American Journal of Plant Sciences
Vol.3 No.6(2012), Article ID:20027,7 pages DOI:10.4236/ajps.2012.36093
Effectiveness of Bacterial and Fungal Isolates to Control Phoma lingam on Oilseed Rape Brassica napus
![]()
1Agriculture Scientific Research Center, Lattakia, Syria; 2Palestine Technical University-Kadoorie (PTUK), Tulkarm, Palestine; 3National Agriculture Research Center (NARC), Jenin, Palestine; 4Department of Biotechnology & Biological Control, Institute for Phytopathology, Christian-Albrechts-University Kiel, Kiel, Germany.
Email: salman_mazen@daad-alumni.de
Received February 28th, 2012; revised March 30th, 2012; accepted April 6th, 2012
Keywords: Biological Control; Oilseed Rape; Phoma lingam; Bacteria
ABSTRACT
Blackleg disease caused by Phoma lingam is an important disease of oil seed rape (Brassica napus) causing losses up to 95%. The efficacy of microbial antagonists against P. lingam in greenhouse was tested. Serratia plymuthica HRO-C48 and Gliocladium catenulatum J1446 were able to reduce the disease intensity of OSR cotelydones infested with P. lingam at rates 44% and 52% respectively. The reduction of the infestation of the root collar in BBCH14/15 was evaluated as well. Plants treated with a suspension of the antagonists (2 × 105 cfu/plant) and inoculated with either pycnidiospore suspension (2 × 107 cfu/ml) or agar disks grown with P. lingam mycelium, showed a reduced infestation rate of 53% - 93% in the presence of S. plymuthica and 46% - 77% in the presence of G. catenulatum. The efficacy of the antagonist depends highly on their concentration inside OSR seeds. Below 105 cfu/seed no significant difference was recorded between control untreated and infested plants.
1. Introduction
Oilseed rape (OSR) Brassica napus is a major crop grown for its oil in many parts of the world including Canada, India, China and Europe [1]. In favourable conditions, B. napus is considered to have the highest yield potential of all Brassica oil crops [2]. Winter and spring varieties of the crop exist. Winter varieties are mainly grown in Europe and China and spring varieties in areas such as Australia and Canada. Winter OSR is an excellent starter crop in cereal rotations, due to its positive impact on disease control and soil improvement. Its cultivation favours the minimum tillage which is of great interest for sustainable agriculture [3].
In the last 20 years, an increase of 260% in OSR production has been recorded worldwide, driven by the enormous economic significance of oilseed crops as a renewable raw material used for a variety of applications in food and non-food areas [4]. In Germany, OSR is the most important oilseed crop, with acreage of more than 1.3 million hectares in 2005. Yields ranged between 2.9 and 3.5 tons per hectare. In 2005, 5.1 million tons of rapeseed was harvested approximating a total seed-oil yield of 2 million tons [3].
With the increased production of oilseed rape (OSR) B. napus spp. napus, reports on pathogen causing diseases have increased simultaneously. Oilseed rape however, is susceptible to many fungal pathogens such as Pyrenopeuiza brassicae Raw. (anamorph C. concetricum Grev.), Leptosphaeria maculans (Desm.) Ges. Et De Not. (anamorph Phoma lingam (Tod.) Desm.), Sclerotiorum (Lib.) de Bary and Verticillium dahliae Kelb. Stem canker (also termed blackleg) caused by P. lingam is of major economic importance in the main OSR growing areas of Australia, Canada and Europe [5]. In some instances (e.g., when chemical fungicidal applications are not effective) heavy losses, as much as 95% might be caused by these fungi [1]. In Europe, the severity of blackleg disease was reported to be significant since 1966 [6]. The disease epidemic differs greatly between seasons, regions and crops. In some instances basal phoma stem canker can potentially cause total crop loss [5,7].
Fungicides are the most effective means for controlling stem canker, but their use must be optimised to achieve maximum economic response and to avoid unnecessary fungicide applications [8]. Beside the side effects to the environment and development of fungicide resistance, use of foliar fungicides in association with cultivars with little or no resistance has proved to be ineffective for controlling phoma stem canker, because the current fungicides have low eradicant activity and are effective as protectants for only a short period [5]. Biocontrol methods might be promising alternatives to reduce the use of fungicides. Various microbial antagonists, including strains of bacteria of the genera Baccillus, Pseudomonas and Serratia and strains of fungi such as Gliocladium and Trichoderma, have shown biocontrol activity against damping-off diseases and Botrytis on Photinia, in conventional Brassica seedling [9].
The aim of this work is to test the efficacy of different biocontrol antagonistic agents (BCA) against the Black leg disease in OSR.
2. Materials and Methods
2.1. Cultivation and Maintenance of Antagonistic Biocontrol Agents
Bacterial isolates Serratia plymuthica HRO-C48, Bacillus subtilis B2g, Pseudomonas fluorescens E9, 1Re2-6, Paenibacillus polymyxa 1P1-2 and Pseudomonas chlororaphis MA342 were grown each in 250 ml Erlenmeyer flasks containing 50 ml of 20 g/l tryptic soy broth (TSB) (Difco Laboratories, USA) supplemented with 100 μg/ml refampicin. The flasks were placed on a rotary shaker (180 rpm) for 48 h at 25˚C. Aliquots of 700 μl of each bacterial isolate were transferred into Eppendorf tubes containing 300 μl of 50% sterile glycerol. The cultures were stored after mixing at –80˚C.
