Food and Nutrition Sciences
Vol. 3 No. 1 (2012) , Article ID: 17081 , 9 pages DOI:10.4236/fns.2012.31010
Bifidobacteria as Potential Functional Starter Cultures: A Case Study by MSc Students in Food Science and Technology (University of Foggia, Southern Italy)
![]()
Department of Food Science, Faculty of Agricultural Science, University of Foggia, Foggia, Italy.
Email: a.bevilacqua@unifg.it
Received June 15th, 2011; revised August 3rd, 2011; accepted August 11th, 2011
Keywords: Bifidobacteria; Functional Starter; Probiotic Properties; Technological Characterization
ABSTRACT
This research paper was the results of activity of MSc students of Food Science and Technology, attending the class “Biotechnology of Functional Starter”. Five strains of bifidobacteria (Bifidobacterium animalis subsp. lactis; B. longum subsp. infantis; B. breve; B. animalis subsp. animalis; B. bifidum) were evaluated in order to assess their suitability as functional starter cultures, by studying the following technological and probiotic traits: growth at different temperatures, NaCl amounts and pH values; acidifying ability; metabolism (arginin deamination, esculin hydrolysis, acetoin production); survival at low pH and in presence of bile salts; hydrophobic properties; antibiotic resistance. After laboratory assays and strain selection through a multivariate analyses, it was highlighted that B. longum subsp. infantis and B. animalis subsp. lactis represent a good compromise as potential functional starter cultures, as B. animalis subp. lactis showed a high growth index at pH 5 and good values at 25˚C and 30˚C, as well as the minimal viability loss at pH 2.5. B. longum subsp. infantis DSMZ 20088 was the best microorganism for its growth index in presence of 6.5% of salt added and at 25˚C and 30˚C.
1. Introduction
Bifidobacteria were isolated for the first time in 1899 by Tissier from the stools of breast-fed infants. These Gram-positive and anaerobic microorganisms, not producing any type of gas, were named for their bifurcate morphology (from Latin bifidum) Bacillus bifidus [1].
Then, Orla-Jensen observed that Bacillus bifidus was able to produce lactic acid and classified it in the family of Lactobacteriaceae under the name of Lactobacillus bifidus. Although in 1924 Orla-Jensen introduced the genus Bifidobacterium as a separate taxon, the name L. bifidus remained until 1970. Except for B. dentium (the etiological cause of dental caries), bifidobacteria are regarded as safe [2]. A number of bifidobacteria now have a long history of safe use as dietary adjuncts; B. adolescentis, B. animalis, B. lactis, B. bifidum and B. breve have GRAS (generally regarded as safe) status [1].
Starter cultures are generally designed to assure food safety, shelf-life, technological and economic feasibility criteria. Apart from these traditional properties, new starter cultures should take into account the risks posed by the formation of biogenic amines in food, the development and spreading of bacterial resistance to antibiotics, protection against harmful bacteria either by the production of antimicrobials (bacteriocins) or acidification. The ability of starter cultures to compete with the natural microbiota of raw materials, as well as technological performances, relies upon the ability to survive in the conditions encountered in food (salt, temperature, pH, preservatives).
Probiotics for human use contribute to organoleptic, rheological and nutritional characteristic of foods. They have also a positive effect on the intestinal microflora of the host.
Their optimal characteristics include tolerance to the conditions present in the gastrointestinal tract (resistance to gastric juices, bile), the ability to adhere to the intestinal surface, the production of antimicrobial substances and the ability to modulate the immune response of host [3].
A new kind of starter cultures are the functional starters, i.e. microorganisms acting at the same time as probiotic and starter. Functional starter cultures are used for the improvement of aroma, to obtain safe products because of their ability to produce bacteriocins, for their ability to enrich food matrix with micronutrients [4].
Some reports are available on the use of lactobacilli as functional starter cultures [4]; however, to the best of our knowledge there are no data on bifidobacteria as functional starter microorganisms. Therefore, the main topic of this research was to study the technological and probiotic characteristics of some strains of bifidobacteria; in particular, we focused on B. animalis subsp. lactis; B. longum subsp. infantis; B. breve; B. animalis subsp. Animalis; B. bifidum, assessing:
1) Growth at different temperatures, NaCl amounts and pH values;
2) Acidifying ability;
3) Metabolism (arginin deamination, esculin hydrolysis, acetoin production) ;
4) Survival at low pH and in presence of bile salts;
5) Hydrophobic properties;
6) Antibiotic resistance.
These assays were used as representative of the technological (growth under different conditions, acidification, metabolism) and probiotic abilities (survival at low pH and in presence of bile salts, resistance to antibiotics, hydrophobicity) to assess the suitability of bifidobacteria as multifunctional starter cultures.
2. Materials and Methods
2.1. Strains
This research focused on 5 strains of bifidobacteria purchased from a Public Collection (Deutsche Sammlung von Mikroorganismem und Zellkulturen’s collection, Braunschweig, Germany, DSMZ): B. animalis subsp. lactis DSMZ 10140; B. longum subsp. infantis DSMZ 20088; B. breve DSMZ 20213; B. animalis subsp. Animalis DSMZ 20104; B. bifidum DSMZ 20456. Before each experiment, the microorganisms were grown in MRS broth (Oxoid Milan, Italy), added with 0.5 g/l of cysteine (Sigma-Aldrich, Milan, Italy) (cMRS) and incubated at 37˚C for 48 h under anaerobic conditions, in order to attain a cell concentration of 9 log cfu/ml.
