Chiayi University, Taiwan
3Department of Microbiology, Immunology and Pharmaceutical Sciences, National Chiayi University, Taiwan
4Department of Chinese Pharmaceutical Science and Chinese Medicine Resources, China Medical University, Taiwan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 19, 2016; Accepted: January 20, 2017; Published: January 23, 2017
ABSTRACT
Biosynthesis of peanut stilbenes, including resveratrol as the secondary metabolites, could be enhanced by subjecting the kernels to germination and wound-stress. Investigations of the bio-elicited peanut sprout powder (BPSP) addressed on characterization of the comprising stilbenes and effectiveness in longevity extension deserves intensive research. In this study, peanut kernels were subjected to germination and wound-stress in preparation of BPSP. The methanol extracts of BPSP were medium pressure liquid chromatographic (MPLC) fractionated and semi-preparative HPLC recovered and followed by instrumental identification and biological activity determinations of the isolated stilbenes. In longevity experiments, 16 female 11-mon-old BALB/c mice and both genders of 12-mon-old ICR mice were daily fed with BPSP- supplemented diets at doses of 0, 0.1 and 0.5 g BPSP/kg bw for 750 and 762 days, respectively. Based on chemical characterization, enriched quantity of stilbenes in the BPSP up to ca. 1% (w/w) was detected. Two new stilbene compounds, namely, 4, 5’- dihydroxy-6’’-hydroxymethyl, 6’’-methylpyrano [2’’, 3’’: 3’, 4’] stilbene and 3, 4, 5’- trihydroxy-6’’, 6’’-dimethylpyrano [2’’, 3’’: 3’, 4’]stilbene along with 5 known stilbenes were isolated. The 7 stilbenes exhibited potent antioxidative and antiglycative activities and varied with structure-activity nature. Based on the resultant survival curves and average lifespans of both mouse models, basal diets supplemented with BPSP are effective to extend mouse longevity by a dose dependent manner. It is of merit to demonstrate that peanut kernels as a potent producer could be bio-elicited to biosynthesize a broad spectrum of bioactive stilbenes to prepare BPSP which is effective to extend mouse longevity as science-evidenced by the two long-term animal experiments.
Keywords:
Bio-Elicited Peanut Sprout Powder (BPSP), Wound-Stress, MPLC, Peanut Stilbenes, Longevity Extension

1. Introduction
In addition to one of worldwide-favored nuts, peanut plant and seeds are capable of biosynthesizing bioactive stilbenes and phytochemicals [1] - [6] . Peanut stilbenes are small molecular secondary metabolites and induced by biotic and/or abiotic elicitations to enhance biosynthesis [6] - [13] . Among those discovered stilbenes, resveratrol is one of the most noticed, due to its virtual biological activities and emerging high values in development of the health-enhancing products. Since the first discovery of peanut resveratrol in 1976 [14] , many other stilbenes originated from peanut have been isolated and identified [8] [10] [15] - [22] . Accordingly, a broad spectrum addressed on enhance- ment of biosynthesis and organic synthesis along with biological and/or pharmaceutical activities characterization of the stilbenes has been extensively and intensively investigated [4] [8] [12] [13] [17] [23] - [28] .
As conducted in our laboratory, subjection of peanut kernels to germination in preparation of peanut sprouts as a functional vegetable has been reported [29] [30] . Extracts of peanut sprouts resulting in anti-obesity effects have also been demonstrated [31] . In comparison, peanut seeds after germination bear higher resveratrol content and antioxidant activities than that of un-germinated seeds [29] [32] [33] [34] . Biosynthesis of peanut stilbenes could be remarkably enhanced by subjecting kernels to germination and wound-stress elicitation [8] . The isolated peanut stilbenes, such as arachidin-1, have exhibited potent anti-inflammatory and anti-cancer along with anti-oxidant activities [8] [23] [24] [26] . In a recent report, arachidin-1 and resveratrol exhibit phytoestrogenic activities and modulate regulatory T cell functions responsible for successful aging of the aged ICR mice [28] . These findings point out a perspective of potency to use the bio-elicited peanut sprout powder (BPSP) in development of value- added and health-beneficial products. In this study, in order to discover the unknown comprising stilbenes and isolate sufficient quantities of pure compounds for chemical and biological activities characterization, BPSP was subjected to medium pressure liquid chromatographic (MPLC) fractionation and followed by semi-preparative HPLC purification. Two new compounds along with 5 known stilbenes have been isolated, identified and followed by determination of antioxidative and antiglycative activities. Importantly, based on the findings that arachidin-1 and resveratrol exhibit phytoestrogenic activities and modulate regulatory T cell functions responsible for successful aging of the aged ICR mice [28] and arachidin-1 is one of the major bioactive stilbenes of BPSP [8] , two long-term longevity experiments have been conducted, respectively. Both BALB/c mice as an in-bred mouse model and ICR mice as an out-bred mouse model were fed with basal diets supplemented with BPSP at various doses for 750 and 762 days.
2. Materials & Methods
2.1. BPSP Preparation and Nutrition Fact Analysis
Basically, the previously reported procedure [8] was followed with modification. In brief, sound kernels (Arachis hypogaea L., a Spanish cultivar) were soaked with water at ambient temperature (25˚C - 28˚C) for 16 h, drained and incubated under dark and humidified condition 3 days for germination. Cotyledons removed from each of the germinated kernels were sliced to create a wound stress and spread onto perforated plates and heated in a forced-air-oven at 120˚C for 45 min to dehydrate and inactivate enzymes. The dried peanut slices were ground into paste with a peanut butter grinder and defatted with n-hexane. BPSP was prepared after solid-solvent separation through filtration and vacuum evaporation in a closed chamber for complete solvent removal. Then, the obtained BPSP was packed in heavy-duty polyethylene bags and frozen- stored (−20˚C) for later use. BPSP was subjected to nutrition fact analysis by Food Inspection and Analysis Center, College of Life Sciences, National Chiayi University.