The fungus Gliocaldiom catenulatum isolate J1446 (commercially produced under the name Prestop® (Verdera, Finland) was grown on Malt extract agar (MEA) media containing 100 mg/l streptomycin and 50 mg/l red Bengal. Cultures were incubated at 22˚C for one week. For spore production, MEA agar disks containing fungal mycelium were grown in 250 ml Erlenmeyer flasks containing 50 ml malt extract liquid media on a rotary shaker (140 rpm) for two weeks at 20˚C. Spore concentration of the fungus was then adjusted to 6 × 108 cfu/ml in 0.85% normal saline.
2.2. In Vitro Antagonistic Tests
Antagonistic activity of the biocontrol agents against P. lingam was determined using dual culture technique. 100 μl of P. lingam spore suspension (2 × 107 Spore/ml) were spread on plates containing V8 agar media. Each antagonist was then streaked on the plate (3 streaks/plate) and incubated either at 10 or 22˚C for 7 days. The control experiments were done by using sterile distilled water instead of antagonists. The effect of each BCA was determined by measuring the inhibition zones of fungal growth. The rating scale was: 4, inhibition zone > 10 mm; 3, inhibition zone 5-10 mm; 2, inhibition zone < 5 mm; 1, growth was stopped at the bacterial-streak line; 0, no inhibition zone and often P. lingam was growing over the bacterial streak [9]. The experiment was repeated using agar disks grown with the fungus instead of fungal spores.
Two V8 agar disks (7 mm diameter) grown with 7 days old P. lingam were placed about 20 mm from each of BCA streak. The plates were further incubated as mentioned above and zones of inhibition were determined according to the above scale.
To test the effect of G. catenulatum, WA agar disks grown with the fungus were used instead of bacteria.
2.3. Inoculation of Cotyledons with P. lingam
Cotyledons were punctured with a needle and 10 μl of pycnidiospore suspension (2 × 107 Spore/ml) was placed on the wound as described by Mithen et al. (1987) [10]. Control plants were inoculated with 10 μl sterilized distilled water. Plants were further incubated in growth chamber cabinets for 14 days. To prevent plant proliferation, growing leaves were removed every two days during the experimental time. Disease incidence was then measured according to Kutcher et al. (1993) [11] in a rating scale from 0 - 6 as follows: 0: no symptoms; 1: lesions on the infection site < 1.5 mm; 2: lesions on the infection site 1.5 - 3.5 mm; 3: lesions on the infection site > 3.0 mm; 4: gray to green tissue collapse 3.1 - 5.0 mm; 5: gray to green tissue collapse > 5.0 mm (≤10 pycnidia); 6: gray to green tissue collapse > 5.0 mm (>10 pycnidia) [12].
2.4. Inoculation of OSR Plantlets with P. lingam
The efficacy of the biocontrol agents on disease development at an earlier stage of plant growth was also tested. For this, OSR plantlets at growth stage of BBCH 14/15 [13] were inoculated at the stem base either with V8 agar disks (7 mm diameter) grown with P. lingam or with 40 μl pycnidiospors (2 × 107 spore/ml). Plants were incubated for 50 days in growth chamber under the above mentioned conditions. Infestation with P. lingam was then determined according to Kutcher et al. (1993) [14]. The volume of disease tissue (VDT) was assessed in a rating scale of 0 - 4 as illustrated in Table 1.
2.5. Colonization Studies
To study the population size of the antagonists in OSR rhizosphere, seeds were treated with the antagonists by soaking 1 g of seeds in 1-ml bacterial suspension for 5 h at 20˚C. The seeds were grown in growth chambers at 22˚C ± 1˚C and 75% ± 5% relative humidity. After different periods of time (1, 10 and 30 days), 5 g of roots with adhering soils from five OSR seedlings of each treatment were placed in an Erlenmyer flask containing 40 ml sterile water and shaked for 1 h at 24˚C. Serial dilutions (10–2, 10–3 and 10–4) were prepared and aliquots (100 μl) were spread on TSA media supplemented with 150 mg/ml refimpicin. Plates were further incubated at 25˚C for 48 h and the number of bacteria was estimated as log cfu/g root fresh weight.

Table 1. Rating scale of P. lingam on OSR leaves.
2.6. Field Experiments
Experiments were conducted in naturally infested fields with P. lingam. The field which was located in Achterwehr, (54˚19'N, 9˚58'E) is owned by the institute of agronomy and crop science, Christian Albrecht University of Kiel. OSR was cultivated in a Randomized Block Design in which four blocks were used as replicates. The area of each block was 14 × 3 m and divided into smaller areas (4.5 × 3 m each).
Before sowing, OSR seeds were treated with the antagonists as follows: S. plymuthica, HRO-C48Rif, S. plymuthica, HRO-C48Rif combined with the fungicide Metconazol (applied two weeks after starting the experiment), G. catenulatum and G. catenulatum combined with the fungicide Metconazol (applied two weeks after starting the experiment). Untreated seeds were used as control. Seed coating with insecticides and fungicides was achieved as follows: seeds were first treated with S. Plymuthica or with G. Catenulatum as mentioned above. After that the insecticide Chinook was added (20.0 ml/kg seeds), followed by adding the fungicide DMM (Dimethomorph) (10 g/kg seeds). While rotating at 400 rpm, talcum blue was added until all liquid is bound to the seeds. The seeds were then dried under laminar flow for 1 h. The disease severity was assessed by counting the number of pycindiospores on OSR leaves.