2.2. Metabolism
The following metabolic properties were assessed:
Deamination of arginine. The deamination of arginine was assessed in the substrate of Abd-El-Malek, buffered at pH 7.0. The composition of the substrate is: tryptone (Oxoid) 5 g/l; yeast extract (Oxoid) 2.5 g/l; glucose (C. Erba) 0.5 g/l; K2HPO4 (J.T. Baker, Milan) 2 g/l; arginine hydrochloride (Sigma-Aldrich) 3 g/l. The substrate was distributed into sterile test tubes (5 ml), inoculated with ca. 7 log cfu/ml of each strain separately and incubated at 37˚C for 96 h. The test was considered positive if, after the addition of Nessler’s reagent (C. Erba), the samples turned to orange.
Hydrolysis of esculin. This assays was performed using aliquots of 5 ml of MRS broth, buffered at pH 6.5 and supplemented with esculin (2 g/l) (Sigma-Aldrich) and ammonium iron citrate (1 g/l) (C. Erba). The samples were inoculated with ca. 7 log cfu/ml of each strain separately and incubated at 37˚C for at least 72 h. The black colour of the medium after the incubation period denoted hydrolysis of esculin.
Production of acetoin. The production of acetoin was determined on glucose phosphate broth consisting of: bacteriological peptone (Oxoid) 5 g/l; glucose 5 g/l; K2HPO4 5 g/l. After inoculation (7 log cfu/ml), the samples were incubated for 4 - 7 days at 37˚C. The test was considered positive if the colour of the substrate turned to red after the addition of a 6%-solution of α-naphthol (Sigma Aldrich) in ethanol and a 16%-aqueous solution of NaOH.
2.3. Effect of NaCl, pH and Temperature
The assay was performed in cMRS broth, adjusted at pH 5.0 through HCl 1.0 N, or added with different concentrations of NaCl (2-4-6.5%). Otherwise, the effect of the temperature was studied in not-modified cMRS broth (pH 6.0 - 6.2), incubated at 25˚C, 30˚C, 37˚C and 44˚C.
The samples were inoculated with 3 log cfu/ml of each strain separately, and incubated at 37˚C (effect of pH and salt) or at 25˚C - 44˚C (effect of the temperature). Aliquots of not-modified MRS broth, inoculated with the five strains and incubated a 37˚C, were used as controls.
Microbial growth was evaluated after 24, 48 and 96 h through absorbance measurement at 600 nm using a Shimadzu UV-visible spectrophotometer (Shimadzu Europe Ltd., Duisburg, Germany). Data were modeled as Growth Index [5], modified as follows by Bevilacqua et al. [6,7]:

where Abss is the absorbance of the samples at different pH values, NaCl concentrations or temperature, and Absc the absorbance of the control sample. All the experiments were conducted in duplicate on two independent batches.
2.4. Acidification
Aliquots of MRS broth of 10 ml were inoculated with 6 log cfu/ml of each strain separately and incubated at 30˚C, 37˚C and 44˚C. The acidifying ability was assessed as decrease of the pH of the medium after 24 and 48 h; pH measurements were performed through a pH-meter Crison mod 2001 (Crison Instruments, Barcelona, Spain). All the experiments were conducted in duplicate over two independent batches.
2.5. Survival at 60˚C for 30 min
Microorganisms were grown in cMRS broth, incubated at 37˚C for 48 h; then, cultures were heat-treated at 60˚C for 30 min in a water-bath. After heat-treatment, 100 μl of these cultures were used to inoculate aliquots of 5 ml of cMRS broth, then incubated at 37˚C for 24 h. Microbial growth was evaluated through absorbance measurement at 600 nm. All the experiments were performed in duplicate.
2.6. Survival at pH 2.5 and in Presence of 0.3% of Bile Salts
Aliquots of saline solution (0.9% NaCl), adjusted at pH 2.5 or added with 0.3% of bile salts (Oxoid), were inoculated with ca. 7 log cfu/ml of each strain separately. Then, the samples were incubated at 37˚C for 3 h. Bifidobacteria viability was evaluated through pour plate method on cMRS agar, incubated at 37˚C for 48 h under anaerobic conditions. The analyses were performed on duplicate over two different batches. Aliquots of saline solution at pH 7 and not containing bile salts, but inoculated with bifidobacteria, were used as controls.
Data were modelled as viability loss referred to controls, as follows:
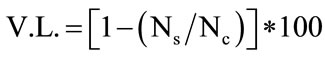
where Ns and Nc were cell counts (log cfu/ml) in the samples acidified at pH 2.5 or added with bile salts and in the control, respectively.
2.7. Antibiotic Resistance
Antibiotic resistance assay was performed through the agar diffusion technique, according to the protocol established by the NCCLS [8]. The strains of bifidobacteria, previously grown in cMRS broth, were streaked on cMRS agar plates through sterile swabs. Then, the disks containing the antibiotics were placed on the surface of the plates.