2.2. MPLC and HPLC Fractionation and Recovery of the BPSP Stilbenes
BPSP 500 g was refluxed with methanol (5 liters) for 2 h and filtered to obtain methanol extract and followed by rotary vacuum evaporation to get a final concentrated volume ca. 150 ml. The concentrate was partitioned twice with 300 ml ethyl acetate (EA). The pooled EA fractions were evaporated to dryness and dissolved in 20 ml of 80% methanol. The dissolved solution was membrane-filtered (0.45 µm) and loaded onto a MPLC column (ODS, 3.6 × 23 cm, Buchi Glass Column) for MPLC (Buchi Pump Manager C-615 with two Pump Module C-601, Buchi Labortechnik, Flawil, Switzerland). MPLC was run with dual solvents of methanol and water from initial 65% methanol and linearly increased to final 75% methanol in 60 min with 25 ml/min of flow rate. The eluted solution was collected every 3 min. Then, the column was eluted for clean-up with 90% methanol for 15 min. The MPLC loading solution and each of the eluted and collected fractions was subjected to analytical HPLC analysis (Hitachi L-7100 pump, L-7455 PDA detector) (Hitachi Co., Ltd., Tokyo, Japan) equipped with an ODS column (Hypersil ODS, 250 × 4.6 mm, 5 μm, Thermo Electron Corp. Hypersil Ltd., Cheshire, UK) run with a dual solvent system of methanol (A solvent) and water (B solvent) with 1 ml/min, 20 µl and 254 nm of flow rate, injection volume and monitoring wavelength, respectively. The mobile phase was programmed and run at 0 min with initial 50% A and linearly increased to 75% A at 5 min; increased to 100% A at 18 min; back to 50% A at 21 min and held for an additional 3 min prior to next sample injection.
Based on the obtained HPLC chromatograms of the loading solution for MPLC [Figure 1(a)] and MPLC separate fractions [Figures 1(f1)-(f6)], the fractions containing the selected peanut stilbenes were concentrated appropriately by vacuum rotary evaporation and membrane-filtered to prepare samples for semi-preparative HPLC fractionation (L-7100 pump, L-7420 UV/Vis detector, L-2200 auto-sampler, Hitachi Co.; and a fraction collector, CHF 121SA, Advantec Co.). Semi-HPLC equipped with an ODS-column (Hyperprep HS C18, 10 mm diameter × 25 cm length, Thermo Electron Corp. Hypersil Ltd., Cheshire, UK) was run at 35˚C (controlled by a column oven) with a dual solvent system containing methanol (A solvent) and water (B solvent) with 100
Figure 1. HPLC chromatograms of the loading solution extracted from bio-elicited peanut sprout powder (a) and the collected fractions (f1 to f6) after subjection of the loading solution to medium pressure liquid chromatographic (MPLC) fractionation.
µl, 3 ml/min and 254 nm of injection volume, flow rate and monitoring wavelength, respectively. The optimal mobile phase was programmed and adjusted according to the fractions destined for further purification. From each fraction, the repeatedly collected elutes corresponding to a targeted peak were pooled and vacuum evaporated to dryness. After further vacuum drying at 40˚C for 2 h, the substance was weighed, cap sealed in brown vial and stored under −20˚C for structural identification and bioactivity determinations. In addition to match of HPLC retention time and PDA spectrum, structures of the known stilbenes were confirmed by dissolving each in CD3OD or CDCl3 for 1H NMR analysis. As indicated in the HPLC chromatograms of the MPLC collected fractions by order of polarity, known stilbenoids of resveratrol (Res) was respectively isolated from MPLC fraction 1 [Figure 1(f1)], arachidin-1 (Ar 1), compound I, arachidin-3 (Ar 3), isopentadieneylresveratrol (IPD) and compound II were isolated from MPLC fraction 2 to 5 [Figures 1(f2)-(f5)] and arahypin-5 (Ap 5) was isolated from fraction 6 [Figure 1(f6)]. Among those, two new unknown compounds, i.e., compound I [Figure 1(f2)] and compound II [Figure 1(f5)] were weighted and subjected to structural identification.
2.3. Structural Identification of the Unknown Compounds
The isolated and weighed dry solids of compounds I and II were dissolved in methanol and CH2Cl2 for UV λmax determination with a spectrophotometer (Hitachi U-2100). ESI-HRMS (EI + VE + LMR) (m/z, M+) analysis of the compounds was run on a LTQ Orbitrap XL Hybrid Ion Trap Mass Spectrometer (Thermo Fisher Scientific Inc., Waltham, MA). Optical rotation measurements employed an automatic polarimeter (Autopol IV, Rudolph Research Analytical, Hackettstown, NJ).
For NMR analyses, compound I (ca. 2.0 mg) and compound II (ca. 2.5 mg) were respectively dissolved in 0.6 ml CD3OD and CDCl3 for sample preparation. All NMR experiments were performed by a Bruker Avance III 500 MHz spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany), equipped with a 5-mm BBFO probe. The 1H and 13C NMR were measured at 298 K operating at 500.13 and 125.75 MHz, respectively. Chemical shift for each hydrogen was recorded with reference to the residual solvent signal of CD3OD (at δH 3.31 and 4.70, δC49.2) and CDCl3 (at δH 7.26, δC 77.2). Coupling constants (J) were reported in Hertz (Hz). All one dimensional (1H and 13C) and two dimensional NMR (COSY, HSQC, and HMBC) measurements were performed with standard Bruker pulse sequences.