Population size of S. plymuthica, HRO-C48 and G. catenulatum in OSR rhizosphere was studied under field conditions. 5 g of roots with adhering soils from five OSR seedlings of each treatment were placed in Erlenmyer flasks containing 40 ml sterile water and shaked for 1 h at 24˚C. Serial dilutions (10–2, 10–3 and 10–4) were prepared and aliquots (100 μl) were spread on TSA media supplemented with 150 μg/ml refimpicin. Plates were further incubated at 25˚C for 48 h and the number of bacteria was estimated as log cfu/g root fresh weight.
2.7. Statistical Analysis
Statistical analyses were done using XlStat program (Adinosoft). Analysis of variance (ANOVA) and significant differences for means were calculated after Tukeys HSD test at (P ≤ 0.05).
3. Results
3.1. In Vitro Inhibition of Mycelial Growth of P. lingam
All of the antagonistic isolates reduced the mycelial growth as well as the growth of pycnidospores of P. lingam on V8 media. The size of the inhibition zone varied according to antagonist and type of media used (Table 2). G. catenulatum was the most effective in inhibiting the mycelial growth of P. lingam on both types of media at 22˚C and caused a very strong inhibition zones >10 mm. However, its effect on pycnidospores was lower (<5 mm). Except for B. subtilis, all bacteria isolates were able to inhibit P. lingam since the bacterium did not grow at 10˚C. As shown in Table 2 only the isolate P. fluorescens (E9) caused week inhibition zone (<5 mm) on both media and temperatures. The effect of P. chororaphis at 22˚C on V8 media was more pronounced in inhibiting the growth of pycnidospores than that at 10˚C (inhibition zone > 10).
3.2. Effect of Antagonists on Cotyledon Infestation with P. lingam
The antagonistic isolates S. plymuthica, P. chlororaphis were the most effective in reducing the infestation of cotyledons of OSR cultivar Talent grown under growth chamber conditions, and were able to reduce the disease infection by 43.3% (Figure 1). In addition to that, the number of healthy plants of cultivar Talnet under growth chamber at 22˚C was significantly higher in the seeds treated with the isolate P. chlororaphis (Figure 1). Treatment with G. catenulatum resulted in a significant reduction of disease intensity (51.6%) compared to untreated control seeds.
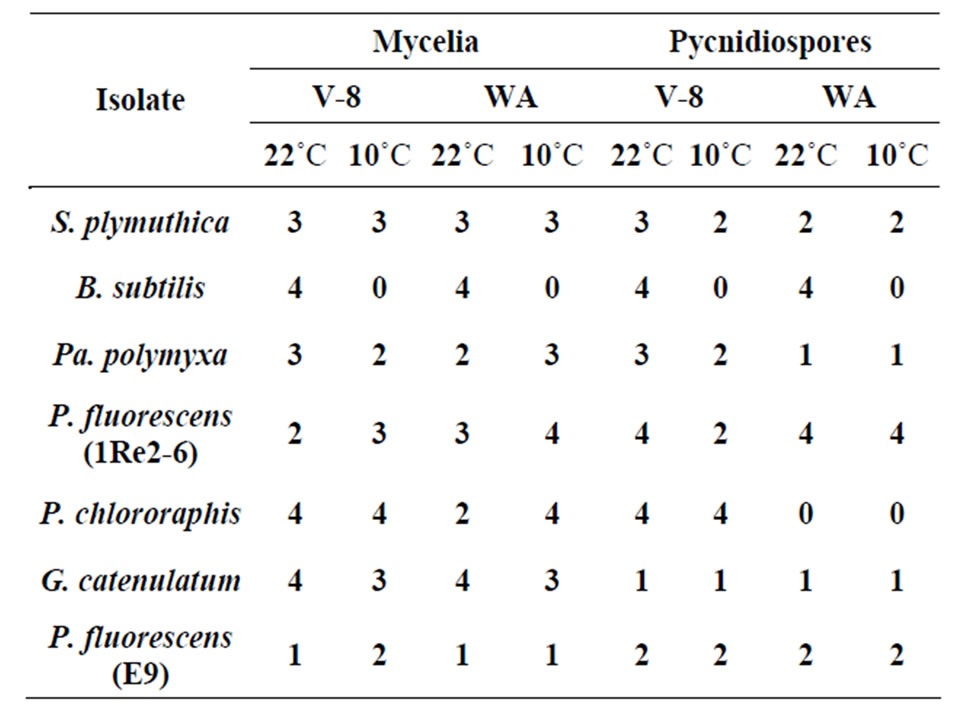
Table 2. In vitro inhibition of P. lingam mycelium and pycndiospores grown on V8 and WA media at 10 and 22˚C.