The plates were incubated at 37˚C for 24 h under anaerobic conditions. At the end of the incubation period, the presence of a halo around the disk of the antibiotic revealed susceptibility of the target towards the antibiotic. All tests were performed in triplicate. The antibiotics were:
1) Ampicillin (33 µg);
2) Vancomycin (70 µg);
3) Erythromycin (78 µg);
4) Gentamicin (40 µg);
5) Streptomycin (100 µg);
6) Chloramphenicol (60 µg);
7) Tetracyclines (80 µg);
8) Ciprofloxacin (10 µg);
9) Trimethoprim (52 µg).
All the antibiotics were purchased from Neo Sensitabs® (Taastrup, Denmark).
2.8. Hydrophobic Properties
The ability to adhere to intestinal mucosa was evaluated indirectly as hydrophobic property, i.e. as the ability of hyrocarbons (hexadecane) to catch cells. The assay was conducted as follows:
1) 10 ml of cell cultures were centrifuged at 4000 rpm for 10 min;
2) then, the supernatant was discarded and the pellet suspend in 25 ml of PBS (0.8 g/l K2HPO4; 0.68 g/l K2HPO4; 8.77 g NaCl, acidified at pH 2.0 with HCl 2.0 N (cell suspension);
3) for each strain two different samples were prepared: control (9.5 ml of cell suspension + 0.5 ml of water) and active sample (9.5 ml of cell suspension + 0.5 ml of hexadecane-C. Erba);
4) the samples were shaken for 10 s and left under static conditions for 10 min;
5) the ability of hexadecane to catch cells was evaluated through absorbance measurement at 600 nm after 30, 60, 90 and 120 min.
All the analysis were performed in duplicate and data modelled as hydrophobic index:

where for each time of analysis ΔAbss and ΔAbsc are the decrease in absorbance in the sample containing the hexadecane and in the control, respectively.
2.9. Statistical Analysis
Data were analyzed through one-way analysis of variance (one-way ANOVA) and Tukey’s test through the software Statistica for Windows, ver. 10.0 (Statsoft, Tulsa, Okhla.).
Moreover, the results of technological and probiotic characterization were used as input values to run a Principal Component Analysis through the add-in-soft component of Excel XLSTAT (Addinsoft, Paris, France).
3. Results and Discussion
3.1. Metabolic Traits
Table 1 reports the results for the metabolic characterization of bifidobacteria. All the strains were able to perform the hydrolysis of esculin but not the deamination of arginin; concerning the production of acetoin, this characteristic was recovered only for two strains: B. animalis subsp. lactis DSMZ 10140 and B. animalis subsp. animalis DSMZ 20104. Finally, bifidobacteria did not survive a heat-shock at 60˚C for 30 min.
3.2. Growth under Different Conditions and Acidification
One of the most important trait for the selection of a suitable starter is the study of growth under conditions simulating food matrix for which the starter is intended to. Keeping in mind a possible applications of bifidobacteria as suitable functional starter cultures for dairy products, the technological challenges performed were the growth under acidic conditions, salt resistance and growth in a wide range of temperature.
Figure 1 shows the data of the assays under acidic conditions (pH 5.0) after 24 h of incubation. B. animalis subsp. lactis DSMZ 10140, B. animalis subsp. animalis DSMZ 20104 and B. breve DSMZ 20213 were not affected by the relatively low pH, as one could infer from Growth Index (ca. 95% - 100%); otherwise, B. longum subsp. infantis DSMZ 20088 and B. bifidum DSMZ 20456 were partially inhibited and experienced Growth Indices of 47% and 63%, respectively.
Table 2 reports growth index values after 24 h referred to the assay in presence with different amounts of salt; the effects of salt relied upon two factors: its concentration and strains. Concerning the effect of concentration, as expected Growth Index decreased with increasing salt amount; moreover, a strain-dependent effect was found, as there were resistant strains (B. animalis subsp. lactis DSMZ 10140, B. animalis subsp. animalis DSMZ 20104, B. breve DSMZ 20213) and salt-affected ones (B. longum subsp. infantis DSMZ 20088, B. bifidum DSMZ 20456). Amongst the resistant strains, the strongest one was B. animalis subsp. animalis DSMZ 20104, which showed a Growth Index of 34.36% with 6.5% of salt added; otherwise, the most sensible strain was B. longum subsp. infantis DSMZ 20088.
Based on the results of salt assay, a resistance hierarchy was built (from the most resistant strain to the most sensible one):
B. animalis subsp. animalis DSMZ 20104 > B. animalis subp. lactis DSMZ 10140-B. breve DSMZ 20213 > B. bifidum DSMZ 20456 > B. longum subsp. infantis 20088.
Then, growth under different temperatures was assessed. For this technological trait a time-dependent effect was highlighted, as evidenced by Figures 2(a) and (b). After 24 h bifidobacteria were not able to grow at 44˚C (Figure 2(a)); moreover, a kind of inhibition was evidenced also at 25˚C and 30˚C. The extent of this effect depended upon the strains: B. animalis subsp. lactis DSMZ 10140, B. longum subsp. infantis DSMZ 20088 and B. breve DSMZ 20213 were partially inhibited only at 25˚C (Growth Index ranging from 33% to 58%); otherwise, B. animalis subsp. animalis DSMZ 20104 did not grow both at 25 and 30˚C.