2.4. Antioxidative Potency (AOP) and Antiglycative Activity Determination
For AOP determination by linoleic acid in an iron/ascorbate system, the previously reported procedure [34] was followed. For comparison on identical molecular basis, 100 µM resveratrol, compound I, arachidin-1, arachidin-3, IPD, compound II, arahypin-5 and butylated hydroxytoluene (BHT) as a reference were respectively prepared by dissolving in methanol and subjected to followed measurements. For antiglycative activity determination, the reported procedure [35] was followed. Briefly, the above 7 stilbenoid compounds were respectively prepared in 5% DMSO at 50 μM for determination. Aminoguanidine (AG) (Sigma Chem. Co., St. Louis, MO) at 3 mM was prepared as a reference and determined concurrently.
2.5. Longevity Experiments with BALB/c and ICR Mice
Two long-term animal experiments with BALB/c mice, an in-bred model, and ICR mice, an out-bred model, have been respectively conducted. Both experiment proposals have been approved by the Institutional Animal Care and Use Committee (IACUC) (Approval No. IACUC-2006005) of the National Chiayi University. For the first experiment with 11-mon-old female BALB/c mice, 48 mice provided by National Laboratory Animal Center, Taipei, Taiwan, after 2-week adaptation, were distributed randomly into three groups, namely, control group (BPSP 0 g/kg bw) (composition of the basal diet was listed in Supplementary Material Appendix Table S1), L-BPSP group (BPSP 0.1 g/kg bw) as a low dose treatment and H-BPSP group (BPSP 0.5 g/kg bw) as a high dose treatment. Four mice were raised and housed in a cage with a 12-h cycle of circadian rhythms and each was ear-tagged for identification. The temperature and relative humidity were controlled at 20˚C - 22˚C and 60% - 65%, respectively. The mice were daily fed with the estimated diets provided on 15 g diet/100 g∙bw basis. The uneaten diets were weighed as a health monitoring purpose and average daily feed intake. Partial amount of soybean protein in the basal diet formula was substituted by BPSP to meet the desired dose levels and adjusted to increase proportion according to the observed slight decrease of feed intake in the later periods. The BALB/c animal experiment started from November 3, 2006 and ended after the last death on November 14, 2008 for 750 days of entire period. Body weight for each survival mouse was determined every 2 weeks and each dead mouse was weighed and subjected to organ resection and combined with carcass for tissue fixation in a formalin jar for later use.
For the second longevity experiment, male and female ICR mice (12-mon-old, an out-bred animal model) obtained from the Laboratory Animal Center, National Taiwan University, Taipei, Taiwan were treated following the same protocol and treatment doses as that done with BALB/c mice. After 2-week adaptation, 10 male and 6 female mice were randomly distributed into a treatment and housed in a low density setting of 2 male/cage and 3 female/cage. Each mouse was weighed and identified with ear punched tagging. For diet preparation, BPSP was introduced in substitution of soybean protein of the basal diet (Supplementary Material Appendix Table S1). The treatment started from December 18, 2008 and ended after the last death on January 26, 2011 for 762 days of entire period.
2.6. Statistics
Means with standard deviation of the determinations (n > 3) are expressed. Data were subjected to one-way analysis of variance (ANOVA) for variance comparison among test groups. Significant differences between means were determined using Duncan’s multiple-range test.
3. Results
3.1. BPSP Preparation and Nutrition Fact
In this study, BPSP preparation was initiated from dormant but of vitality peanut kernels transited to physiological and biochemical activation of germination-related metabolism and followed by wound-stress, heat treatment, grinding, defatting and powder packaging for storage. As subjected to compositional analysis to indicate nutrition fact of BPSP, its energy, protein, carbohydrate (sugars), fat (saturated fat and trans fat) and sodium per 100 g BPSP are 366 Kcal, 55 g, 30 g (1.1 g), 2.6 g (0.5 and 0 g) and 17 mg, respectively. BPSP is a high protein powder and used to substitute soy protein to formulate BPSP-supplemented diets (Supplementary Material Appendix Table S1) in the followed animal experiments.
3.2. Isolation and Identification of the Recovered BPSP Stilbenes
As shown in Figure 1(a) of HPLC chromatogram, presence of a complexity of comprising components in the BPSP methanol extracts after ethyl acetate partition is expected. The complex composition of the BPSP extract has been effectively separated by MPLC fractionation [Figures 1(f1)-(f6)]. From those collected fractions, series of isolation of the comprising stilbenes by semi-preparative HPLC were further accomplished. In reference of HPLC retention time, UV-spectrum shown by PDA detection and confirmation with 1H NMR analysis, 5 known peanut stilbenes, namely, trans-res- veratrol (Res), trans-arachidin-1 (Ar 1), trans-arachdin-3 (Ar 3), trans-isopentadieneyl resveratrol (IPD) and trans-arahypin-5 (Ap 5) were isolated (Figure 2). Two new compounds, i.e., compound I [Figure 1(f2)] and compound II [Figure 1(f4) and Figure 1(f5)] were subjected to spectroscopic analyses and structural elucidation. As estimated based on recovery yields, contents of resveratrol, arachidin-1, arachidin-3, IPD, arahypin-5, compound I and compound II are ca. 50, 2400, 1800, 3800, 980, 25 and 110 µg/g BPSP, respectively.