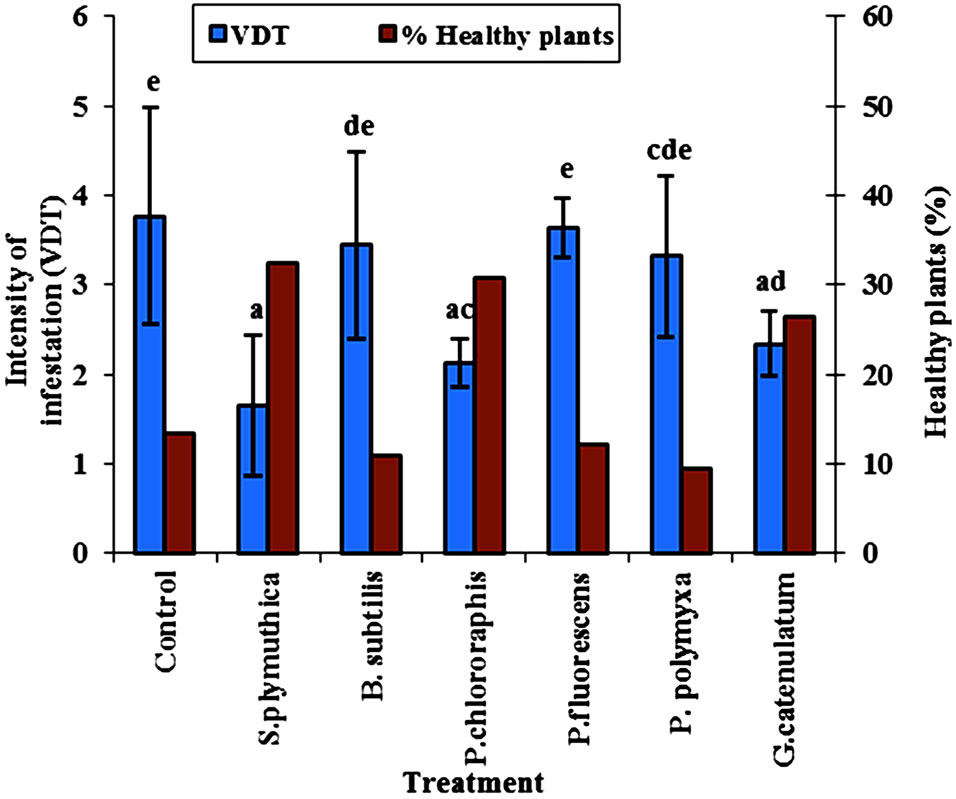
Figure 1. Effect of seed biopriming with different antagonists on P. lingam intensity on cotyledon of OSR cultivars Talent grown in growth chamber at 22˚C. Disease intensity (scale 0-6) and the percent of healthy plants were recorded after inoculating the cotyledons 2 × 105 cfu/plant of a spore suspension of 2 × 107 cfu/ml of P. lingam. Results of different letters are significantly different after Tukey HSD test at P < 0.05, n = 45 plant per treatment).
The results showed a pronounced increase in the percent of healthy plants of OSR in the presence of P. chlororaphis. In seeds treated with S. plymuthica or G. catenulatum the percent of healthy plants was higher than that in seeds treated with the other antagonists (Figure 1).
3.3. Efficacy of Antagonists on P. lingam Infection on Stem Base
Data in Figure 2 shows the effect of the BCAs on reduction of root collar infection. The volume of diseased tissue (VDT) in plants infested with spore suspension of the pathogen was significantly lower (P < 0.05) in OSR seeds treated with S. plymuthica, P. chlororaphis and G. catenulatum (1.14 ± 0.8, 1.08 ± 0.4 and 1.09 ± 0.7, respectively). In the presence of P. fluorescens the VDT (1.81 ± 0.7) did not differ significantly from the control untreated plants (VDT = 2.04 ± 1.1). In OSR plants infested with V8 agar pieces grown with P. lingam, the antagonists reduced significantly the VDT. The effect P. fluorescens was lower (but not significantly different) than the effects of the other antagonists (Figure 2).
3.4. Colonization of the Rhizosphere with S. plymuthica and G. catenulatum
Our results showed that the isolates S. plymuthica, P. chlororaphis and P. fluorescens are rhizosphere competent antagonist since they were able to colonize the rhizosphere of ORS in a short period of time. Root colonization was also recorded after seed treatment with B.