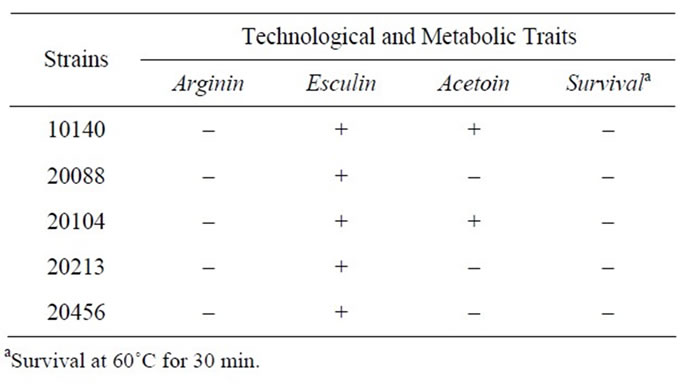
Table 1. Metabolic traits and survival after a heat shock (60˚C for 30 min).
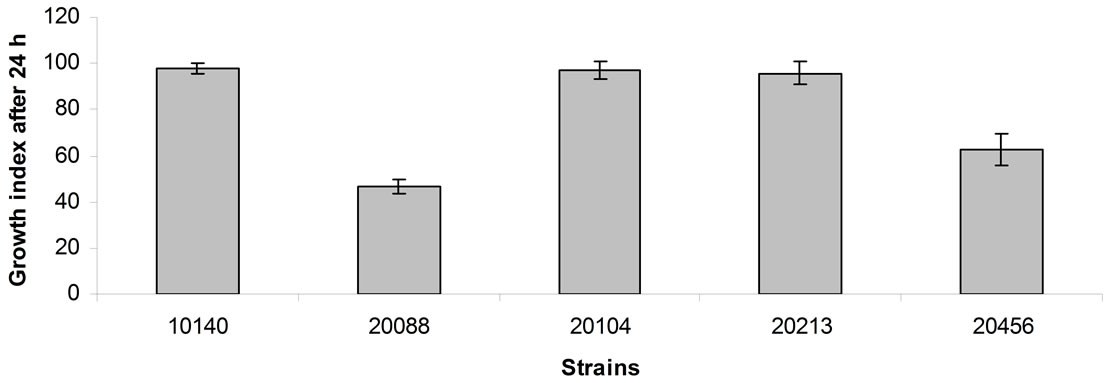
Figure 1. Growth index of bifidobacteria after 24 h of incubation at 37˚C in cMRS adjusted at pH 5. Mean value ± standard deviation.
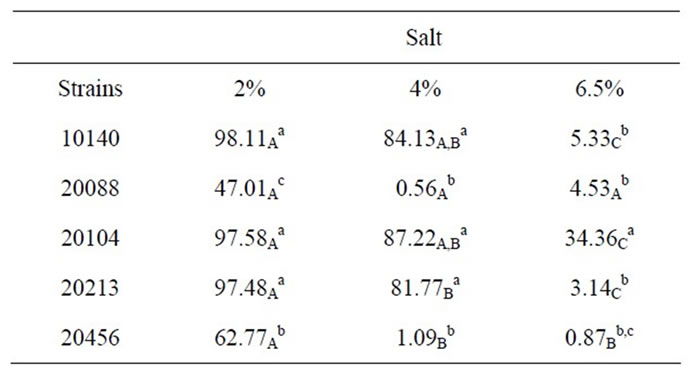
Table 2. Growth index after 24 h of bifidobacteria in MRS broth containing different amounts of salt. Small letters for each column indicate significant differences; otherwise capital letters indicate differences in a row (one-way ANOVA and Tukey’s test, P < 0.05).
After 48 h (Figure 2(b)), the growth at 25 and 30˚C was generally similar to that recovered for the control (i.e. the sample at 37˚C) (Growth Index ca. 100%), while bifidobacteria began to growth at 44˚C, although Growth Index revealed a kind of inhibition. A strong difference between Growth index after 24 h and that after 48 h was recovered for B. animalis subsp. animalis DSMZ 20104, due probably to a significant effect of the temperature on the lag phase of the population, thus suggesting that this strain could acquire temperature resistance only after a prolonged exposure.
The main ability of lactic acid bacteria and related bacteria (like bifidobacteria) is the decrease of the pH of the medium, due to the production of the end-products of the primary metabolism.
For the strains under investigation, the acidifying ability was evaluated at different temperatures (30˚C, 37˚C and 44˚C) to highlight the best temperature for the primary metabolism; the results are shown in Figure 3. No significant differences were recovered amongst the different strains (P > 0.05); otherwise, the assay showed a possible effect of the temperature. In fact, the decrease of pH was ca. 2.3 both at 30˚C and 37˚C; otherwise ΔpH values were significantly lower at 44˚C (ca. 2), thus confirming a slight inhibitory effect at this temperature, as evidenced by growth assay at 44˚C.