For the compound I, a light grey powder was obtained with UV (CH2Cl2) λmax (log ε): 340 nm (4.32); [α]D22 +6.67 (c 0.1, CH2Cl2); ESI-HRMS [(EI+VE+LMR, M+) m/z 310.1212, C19H18O4 with theoretical mass 310.1205]. After subjection to 1D and 2D NMR spectroscopic analysis, 1H and 13C chemical shifts are shown in Table 1 and the related 1D (1H and 13C) and 2D (COSY, HSQC, HMBC) spectra are shown in Supplementary Material. 1H NMR spectrum of I indicated the presence of a 1,4-disubstituted phenyl with protons at δH 6.78 (d, 2H, J = 8.8 Hz), 7.35 (d, 2H, J = 8.8 Hz), a 1,3,4,5-tetrasubstituted phenyl with protons at δH6.48 (s) and 6.49 (s), a trans-olefin with protons at δH 6.75 and 6.96 (each d, J = 15.5 Hz), and a cis-olefin with protons at δH 5.54 and 6.74 (each d, J = 9.9 Hz) and a gem-hydroxyl methyl moiety with methylene protons at δH 3.52, 3.62 (each d, 2H, J = 11.5 Hz) and methyl protons at δH 1.35 (3H, s). The proton correlations were confirmed by a COSY experiment. The 13C NMR spectrum of compound I (Table 1) displayed 19 carbons, of which 14 were assigned to a stilbene moiety and 5 were assigned to a gem-hydroxymethyl-methylpyran unit.
The HMBC spectrum indicated correlations of H-7 to C-2 (δC 128.9) and C-6 (δC 128.9); H-8 to C-2’ (δC 106.74) and C-6’ (δC 106.9); H-5’’ to C-6’’ (δC 79.8) and C-4’ (δC 110.1); H-7’’ to C-6’’ (δC 79.8); and H-8’’ to C-6’’ (δC 79.8) and C-7’’ (δC 68.49). Based on the above instrumentally supported evidence, compound I was identified as 4, 5’- dihydroxy-6’’-hydroxymethyl, 6’’-methylpyrano [2’’, 3’’: 3’, 4’]stilbene (Figure 2). It is a new assigned natural compound.
Figure 2. Chemical structures of stilbenes isolated from the bio-elicited peanut sprout powder.
Table 1. 1H and 13C NMR chemical shifts respectively analyzed by 500 and 125 MHz of the compounds I and II isolated from the bio-elicited peanut sprout powder.
aJ: coupling constant in Hz; s: singlet; d: doublet.
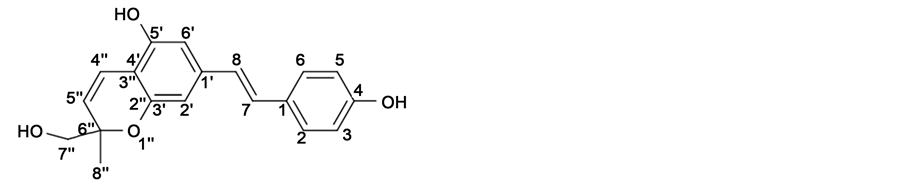
Compound I

Compound II
For structural elucidation of the compound II, a light grey powder was obtained with UV (MeOH) λmax (log ε): 340 nm (4.74); ESI-HRMS [(EI+VE+LMR, M+) m/z 310.1200, C19H18O4 with theoretical mass 310.1205]. 1H and 13C chemical shifts of compound II are shown in Table 1. The related 1D and 2D spectra are also shown in Supplementary Material. 1H NMR spectrum of II indicated signals of three hydroxyl protons (δH 9.78, 9.80 and 10.06), a 1,3,4-trisubstituted phenyl with protons at δH6.85 (d, J = 8.2 Hz), 6.92 (d, J = 8.2 Hz), and 7.03 (s), a 1,3,4,5-tetrasubstituted phenyl with protons at δH 6.43 (s) and 6.58 (s), a trans-olefin with protons at δH6.76 and 6.90 (each d, J = 16.5 Hz), and a cis-olefin with protons at δH 5.59 and 6.61 (each d, J = 9.9 Hz) and protons at 1.44 (6H, s) corresponding to a gem-dimethylpyran group. The proton correlations were confirmed by a COSY experiment. The 13C NMR spectrum of compound II (Table 1) displayed 19 carbons, of which 14 were assigned to a stilbene moiety and 5 to a gem-dimethylpyran unit.
The HMBC spectrum indicated correlations of H-2 and H-5 to C-3 (δC 143.6) and C-4 (δC 143.7), and H-6 to C-4, both H-7 and H-8 are correlated to C-1 (δC 130.8) and C-1’ (δC 138.6). The gem-dimethylpyran moiety was determined to be attached to the 1,3,4,5-tetrasubstituted benzene ring by the long-range correlations of H-4’’ to C-3’ (δC 151.4), C-4’ (δC 109.0) and C-5’ (δC 154.1) as well as H-5’’to C-4’. Accordingly, based on the above instrument-supported data, compound II was identified as 3, 4, 5’-trihy- droxy-6’’, 6’’-dimethylpyrano [2’’, 3’’: 3’, 4’]stilbene (Figure 2). It is a new assigned natural compound.
3.3. AOP and Antiglycative Activities of the Recovered BPSP Stilbenes
As compared on the same molecular basis (100 µM), in comparison to BHT, all test stilbenes have exhibited potent AOP activities [Figure 3(a)]. Apparently, among the test stilbenes, AOP of each molecule varied with dependence on each own chemical structure. As a comparison was made on antiglycative activity, the test 7 peanut stilbenes [Figure 3(b)], archidin-1, arachidin-3 and compound II exhibited much higher activity than that of 3 mM AG.
3.4. Longevity Extension of BALB/c and ICR Mice
For the 11-mon-old female BALB/c mice, an in-bred animal model, continuously fed with BPSP-supplemented normal diets for 750 days until the last death, survival cures are shown in Figure 4(a). Longevities of the mice fed with BPSP-supplemented nor- mal diets were longer than mice fed with the control diet. As further comparison of overall life spans, average lifespans of the mice fed with BPSP-supplemented diets were longer than that of normal diet. For the mice fed with BPSP-supplemented diets at 0.5 g BPSP/kg∙bw [Figure 4(b)], ca. 50 days of longevity was extended.