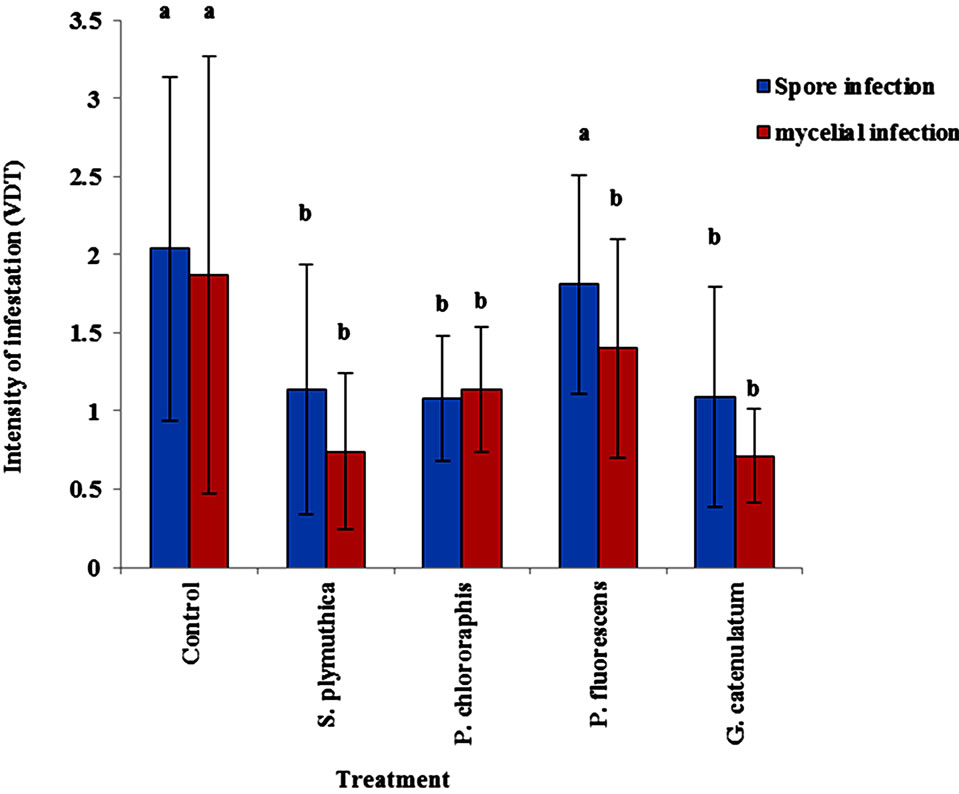
Figure 2. Effect of different BCAs on infection of stem base. OSR seeds cultivar Talent were treated with the antagonists at 106 cfu/seed and grown for 50 days in growth chamber at 12 h day light, 20˚C - 24˚C and 70% - 75% humidity. When plantlets reached the phonological stage of BBCH 14/15, P. lingam was applied as mycelium grown on V8 medium. Data of same letters are not significantly different after Tukey HSD test at P < 0.05.
subtilis und P. polymyxa (2 - 1.2 × 106 cfu/ml). However, the colonization was lower than that of S. plymuthica, P. chlororaphis and P. fluorescens. After 8 day of plant growth under greenhouse conditions, the average number of bacteria was 1.2 × 105 cfu/g root fresh weight. At the end of the experiment (30 days), colonization rate was 5.2 × 104 cfu/g root fresh weight (Figure 3).
3.5. Efficacay of Antagonists against P. lingam under Field Conditions
In plants treated with the antagonists, the disease intensity was lower than that of the control untreated plants. As shown in (Figure 4) the average number of pycnidiopore after two weeks of planting was 21.3 pycnidia/ plant in the control untreated plants and 2.5 and 1.5 pycnidia/plant in plants treated with S. plymuthica and G. catenulatum respectively. The combined treatments of S. plymuthica and G. catenulatum with the fungicides resulted in a pronounced reduction of disease infestation (0.0 and 0.9 pycnidiospore/plant respectevily).
The highest number of pycnidiospores was recorded after two weeks of fungicide application. After that the disease intensity decreased and no significant differences were recorded between the numbers of pycnidiospores in control plants and in plants treated with the antagonist or the antagonists combined with the fungicide.
Six months after sowing, the disease intensity was reduced and one month later no disease on leaves was recorded. The disease intensity was significantly lower in treated seeds compared to the control untreated seeds.
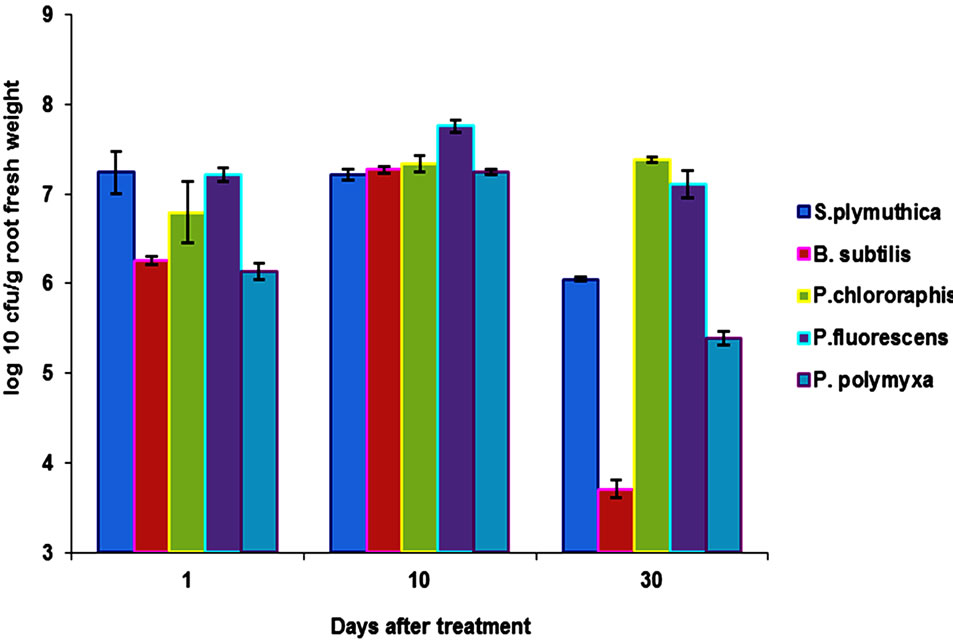
Figure 3. Comparison of root colonization of OSR roots by different antagonists. Data are the means (±SD) of 5 plants taken after 1, 10 and 30 days of incubation under growth chamber cabins at 22˚C and 12 h day/night regimes. Seeds were bioprimed with the antagonists by soaking the seeds in bacterial suspensions (2 × 1010 cfu/ml) for 5 h and dried at room temperature for two days.