Fermented dairy products are still the major food vehicles in which probiotic bifidobacteria are delivered. A number of parameters, including interactions with other starter and probiotic strains, salt, sugars, temperature, flavouring and colouring compounds can influence the survival of bifidobacteria in these products [1]. However, pH and oxygen exert the greatest influence on Bifidobacterium throughout storage [1]; oxygen effect was not assessed in this research, as oxygen sensitivity can be addressed by strain selection and with appropriate pack-
 (a)
(a) (b)
(b)
Figure 2. Growth index value in cMRS as a function of the temperature. (a) Growth index after 24 h; (b) Growth index after 48 h.
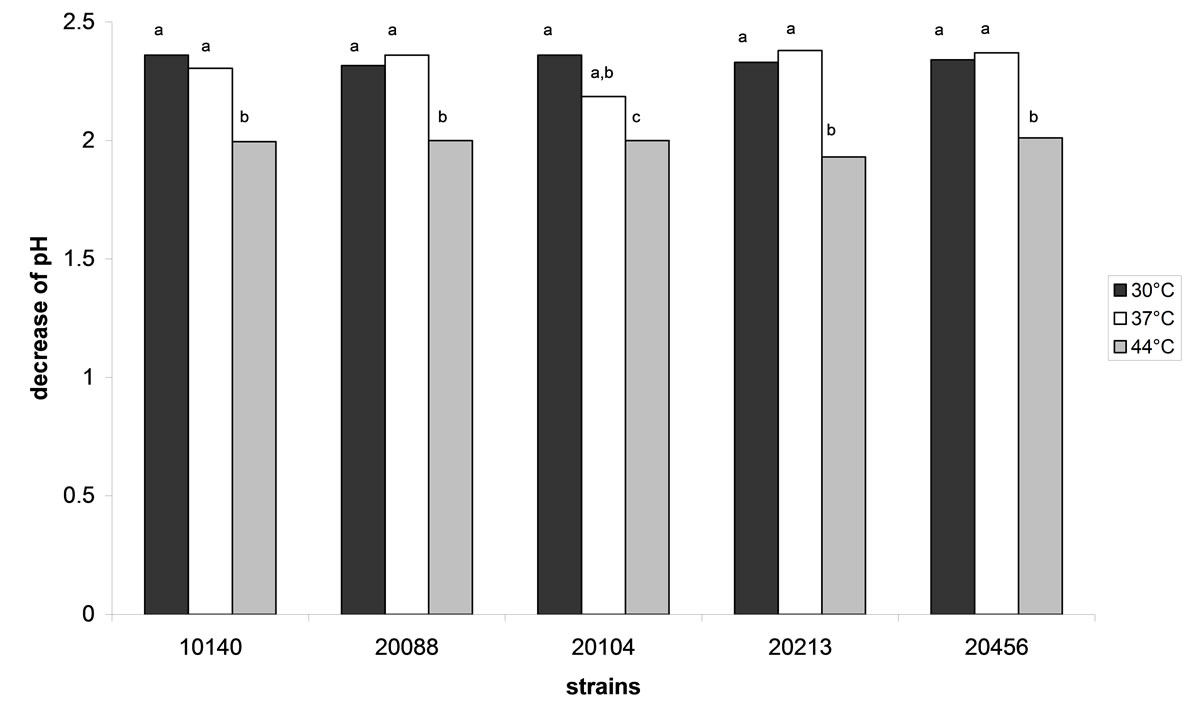
Figure 3. Decrease of pH performed by bifidobacteria after 48 h in cMRS at different storage temperatures. Different letters for each strain indicate significant differences (one-way ANOVA and Tukey’s test, P < 0.05).
aging techniques and materials.
The physiological mechanisms behind acid tolerance of B. animalis/lactis was addressed by several authors; for example B. lactis can survive well in fermented dairy product throughout their shelf life if the pH is prevented from dropping below 4.1 [9]. The results of this paper shows that some strains were able to grow at pH 5.0, thus suggesting a possible consociation with other starters, as proposed by Altieri et al. [10].
3.3. Probiotic Properties
Table 3 shows cell numbers of bifidobacteria at pH 2.5 and with 0.3% of bile salts added. Bile salts did not affect the target strains, as highlighted by the statistical analysis; otherwise, the acidic pH determined a strong reduction of cell count. The use of a standardized index (viability loss)

Table 3. Cell numbers of bifidobacteria (log cfu/ml) in saline solution adjusted at pH 2.5 or added with bile salts (0.3%) after 3 h at 37˚C. For each strains letters indicate significant differences (one-way ANOVA and Tukey’s test, P < 0.05).
(Figure 4) highlighted that viability loss was ca. 100% for B. bifidum DSMZ 20456 and B. breve DSMZ 20213. A lower effect was recorded for the other strains, corresponding to a viability loss of 30% for B. animalis subsp. lactis DSMZ 10140 and 50% for B. animalis subsp. animalis DSMZ 20104 and B. longum subsp. infantis DSMZ 20088.
Concerning the effect of bile salts, the results of this paper confirmed some literature reports on the bioactivity of bile salts towards bifidobacteria. Vinderola and Reinheimer [11] reported for some strains of B. longum and B. bifidum a survival of ca. 75% in presence of bile salts. This characteristic could be a common property of some probiotic strains, both wild and collection isolates; for example Bevilacqua et al. [7] characterized some probiotic strains of Lactobacillus plantarum from olives and found that after 3 h bile salt did not exert any effect on lactobacilli.