For the experiment of longevity extension with an out-bred animal model for both genders, 12-mon-old male and female ICR mice fed with the same above-described diets for 762 days. As generally observed, longevity of the mice as affected by gender was limited and the integrated survival curves are shown in Figure 4(c). It is obvious to observe that longevity of the mice fed with BPSP-supplemented basal diets were longer, with a dose dependent manner, than mice fed with the control basal diet. As compared, average lifespans of the mice fed with BPSP-supplemented diets was longer


Figure 3. Antioxidative potencies (a) test at 100 μM of resveratrol (Res), compound I (Com I), arachidin-1 (Ar 1), arachidn-3 (Ar 3), isopentadienylresveratrol (IPD), compound II (Com II), arahypin-5 (Ap 5) and butylated hydroxytoluene (BHT) and antiglycative activities (b) test at 50 μM and aminoguanidine (AG) at 3 mM as a reference (n = 3).


Figure 4. Survival curves and average lifespans of the 11-mon-old female BALB/c mice (a) and (b) and 12-mon-old male and female ICR mice (c) and (d) respectively fed daily with basal diets supplemented with bio-elicited peanut sprout powder (BPSP) for 750 and 762 days; control: 0 g BPSP/kg bw; L: 0.1 g BPSP/kg bw; H: 0.5 g BPSP/kg bw.
than that of basal diet [Figure 4(d)]. For the mice fed with 0.5 g BPSP/kg bw, ca. 96 days of the mouse lifespan was extended.
4. Discussion
With an attempt to effectively isolate the complex compositional compounds from BPSP (Figure 1), MPLC is capable of resolving whole overlapping compounds into fractions and enabling subsequent semi-pre HPLC purification to obtain 7 compounds (Figure 1 and Figure 2). Trans-resveratrol (Res), trans-arachidin-1 (Ar 1), trans- arachdin-3 (Ar 3) and trans-isopentadieneyl resveratrol (IPD) have been previously reported in our laboratory [8] . Those stilbenes along with trans-arahypin-5 (Ap 5) and many other stilbene compounds of peanut origins have also been discovered in different research laboratories [10] [12] [13] [17] [20] [22] . A new compound with prenylatedbenzenoid and but-2-enolide moieties, termed SB-1, has been isolated from peanut kernels challenged with species of Aspergillus [18] . Arahypin-5 (4,5’-dihydroxy-6’’,6’’- dimethylpyran [2”,3”:3’,4’]stilbene) has been isolated from peanut seeds challenged with Aspergillus caelatus [20] . Other new arahypins and other stilbenes have also been isolated from fungal-challenged peanut seeds [12] [13] . Even peanut stilbene compounds have long been extensively and intensively investigated, two new peanut stilbene compounds, i.e., 4, 5’-dihydroxy-6’’-hydroxymethyl, 6’’-methylpyrano [2’’, 3’’: 3’, 4’]stilbene (compound I) and 3, 4, 5’-trihydroxy-6’’, 6’’-dimethylpyrano [2’’, 3’’: 3’, 4’]stilbene (compound II) (Figure 2) have been isolated, identified and assigned.
In addition to chemical characterization, a wide variety of biological activities of the selected natural and/or synthetic peanut-related stilbenes has been investigated and demonstrated [12] [13] [22] [25] [26] [27] [28] . In particular, presence of resveratrol and other bioactive phytochemicals related to consumer health has been highlighted [3] [6] . As subjection of the 7 peanut stilbenes isolated in this study to AOP and antiglycative activity characterization (Figure 3), all test compounds have exhibited potent activities and varied on structure-activity nature of each molecule. Antioxidant activity of a molecule is the most noticed characteristics of a molecule to be recognized as a bioactive candidate. Glycation of serum albumin and functional proteins under high-blood- sugar condition, such as enzymes and membrane proteins, leading to metabolic syndrome and subsequent chronic diseases is always an important issue of human health. Among the test stilbenoids shown in Figure 3, it is of importance to point out that arachidin-1 and arachidin-3 have exhibited considerably higher AOP and antiglycative activities than other stilbenes. As reported, arachidin-1 and arachidin-3 are major stilbenes of BPSP and bear potent antioxidant and anti-inflammatory activities have been demonstrated [8] [23] . In further, a broad spectrum of potent biological activities of arachidin-1 has been demonstrated [4] [8] [23] [24] [26] [28] . In the future, an intensive structure-activity investigation addressed on nature of each compound remained research interest. For BPSP, presence of a wide spectrum of various peanut stilbenes is no doubt closely related to reflect its unique properties.
In longevity experiments with the aged mice of in-bred and out-bred models for 750 and 762 days, it is obvious that longevity of the mice fed with BPSP-supplemented basal diets were longer than mice fed with the control basal diet (Figure 4). Overall average life spans of the mice fed with BPSP-supplemented diets at 0.5 g BPSP/kg∙bw were longer than that of basal diet by ca. 50 and 96 days for both mouse models, respectively. Based on the obtained survival curves or average life spans as science-based evidences, the effect of dietary supplementation of BPSP in longevity extension was also obvious. These findings are supporting an old Chinese tale that “Peanut also named life longer nut” as well as a phrase that “A longer life with peanuts” promoted by The Peanut Institute [3] .
As expected, approaches by dietary interventions to extend mouse longevity by antioxidant supplementation, calorie restriction or complex dietary supplements are of virtue [36] [37] [38] . In this study, longevity extension of either in-bred or out-bred mouse models was achieved by dietary BPSP supplementation (Figure 4). This is of significance and perspective to raise the link that human longevity extended by dietary intake of the BPSP-like supplements is likely. As reported recently, arachidin-1 and resveratrol modulate regulatory T cell functions responsible for successful aging in aged ICR mice mainly due to their phytoestrogenic activities [28] . In that report, when 12‑week-old ICR mice were fed with BPSP-supplemented diets for 48 weeks, their circulating Treg populations, the gene expression levels of CTLA-4 and TGF-β were significantly elevated. Arachdin-1 is one of the major stilbenes of BPSP [8] . Therefore, the studies support the beneficial roles of arachidin-1, resveratrol and other BPSP stilbenes in facilitating a successful aging immune status and contribute longevity extension.