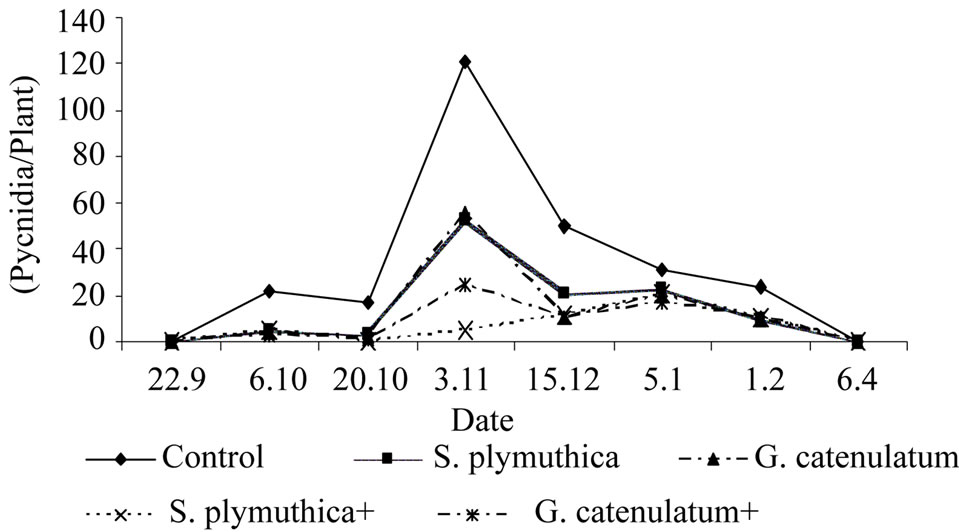
Figure 4. Effect of seed treatment with S. plymuthica and G. catenulatum on disease intensity of P. lingam on leaves of OSR grown under field conditions. The experiment was conducted in 2005/2006. OSR seeds were treated either with S. plymuthica (7.9 × 106 cfu/seed) or with G. catenulatum (8.5 × 105 cfu/seed). After two weeks of sowing the fungicide Caramba (60% Metconazol, 0.7 l/ha) was applied.
Interestingly, no significant difference in disease intensity was seen when the fungicide was combined with the biocontrol agents.
The disease incidence was also calculated at two time points (03.11 and 15.12). In control plants infested with P. lingam the disease incidence was 74% and 68% and in plants treated with the S. plymuthica combined with the fungicide the disease incidence was 12.5% and 25%.
4. Discussion
In this study, several antagonistic microorganisms including bacteria and fungi were tested for their efficacy against the black leg disease caused by P. lingam on oilseed rape. The first step toward successful selection of effective biocontrol agents was done by testing the effect of the antagonists against the pathogen in dual culture assays.
As revealed from the dual culture assays, the biocontrol agents differ in their efficacy against P. lingam. The size of inhibition zone varied according to the incubation temperature. Although no general relationship exist between the ability of a microoganism to inhibit a pathogen in vitro and to suppress disease caused by that pathogen in ad planta [15,16], some authors [17,18] have shown that the antagonistic efficacy might be correlated with the in vivo effect of the antagonists. It was found that the results of dual culture assay depend highly on culture media [19,20]. In this work the size of the zone of inhibittion caused by the biocontrol agents was affected by the type of media used (V8 and WA media). Because the pathogen P. lingam infects mostly winter rape at low temperatures (10˚C) the effect of temperature on antagonism was also tested. Except for B. subtilis and P. polymyxa most isolated with high efficacy at 22˚C were also able to inhibit mycelial growth at 10˚C.
P. lingam is able to infect all parts of OSR at all stages of plant development [21]. For example an infection in autumn might cause later an infection of root collar and stem base [22]. The fungus is able to spread from the infected leaves to other parts of the plant causing the disease symptoms [23]. For this reason inhibition of the fungus on cotyledons was studied.
To test the effect of the pathogen on emerging leaves, the leaves of the OSR plants in the physiological growth stage BBCH1-12 were injured mechanically by a sterile needle. A spore suspension was then spread on the wounds and the disease symptoms were monitored after 14 days [11,24]. In ad planta tests, more than 50% reduction of the disease caused by P. lingam on leaves of OSR seedling grown in growth chamber was recorded in seeds treated with S. plymuthica, P. chlororaphis, P. fluorescens and G. catenulatum. The percent of healthy plants increased significantly from less than 10% to 40% - 50% in the presence of the antagonists. However, the efficacy of the gram positive isolates was lower than that of the gram negative bacterial strains.
The isolate S. plymuthica was the most effective in controlling the disease caused by P. lingam on root collar and stem base. The disease intensity was reduced by 54% and 63% in plants grown in greenhouse and growth chamber conditions. In seeds treated with G. catenulatum, P. fluorescens und P. chlororaphis disease reduction was 52%.