The results of the survival under acidic conditions confirmed bifidobacteria sensitivity towards low pH, as reported by literature [11,12].
Finally, bifidobacteria showed an hydrophobic index of 0% - 5%, thus confirming the idea of Gramovà et al. [12], i.e. although bifidobacteria can survive the passage through the gastrointestinal tract, they do not colonize the gastrointestinal tract permanently.
3.4. Antibiotic Resistance
Figures 5(a) and (b) shows antibiotic resistance of bifiobacteria; results of Kirby-Bauer test were modelled as
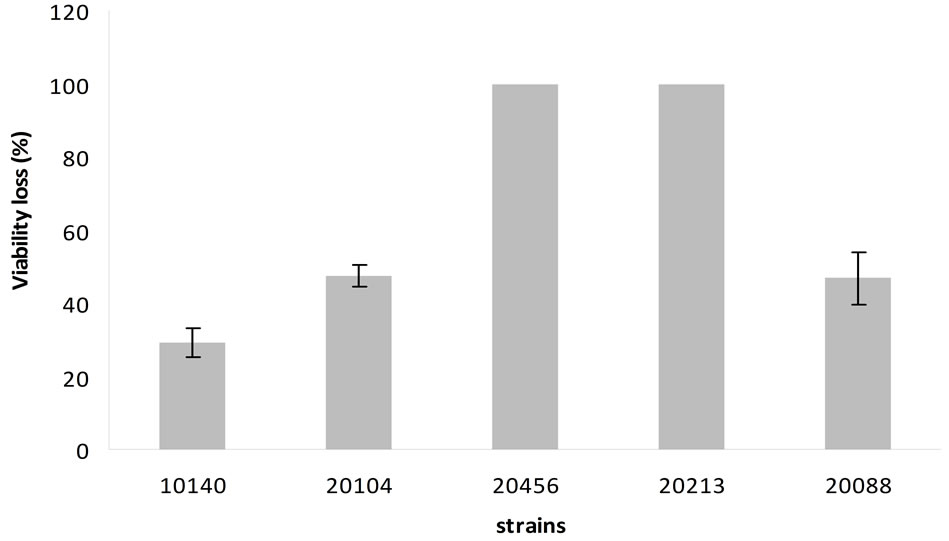
Figure 4. Percentage of viability loss at pH 2.5. Mean value ± standard deviation.
 (a)
(a)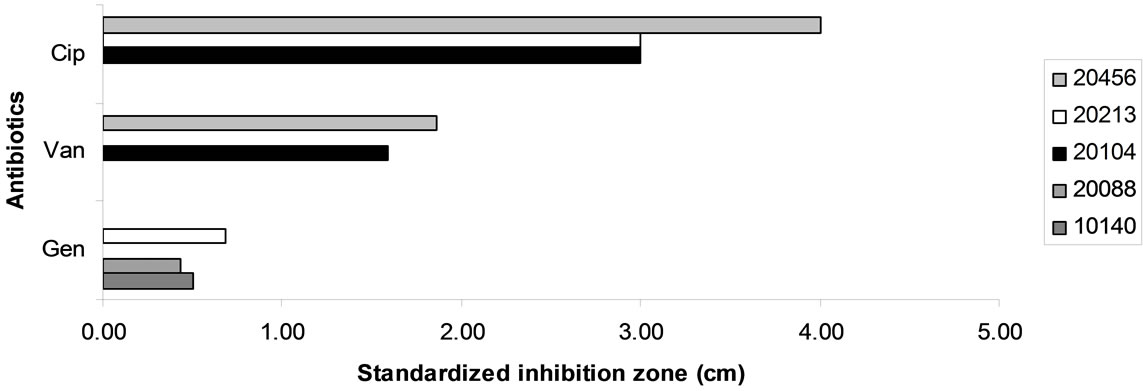 (b)
(b)
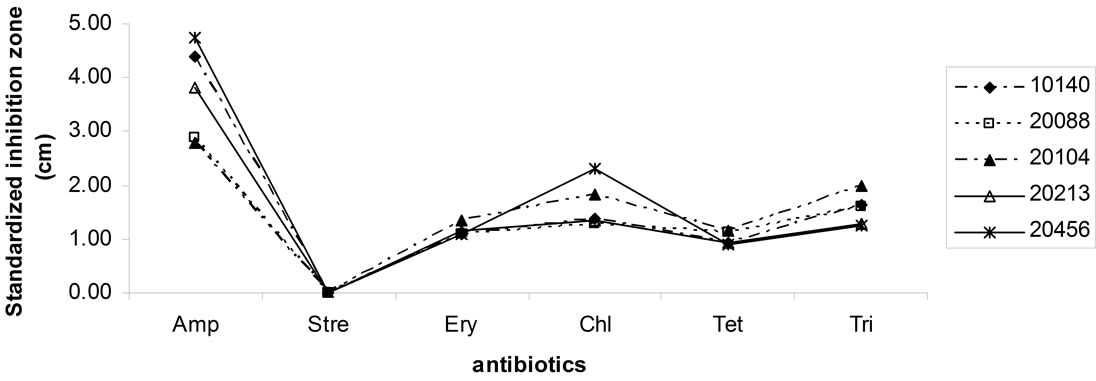
Figure 5. Antibiotic resistance of bifiobacteria. Data are expressed as inhibition zone (ray) (cm) referred to a standardized amount of 100 μg.
standardized inhibition zone index, i.e. as inhibition zone (ray of the halo around the disc of the antibiotic) referred to a standard concentration of 100 μg (the highest level of antibiotics used, corresponding to the amount of streptomycin). Bifidobacteria showed resistance towards streptomycin, but not against ampicillin, erythromycin, chloramphenicol, tetracyclines and trimethoprim; the most effective antibiotic was ampicillin.