5. Conclusion
It is of novelty that the subjection of peanut kernels to germination and wound-stress is remarkably activating biosynthesis of peanut stilbenes. Through MPLC to facilitate the followed isolation and recovery of the stilbenes from BPSP, high yield up to ca. 1.0% (w/w) of two new stilbenes, identified for the first time, and 5 new compounds were recovered. All recovered stilbenes exhibited potent anti-oxidative and anti-glycative activities. With an attempt to use BPSP as a dietary supplement, it is of merit to demonstrate that BPSP is effective to extend mouse longevity as science-evidenced by the two long-term animal experiments. In addition to the fact that phytoestrogenic stilbenes, arachidin-1 and resveratrol exhibit phytoestrogenic activities and modulate regulatory T cell functions responsible for successful aging in aged ICR mice [28] , co-present components of BPSP including other phenolic compounds all contribute to the observed effectiveness of lifespan extension. In the future, continuous investigations with BPSP addressed on investigations of the specified longevity involved mechanisms and related nutraceutical products development are of worth.
Acknowledgements
We acknowledge financial support from the Ministry of Science and Technology, Republic of China (NSC 98-2622-B-415-003-CC2; NSC 100-2313-B-415-004-MY3; NSC 101-2622-B-415-002-CC2). Gratitude is extended to Ms Show-Phon Learn for helpful assistance in the animal experiments.
Supplementary Material
Supplementary data associated with this article are presented in the Appendix.
Declarations
1) Ethics approval and consent to participate
Not applicable.
2) Availability of data and material
Datasets generated or analyzed in this study are included in this published article and raw data of the current study are available from the corresponding author on reasonable request.
Authors’ Contributions
R.Y.Y.C. supervised, designed experiments and written manuscript; P.C.C. purified new compound and instrumental analyses; J.C.C. conducted and managed progress of experiments; Y.J.L. elucidated chemical structure; C.W.H. conducted product preparation and characterization; J.Y.W carried NMR analysis and data collection; S.M.L. carried out parts of longevity animal experiments; Y.L.L. carried out parts of new compound elucidation; B.B.C.W. carried out parts of animal experiments
Cite this paper
Chiou, R.Y.-Y., et al. (2017) Discovery of New Stilbene Antioxidants of the Bio-Elicited Peanut Sprout Powder (BPSP) and Longevity Extension of Mice Fed with BPSP-Supplemented Diets. Food and Nutrition Sciences, 8, 141-162. http://dx.doi.org/10.4236/fns.2017.81010
References
- 1. Cassidy, A., Hanley, B. and Lamuela Raventos, R.M. (2000) Isoflavones, Lignans and Stilbenes—Origins, Metabolism and Potential Importance to Human Health. Journal of Science of Food and Agriculture, 80, 1044-1062.
https://doi.org/10.1002/(SICI)1097-0010(20000515)80:7<1044::AID-JSFA586>3.0.CO;2-N - 2. King, J.C., Blumberg, J., Ingwersen, L., Jenab. M. and Tucker, K.L. (2007) Tree Nuts and Peanuts as Components of a Healthy Diet. Journal of Nutrition, 138, 1736S-1740S.
- 3. The Peanut Institute (2007) A Long Life with Peanuts. Food Thought, 11, 1-4.
- 4. Lopes, R.M., Agostini-Costa, T.S., Gemenes, M.A. and Silvera, D. (2011) Chemical Composition and Biological Activities of Arachis Species. Journal of Agricultural and Food Chemistry, 59, 4321-4330.
https://doi.org/10.1021/jf104663z - 5. Pokkaew, R., Wang, S.H., Liu, C.D., Huang, F.L., Chang, J.C., Lo, C.Y. and Chiou, R.Y.Y. (2013) Properties and Characterization of Antioxidant and Antiglycative Activities for the Multiple Harvests of Aquatic and Field-Cultivated Peanut Leaves and Stems. Journal of Functional Foods, 5, 327-336.
https://doi.org/10.1016/j.jff.2012.11.003 - 6. Sales, J.M. and Resurreccion, A.V.A. (2014) Resveratrol in Peanuts. Critical Reviews of Food Science and Nutrition, 54, 734-770.
https://doi.org/10.1080/10408398.2011.606928 - 7. Chung, I.M., Park, M.R., Chun, J.C. and Yun, S.J. (2003) Resveratrol Accumulation and Resveratrol Synthase Gene Expression in Response to Abiotic Stresses and Hormones in Peanut Plants. Plant Science, 164, 103-109.
https://doi.org/10.1016/S0168-9452(02)00341-2 - 8. Chang, J.C., Lai, Y.H., Djoko, B., Wu, P.L., Liu, C.D., Liu, Y.W. and Chiou, R.Y.Y. (2006) Biosynthesis Enhancement and Antioxidant and Anti-inflammatory Activities of Peanut (Arachis hypogaea L.) Arachidin-1, Arachidin-3, and Isopentadienylresveratrol. Journal of Agricultural and Food Chemistry, 54, 10281-10287.
https://doi.org/10.1021/jf0620766 - 9. Rudolf, J.L. and Resurreccion, A.V. (2007) Optimization of Trans-Resveratrol Concentration and Sensory Properties of Peanut Kernels by Slicing and Ultrasound Treatment, Using Response Surface Methodology. Journal of Food Science, 72, S450-S462.
https://doi.org/10.1111/j.1750-3841.2007.00467.x - 10. Sobolev, V.S. (2008) Localized Production of Phytoalexins by Peanut (Arachis hypogaea) Kernels in Response to Invasion by Aspergillus Species. Journal of Agricultural and Food Chemistry, 56, 1949-1954.