For effective protection against plant pathogens, the antagonist must be able to colonize successfully the rhizosphere of the plant [25]. The antagonists must compete with other microorganisms in the root system of the plants in order inhibit the attack of pathogens [26]. It was found that colonization patterns differ between different antagonistic microorganisms [27]. In our experiments, OSR roots were highly colonized by isolates S. plymuthica, P. chlororaphis and P. fluorescens after seed treatments with 1.2 - 4 × 107 cfu/ml bacterial solutions. Sasse (1997) [20] found that root colonization of rape seed plants in seeds treated with fluorescent pseudomonads (8 × 105 cfu/seed) was 1.8 × 106 and 5.1 × 105 cfu/g root fresh weight after 10 and 30 day respectively. Other authors found that the concentration of P. fluorescens was reduced from 106 bis 108 cfu/g root fresh weight after 7 day of plant emergence to 104 bis 105 cfu/g root fresh weight after 60 day of plant growth [28]. The density of rhizospheric bacteria differ strongly accorcing to the root zone of plants as well as the age of the plant [29].
Berg et al. (1996) [9] found that Bacillus spp. were able to colonize the roots of Photinia after 28 day of growth (104 - 105 cfu/g root fresh weight). In in-vitro experiments Brückner (1998) [28] found that the number of bacterial cells of B. subtilis and P. fluorescens in the rhizosphere of winter oilseed rape, reduced from 106 and 108 cfu/g root fresh weight after 7 days of planting to 104 and 105 cfu/g root fresh weight after 60 of planting.
The total number of different bacterial cells was determined in the rhizosphere of OSR plants grown in growth chamber or in greenhouse. An average of 74 × 108 cfu/g root fresh weight was recorded. These results are in agreement with Kleeberger et al. (1983) [30], who recorded viable counts of bacteria of 107 - 108 cfu/g root fresh weight in roots of barley and wheat. Our results are also in agreement with Berg et al. (1996) [9] who determined a bacterial number of 1.5 × 108 cfu/g root fresh root weight of OSR plants.
5. Acknowledgements
The authors would like to thank Dr. Mohammed Abu-Eid, Director General, National Agriculture Research Center (NARC); Ministry of Agriculture, Palestine for his technical assistance to publish this work.
REFERENCES
- R. Grison, B. Grezes-Besset, M. Schneider, N. Lucante, L. Olsen, J.-J. Leguay and A. Toppan, “Field Tolerance to fungal Pathogens of Brassica napus Constitutively Expressing a Chimeric Chitinase Gene,” Nature Biotechnology, Vol. 14, No. 5, 1996, pp. 643-646. doi:10.1038/nbt0596-643
- N. J. Mendham and P. A. Salisbury, “Physiology: Crop Development, Growth and Yield,” In: D. S. Kimber and D. I. Mcgregor, Eds., Bmssica Oilseeds, Centre for Agricultural Bioscience International, Wallingford, 1995, pp. 11-64.
- Eurostat, “Eurostat Website and National Data”. http://epp.eurostat.ec.europa.eu
- D. S. Kimber and D. I. McGregor, “The Species and Their Origin, Cultivation and World Production,” In: D. S. Kimber and K. I. McGregor, Eds., Brassica Oilseeds, Production and Utilization, Centre for Agricultural Bioscience International, Wallingford, 1995, pp. 1-7.
- J. S. West, P. D. Kharbanda, M. J. Barbetti and B. D. L. Fitt, “Epidemiology and Management of Leptosphaeria maculans (Phoma Stem Canker) on Oilseed Rape in Australia, Canada and Europe,” Plant Pathology, Vol. 50, No. 1, 2001, pp. 10-27. doi:10.1046/j.1365-3059.2001.00546.x
- M. L. Pilet, R. Delourme, N. Foisset and M. Renard, “Identification of Loci Contributing to Quantitative Field Resistance to Blackleg Disease, Causal Agent Leptosphaeria maculans (Desm.) Ces. et de Not., in Winter Rapeseed (Brassica napus L.),” Theorotical Applied Genetics, Vol. 96, No. 1, 1998, pp. 23-30. doi:10.1007/s001220050704
- B. D. L. Fitt, H. Brun, M. J. Barbetti and S. R. Rimmer, “World-Wide Importance of Phoma Stem Canker (Leptosphaeria maculans and L. biglobosa) on Oilseed Rape (Brassica napus),” European Journal of Plant Pathology, Vol. 114, No. 1, 2006, pp. 3-15. doi:10.1007/s10658-005-2233-5
- Y. Zhou, B. D. L. Fitt, S. J. Welham, P. Gladders, C. E. Sansford and J. S. West, “Effects of Severity and Timing of Stem Canker (Leptosphaeria maculans) Symptoms on Yield of Winter Oilseed Rape (Brassica napus) in the UK,” European Journal of Plant Pathology, Vol. 105, No. 7, 1999, pp. 715-728. doi:10.1023/A:1008761219493
- G. Berg, “Rhizobacteria of Oilseed Rape Antagonistic to Verticillium dahliae var. longisporum STARK,” Journal of Plant Disease and Protection, Vol. 103, No. 1, 1996, pp. 20-30.
- R. F. Mithen, B. G. Lewis, R. K. Heaney, G. R. Fenwick, “Resistance of Leaves of Brassica Species to Leptosphaeria maculans,” Transactions of the British Mycological Society, Vol. 88, No. 4, 1987, pp. 525-531. doi:10.1016/S0007-1536(87)80036-0
- B. Volke, “Leptosphaeria maculans, der Erreger der Wurzelhalsund Stengelfäule an Raps: Verbreitung Verschiedener Pathogenitätsgruppen in Europa, Quantifizierung des Befalls und Schadwirkug im Freiland,” Ph.D. Thesis, Göttingen, 1999.