Figure 5(b) reports standardized inhibition zone for the antibiotics showing a different effect towards the different strains of bifidobacteria. B. animalis subsp. lactis DSMZ 10140 and B. longum subsp. infantis 20088 were resistant to ciprofloxacin and vancomycin; otherwise, gentamycin was not effective against B. bifidum DSMZ 20456 and B. animalis subsp. animalis 20104, but it inhibited B. animalis subsp. lactis DSMZ 10140, B. longum subsp. infantis DSMZ 20088 and B. breve 20213.
The role of antibiotic resistance in probiotic bacteria is a matter discussed and controversial; in the last years it was reported that it could be a negative trait, as it is coded by genes of plasmids [13]. Therefore, it could be transferred to dangerous microorganisms in the large intestine. The role of this assay for probiotic selection is not clear and generally it is not used for the final selection of suitable strains. It is assessed in order to characterize the strains, but not to choose the starter.
3.5. Strain Selection
As a final step of this research, strain selection was performed through a multivariate approach. i.e. using the Principal Component Analysis (Figure 6). This approach was suitable for data analysis, as it was able to explain the 82% of variability; concerning the correlation of the input variables to the components, growth at 25˚C, at 30˚C and with 6.5% of NaCl added were related to the component 1 (the correlation coefficients were respecttively 0.950, 0.777 and 0.903). Growth at pH 5 was related to component 2 (R2, 0.816); the correlation of viability loss at pH 2.5 (V.L.) with component 2 was partial (R2, 0.527).
The distribution of strains in the factorial space of PCA highlighted that there was not an optimal strain, showing the best performances in all the conditions; however, B. longum subsp. infantis DSMZ 20088 and B. animalis subsp. lactis DSMZ 10140 represent a good compromise, as B. animalis subp. lactis showed a high Growth Index at pH 5 and good values at 25˚C and 30˚C, as well as the minimal viability loss at pH 2.5. B. longum
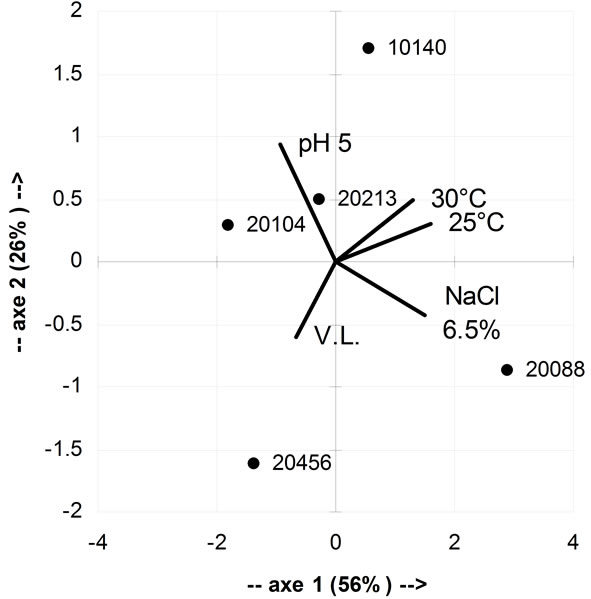
Figure 6. Principal component analysis, using as input value growth at 25˚C, 30˚C, with 6.5% of NaCl added, at pH 5 and viability loss (V.L.) at pH 2.5.
subsp. infantis DSMZ 20088 was the best microorganism for its growth index in presence of 6.5% of salt added and optimal growth at 25˚C and 30˚C. Finally, the worst strain, concerning both technological and probiotic traits, was B. bifidum DSMZ 20456.
4. Conclusion
This paper proposes a case study on the selection of potential multifunctional starter cultures amongst some colction strains of bifidobacteria. Both technological and probiotic properties were assessed and after laboratory assays, B. longum subsp. infantis and B. animalis subsp. lactis were found to be a good compromise as potential functional starter cultures. B. nimalis subp. lactis showed a high growth index at pH 5 and good values at 25˚C and 30˚C, as well as the minimal viability loss at pH 2.5, whereas B. longum subsp. infantis DSMZ 20088 was the best microorganism for its growth index in presence of 6.5% of salt added and at 25˚C and 30˚C.
5. Note by Supervisor
Biotechnology of Functional Starter a class for MSc and Bachelor students of Food Technology degree (University of Foggia). Due to the fact that students acquired during bachelor degree the basic knowledge of lactic acid bacteria I decided to organize this class without lessons but in lab, taking into account that “learning by doing” is a basic rule for future food technologists.