https://doi.org/10.1021/jf703595w - 11. Hasan, M.M., Cha, M., Bajpai, V.K. and Baek, K.H. (2013) Production of a Major Stilbene Phytoalexin, Resveratrol in Peanut (Arachis hypogaea) and Peanut Products: A Mini Review. Reviews in Environmental Science and Biotechnology, 12, 209-221.
https://doi.org/10.1007/s11157-012-9294-7 - 12. Liu, Z., Wu, J. and Huang, D. (2013) New Arahypins Isolated from Fungal-Challenged Peanut Seeds and Their Glucose Uptake-Stimulatory Activity in 3T3-L1 Adipocytes. Phytochemical Letters, 6, 123-127.
https://doi.org/10.1016/j.phytol.2012.11.004 - 13. Liu, Z., Wu, J. and Huang, D. (2013) New Stilbenoids Isolated from Fungus Challenged Black Skin Peanut Seeds and Their Adipogenesis Inhibitory Activity in 3T3-L1 Cells. Journal of Agricultural and Food Chemistry, 61, 4155-4161.
https://doi.org/10.1021/jf400144s - 14. Keen, N.T. and Ingham, J.L. (1976) New Stilbene Phytoalexins from American Cultivars of Arachis hypogaea. Phytochemistry, 15, 1794-1795.
https://doi.org/10.1016/S0031-9422(00)97495-8 - 15. Cooksey, C.J., Garrat, P.J., Richards, S.F. and Strange, R.N. (1988) A Dienyl Stilbene Phytoalexin from Arachis hypogaea. Phytochemistry, 27, 1015-1016.
https://doi.org/10.1016/0031-9422(88)80263-2 - 16. Sobolev, V.S., Cole, R.J., Dorner, J.W. and Yagen, B. (1995) Isolation, Purification, and Liquid Chromatographic Determination of Stilbene Phytoalexins in Peanuts. Journal of AOAC International, 78, 1177-1182.
- 17. Ku, K.L., Chang, P.S., Cheng, Y.C. and Lien, C.Y. (2005) Production of Stilbenoids from the Callus of Arachis hypogaea: A Novel Source of the Anticancer Compound Piceatannol. Journal of Agricultural and Food Chemistry, 53, 3877-3881.
https://doi.org/10.1021/jf050242o - 18. Sobolev, V.S., Deyrup, S.T. and Gloer, J.B. (2005) New Peanut (Arachis hypogaea) Phytoalexin with Prenylated Benzenoid and But-2-Enolide Moieties. Journal of Agricultural and Food Chemistry, 54, 2111-2115.
https://doi.org/10.1021/jf052948o - 19. Medina-Bolivar, F., Condori, J., Rimando, A.M., Hubstenberger, J., Shelton, K., O’Keefe, S.F., Bennett, S. and Dolan, M.C. (2007) Production and Secretion of Resveratrol in Hairy Root Cultures of Peanut. Phytochemistry, 8, 1992-2003.
https://doi.org/10.1016/j.phytochem.2007.04.039 - 20. Sobolev, V.S., Neff, S.A. and Gloer, J.B. (2009) New Stilbenoids from Peanut (Arachis hypogaea) Seeds Challenged by an Aspergillus caelatus Strain. Journal of Agricultural and Food Chemistry, 57, 62-68.
https://doi.org/10.1021/jf802891v - 21. Sobolev, V.S., Neff, S.A. and Gloer, J.B. (2010) New Dimeric Stilbenoids from Fungal-Challenged Peanut (Arachis hypogaea) Seeds. Journal of Agricultural and Food Chemistry, 58, 875-881.
https://doi.org/10.1021/jf903410e - 22. Sobolev, V.S., Krausert, N.M. and Gloer, J.B. (2016) New Monomeric Stilbenoids from Peanut (Arachis hypogaea) Seeds Challenged by an Aspergillus flavus Strain. Journal of Agricultural and Food Chemistry, 64, 579-584.
https://doi.org/10.1021/acs.jafc.5b04753 - 23. Djoko, B., Chiou, R.Y.Y., Shee, J. J. and Liu, Y.W. (2007) Characterization of Immunological Activities of Peanut Stilbenoids, Arachidin-1, Piceatannol, and Resveratrol on Lipopolysaccharide-Induced Inflammation of RAW 264.7 Macrophages. Journal of Agricultural and Food Chemistry, 55, 2376-2383.
https://doi.org/10.1021/jf062741a - 24. Huang, C.P., Au, L.C., Chiou, R.Y.Y., Chung, P.C., Chen, S.Y., Tang, W.C., Chang, C.L., Fang, W.H. and Lin, S.B. (2010) Arachidin-1, a Peanut Stilbenoid, Induces Programmed Cell Death in Human Leukemia HL-60 Cells. Journal of Agricultural and Food Chemistry, 58, 12123-12129.
https://doi.org/10.1021/jf102993j - 25. Park, B.H., Lee, H.J. and Lee, Y.R. (2011) Total Synthesis of Chiricanine A, Arahypin-1, Trans-Arachidin-2, Trans-Arachidin-3, and Arahypin-5 from Peanut Seeds. Journal of Natural Products, 74, 644-649.
https://doi.org/10.1021/np100696f - 26. Sobolev, V.S., Khan, S.I., Tabanca, N., Wedge, D.E., Manly, S.P., Cutler, S.L., Coy, M.R., Becnel, J.J., Neff, S.A. and Gloer, J.B. (2011) Biological Activity of Peanut (Arachis hypogaea) Phytoalexins and Selected Natural and Synthetic Stilbenoids. Journal of Agricultural and Food Chemistry, 59, 1673-1682.
https://doi.org/10.1021/jf104742n - 27. Kwon, J.Y., Seo, S.G., Heo, Y.S., Yue, S., Cheng, J.X. and Lee, K.W. (2012) Piceatannol, Natural Polyphenolic Stilbene, Inhibits Adipogenesis via Modulation of Mitotic Clonal Expansion and Insulin Receptor Dependent Insulin Signaling in Early Phase of Differentiation. Journal of Biochemical Chemistry, 287, 11566-11578.