- P. A. Delwiche, “Genetic Aspects of Blackleg (Leptosphaeria maculans) Resistance in Rapeseed (Brassica napus),” Ph.D. Thesis, Wisconsin, 1980.
- E. Weber and H. Blfiholder, “Erläuterungen zu den BBCHDezimal-Codes für die Entwicklungsstadien von Mais, Raps, Faba-Bohne, Sonnenblume und Erbse—Mit Abbildungen,” Gesunde Pflanzen, Vol. 42, No. 9, 1990, pp. 308-321.
- H. R. Kutcher, C. G. J. Van Denberg and S. R. Rimmer, “Variation in Pathogenicity of Leptosphaeria maculans on Brassica spp. Based on Cotyledon and Stem Reactions,” Canadian Journal of Plant Pathology, Vol. 15, No. 4, 1993, pp. 253-258. doi:10.1080/07060669309501920
- R. Campbell, “Biological Control of Soil-Borne Diseases: Some Present Problems and Different Approaches,” Crop Protection, Vol. 13, No. 1, 1994, pp. 4-13.
- J. W. Kloepper, “Development of in Vivo Assays for Prescreening Antagonists of Rhizoctonia solani on Cotton,” Phytopathology, Vol. 81, No. 9, 1991, pp. 1006-1013. doi:10.1094/Phyto-81-1006
- D. R. Fravel, “Role of Antibiosis in the Biocontrol of Plant Diseases,” Annual Reviews of Phytopathology, Vol. 26, No. 1, 1988, pp. 75-91. doi:10.1146/annurev.py.26.090188.000451
- R. Stanley, M. Brown, N. Poole, M, Rogerson D. C. Sigee, C. Knight, C. C. Ivuin, H. A. S. Epton and C. Leifert, “Biocontrol of Postharvest Fungal Diseases on Dutch White Cabbage by Pseudomonas and Serratia Antagonists in Storage Trials,” Plant Pathology, Vol. 43, No. 4, 1994, pp. 605-611. doi:10.1111/j.1365-3059.1994.tb01597.x
- M. Heupe, “Biologische Bekämpfung Bodenbürtiger Wurzelbranderreger der Zuckerrübe (Beta vulgaris L. ssp. Vulgaris var. Altissima Doell) Durch den Einsatz Mikrobieller Antagonisten,” Ph.D. Thesis, Göttingen, 1992.
- A. Sasse, “Untersuchungen zur Biologischen Bekämpfung des Rapswelkeerregers (Verticillum dahliae Kleb.) Durch den Einsatz Mikrobieller Antagonisten,” Ph.D. Thesis, Göttingen, 1997, p. 133.
- R. K. Gugel and G. A. Petrie, “History, Occurrence, Impact and Control of Blackleg of Oilseed Rape,” Canadian Journal of Plant Pathology, Vol. 14, No. 1, 1992, pp. 36- 45. doi:10.1080/07060669209500904
- K. E. Hammond, B. G. Lewis and T. Musa, “A Systemic Pathway in the Infection of Oilseed Rape Plants by Leptosphaeria maculans,” Plant Pathology, Vol. 34, No. 4, 1985, pp. 357-365. doi:10.1111/j.1365-3059.1985.tb01407.x
- K. E. Hammond and B. G. Lewis, “The Establishment of Systemic Infection in Leaves of oilseed Rape by Leptosphaeria maculans,” Plant Pathology, Vol. 36, No. 2, 1987, pp. 135-147. doi:10.1111/j.1365-3059.1987.tb02213.x
- P. A. Delwiche and P. H. Williams, “Screening for Resistance to Blackleg of Crucifers in the Seedling Stage,” Cruciferae News letter, Vol. 4, 1979, p. 24.
- D. M. Weller, “Biological Control of Soilborne Plant Pathogens in the Rhizosphere with Bacteria,” Annual Reviews of Phytopathology, Vol. 26, No. 1, 1988, pp. 379-407. doi:10.1146/annurev.py.26.090188.002115
- Y. Elad and I. Chet, “Possible Role of Competition for Nutrients in Biocontrol of Pythium Damping-Off by Bacteria,” The American Phytopathological Society, Vol. 77, No. 2, 1987, pp. 190-195. doi:10.1094/Phyto-77-190
- E. A. Milus and C. S. Rothrock, “Rhizosphere Colonization of Wheat by Selected Soil Bacteria over Diverse Environments,” Canadian Journal of Microbiology, Vol. 39, No. 3, 1993, pp. 335-341. doi:10.1139/m93-047
- S. Brückner,“Möglichkeiten Einer Biologischen Kontrolle von Verticillium. Dahliae an Winterraps mit Hilfe Antagonistischer Rhitosphärenbakterien,” Ph.D. Thesis, Universität Rostock, Rostock,1998.
- E. Liljeroth, S. L. G. E Burgers and J. A. Van Veen, “Changes in Bacterial Populations along Roots of Wheat (Triticum aestivum L.) Seedlings,” Biology and Fertility of Soils, Vol. 10, No. 4, 1991, pp. 276-280. doi:10.1007/BF00337378
- A. Kleeberger, A. H. J. Carstorp and W. Klinmüller, “The Rhizosphere Microflora of Wheat and Barley with Special Reference to Gram-Negative Bacteria,” Archives of Microbiology, Vol. 136, No. 4, 1983, pp. 306-311. doi:10.1007/BF00425222