Generally, each meeting was organized as follows:
1) a brief introduction on the impact and importance of the particular technique or protocol under investigation;
2) the experiment (students were responsible for all the experiment, from sample and media preparation to results interpretation and data modeling);
3) a final brain-storming on the problems of the analyzed technique, as well as a discussion on how model and write in a clear way the results of the experiments.
The last meeting of the class was used to plan the present research paper; then, students were divided into three groups, each of them involved in writing a part (Introduction, Materials and Methods, Results and Discussion). The present paper is the result, after a critical review and correction, of their “learning by doing”.
Bevilacqua Antonio: Student supervisor, lecturer for the class “Biotechnology of Functional Starter”.
Maria Rosaria Corbo: Associate Professor of Microbiology.
Milena Sinigaglia: Full Professor of Microbiology. Head of the Department of Food Science.
Cagnazzo, Caldarola, Ciuffreda, Dragano, Lauriola, Franchino, Pacifico. Students for MSc degree in Food Science and Technology. University of Foggia.
REFERENCES
- R. Crittenden, “An Update of Probiotic Bifidobacteria,” In: S. Salminen, A. von Wright and A. Ouwehand, Eds., Lactic Acid Bacteria: Microbiological and Functional Aspects, 3rd Edition, CRC Press, Roca Raton, 2004. doi:10.1201/9780824752033.ch3
- J. O’Brien, R. Crittenen, A. C. Ouwerhand and S. Salminen, “Safety Evaluation of Probiotics,” Trends in Food Science and Technology, Vol. 10, No. 12, 1999, pp. 418- 424. doi:10.1016/S0924-2244(00)00037-6
- I. Jankovic, W. Sybesma, P. Phothirath, E. Ananta and A. Mercenier, “Application of Probiotics in Food ProductsChallenges and New Approaches,” Current Opinion in Biotechnology, Vol. 21, No. 2, 2010, pp. 175-181. doi:10.1016/j.copbio.2010.03.009
- A. Bevilacqua, G. Caggianello, A. Marchesiello, D. Paglialonga, G. Petrella, R. Ruotolo, L. Trivisano, G. Varva, M. R. Corbo and M. Sinigaglia, “Preliminary Assays for the Selection of a Multifunctional Starter for a Dairy Product: A Case Study by Food Science and Technology MSc Students, University of Foggia (Southern Italy),” Advances in Food Sciences, Vol. 32, No. 4, 2010, pp. 238-246.
- M. Blaszyk and R. A. Holley, “Interaction of Monolaurin, Eugenol and Sodium Citrate on Growth of Common Meat Spoilage and Pathogenic Organisms,” International Journal of Food Microbiology, Vol. 39, No. 3, 1998, pp. 175- 183.
- A. Bevilacqua, M. Perricone, M. Cannarsi, M. R. Corbo and M. Sinigaglia, “Technological and Spoiling Characteristics of the Yeast Microflora Isolated from Bella di Cerignola Table Olives,” International Journal of Food Science and Technology, Vol. 44, No. 11, 2009, pp. 2198- 2207. doi:10.1111/j.1365-2621.2009.02060.x
- A. Bevilacqua, C. Altieri, M. R. Corbo, M. Sinigaglia and L. I. I. Ouoba, “Characterization of Lactic Acid Bacteria Isolated from Italian Bella di Cerignola Table Olives: Selection of Potential Multifunctional Starter Cultures,” Journal of Food Science, Vol. 75, No. 8, 2010, pp. M536- M544. doi:10.1111/j.1750-3841.2010.01793.x
- National Committee for Clinical Laboratory Standards, “Performance Standards for Antimicrobial Disc Susceptibility Test: Tentative Standards,” NCCLS, Vallinova, 1993.
- R. Crittenden, A. Laitila, P. Forssell, J. Mättö, M. Saarela, T. Mattila-Sandholm and P. Myllärinen, “Adhesion of Bifidobacteria to Granular Starch and its Implications in Probiotic Technologies,” Applied and Environmental Microbiology, Vol. 67, No. 8, 2001, pp. 3469-3475. doi:10.1128/AEM.67.8.3469-3475.2001
- C. Altieri, A. Bevilacqua, D. D’Amato, M. A. Del Nobile and M. Sinigaglia, “Modelling the Survival of Starter Lactic Acid Bacteria and Bifidobacterium bifidum in Single and Simultaneous Cultures,” Food Microbiology, Vol. 25, No. 5, 2008, pp. 729-734.
- C. G. Vinderola and J. A. Reinheimer, “Lactic Acid Bacteria and Probiotic Bacteria: A Comparative “in Vitro” Study of Probiotic Characteristics and Biological Barrier Resistance,” Food Research International, Vol. 36, No. 9-10, 2003, pp. 895-904. doi:10.1016/S0963-9969(03)00098-X
- M. Gramovà, E. Vlkovà, V. Radà and I. Houtovà, “Survival of Bifidobacteria in Adult Intestinal Tract,” Folia Microbiologica, Vol. 55, No. 3, 2010, pp. 281-285. doi:10.1007/s12223-010-0042-5
- D. Czeruka, T. Piche and P. Rampal, “Review Article: Yeast as Probiotics—Saccharomyces boulardii,” Alimentary Pharmacology and Therapeutics, Vol. 26, No. 6, 2007, pp. 767-778. doi:10.1111/j.1365-2036.2007.03442.x