- 28. Weng, B.B.C., Lin, W.S., Chang, J.C. and Chiou, R.Y.Y. (2016) The Phytoestrogenic Stilbenes, Arachidin-1 and Resveratrol, Modulate Regulatory T Cell Functions Responsible for Successful Aging in Aged ICR Mice. International Journal of Molecular Medicine, 38, 1895-1904.
- 29. Wang, K.H., Lai, Y.H., Chang, J.C., Ko, T.F., Shyu, S.L. and Chiou, R.Y.Y. (2005) Germination of Peanut Kernels to Enhance Resveratrol Biosynthesis and Prepare Sprouts as a Functional Vegetable. Journal of Agricultural and Food Chemistry, 53, 242-246.
https://doi.org/10.1021/jf048804b - 30. Lin, B.S., Lien, T.F., Chao, M.R., Lai, T.Y., Chang, J.C., Chou, S.J., Liao, H.F. and Chiou, R.Y.Y. (2008) Toxicological and Nutraceutical Assessments of Peanut Sprouts as Daily Supplements to Feed Sprague-Dawley Rats for 18 Weeks. Journal of Science of Food and Agriculture, 88, 2201-2207.
https://doi.org/10.1002/jsfa.3335 - 31. Kang, N.E., Ha, A.W., Woo, H.W. and Kim, W.K. (2014) Peanut Sprouts Extract (Arachis hypogaea L.) Has Anti-Obesity Effects by Controlling the Protein Expressions of PPARγ and Adiponectin of Adipose Tissue in Rats Fed High-Fat Diet. Nutritional Research and Practice, 8, 158-164.
https://doi.org/10.4162/nrp.2014.8.2.158 - 32. Sobolev, V.S. and Cole, R.J. (1999) Trans-Resveratrol Content in Commercial Peanuts and Peanut Products. Journal of Agricultural and Food Chemistry, 47, 1435-1439.
https://doi.org/10.1021/jf9809885 - 33. Sanders, T.H., McMichael, R.W. and Hendrix, K.W. (2000) Occurrence of Resveratrol in Edible Peanuts. Journal of Agricultural and Food Chemistry, 48, 1243-1246.
https://doi.org/10.1021/jf990737b - 34. Hsu, W.C., Cho, P.J., Wu, M.J. and Chiou, R.Y.Y. (2002) A Rapid and Small-Scale Method for Estimating Antioxidative Potency of Peanut Sprouts. Journal of Food Science, 67, 2604-2608.
https://doi.org/10.1111/j.1365-2621.2002.tb08785.x - 35. Wang, S.H., Chang, J.C., Pokkaew, R., Lee, J.F. and Chiou, R.Y.Y. (2011) Modified Fast Procedure for the Detection and Screening of Antiglycative Phytochemicals. Journal of Agricultural and Food Chemistry, 59, 6906-6912.
https://doi.org/10.1021/jf201103t - 36. Sadowska-Bartosz, I. and Bartosz, G. (2014) Effect of Antioxidants Supplementation on Aging and Longevity. BioMed Research International, 2014, Article ID: 404680.
https://doi.org/10.1155/2014/404680 - 37. Lemon, J.A., Boreham, D.R. and Rollo, C.D. (2005) A Complex Dietary Supplement Extends Longevity of Mice. Journal of Gerontology Biological Sciences, 60A, 275-279.
https://doi.org/10.1093/gerona/60.3.275 - 38. Minor, R.K., Allard, J.S., Younts, C.M., Ward, T.M. and de Cabo, R. (2010) Dietary Interventions to Extend Life Span and Health Span Based on Calorie Restriction. Journal of Gerontology Biological Sciences, 65, 695-703.
https://doi.org/10.1093/gerona/glq042
Abbreviations
AG, aminoguanidine;
Ar 1, arachidin-1;
Ar 3, arachidin-3;
IPD, isopentadienylresveratrol;
Ap 5, arahypin-5;
AOP, antioxidative potency;
BPSP, bio-elicited peanut sprout powder;
MPLC, medium pressure liquid chromatography;
NMR, nuclear magnetic resonance;
Res, resveratrol.
Appendix
Table S1. Composition of basal murine diets.
aAIN-76; bAIN-76.
1H compound I

Figure S1. NMR data for compound I: 1H.

Figure S2. NMR data for compound I: 13C.

Figure S3. NMR data for compound I: COSY.

Figure S4. NMR data for Compound I: HMBC.

Figure S5. NMR data for Compound I: HSQC.

Figure S6. NMR data for compound II: 1H.

Figure S7. NMR data for compound II: 13C.

Figure S8. NMR data for Compound II: COSY.

Figure S9. NMR data for Compound II: HMBC.

Figure S10. NMR data for Compound II: HSQC.
Submit or recommend next manuscript to SCIRP and we will provide best service for you:
Accepting pre-submission inquiries through Email, Facebook, LinkedIn, Twitter, etc.
A wide selection of journals (inclusive of 9 subjects, more than 200 journals)
Providing 24-hour high-quality service
User-friendly online submission system
Fair and swift peer-review system
Efficient typesetting and proofreading procedure
Display of the result of downloads and visits, as well as the number of cited articles
Maximum dissemination of your research work
Submit your manuscript at: http://papersubmission.scirp.org/
Or contact fns@scirp.org







