Open Journal of Medical Microbiology
Vol.2 No.3(2012), Article ID:22826,6 pages DOI:10.4236/ojmm.2012.23016
Antigen Detection in Canine Blastomycosis: Comparison of Different Antibody-Antigen Combinations in Two Competitive ELISAs
Department of Biological Sciences, Idaho State University, Pocatello, USA
Email: andrdeb2@isu.edu
Received August 2, 2012; revised September 3, 2012; accepted September 10, 2012
Keywords: Blastomycosis; Antigen Detection; Lysate Antigen and Antibody; ELISA
ABSTRACT
This present study was designed to evaluate four different Blastomyces dermatitidis antibody-antigen combinations (B5896 and T-58 antibodies and B5896 and WI-R antigens) for the detection of antigen in 36 urine specimens from dogs with blastomycosis using a standard indirect ELISA (STD) and a biotin-streptavidin ELISA (B-SA). The antigen detection sensitivity values ranged from 81% (B-SA: T-58 Ab + WI-R Ag) to 100% (STD and B-SA: B5896 Ab + WI-R Ag; B5896 Ab + B5896 Ag) with the antibody-antigen combinations in the two assays. Optimal detection was evidenced when the B5896 Ab was allowed to react with the urine specimens for 30 min at 37˚C and then placed in the B-SA ELISA plates containing the B5896 Ag. The greatest absorbance value obtained with this antibody-antigen combination was 0.903 (range of 0.596 - 0.903) as compared to the control value of 1.246. The difference between the control absorbance and the test absorbance values was 0.343 which was considerably greater than the control-test values with the other combinations. This study thus showed that the results obtained in antigen detection assays are dependent upon the antibody used to react with the urine specimens as well as the antigen used in the enzyme immunoassay.
1. Introduction
Blastomycosis is a systemic fungal infection of mammals, especially dogs and humans, caused by the thermally dimorphic fungal organism Blastomyces dermatitidis. The disease is initiated by the inhalation of an infectious spore produced by the filamentous phase of the fungus [1]. The organism exists in this stage in nature or in the laboratory at 25˚C and has the ability to convert to the yeast phase at 37˚C in the lungs of the infected host. The disease may be self-resolving, or it may exist as an acute infection characterized by cough, chills, headaches, malaise, fever, chest pain, night sweats and weight loss or may also progress to a chronic state where it may be misdiagnosed as tuberculosis or other diseases. If the disease is not diagnosed or goes untreated while in the lungs it may become invasive and disseminate to other organs and possibly to the central nervous system where fatal meningitis may develop [2-6]. In dogs a B. dermatitidis infection may disseminate to the eyes, bones, skin or lymph nodes and produce an immune response directed against the yeast phase antigens [7,8]. Blastomycosis, as well as other systemic mycoses, are termed “emerging fungal threats” since they can not only infect individuals with normal immune systems, but also are a cause for concern in hosts with deficiency diseases that compromise the immune system; therefore, a prompt diagnosis and treatment is important to the survival of the patient [2,5,6].
There has been a considerable amount of interest among researchers with respect to developing improved methods to combat the dramatic increase in the systemic fungal diseases [2,3]. With respect to blastomycosis the development of rapid, specific and sensitive clinical diagnostic tests has been limited and diagnosis still relies a great deal on histopathologic or cytologic direct visualization or culturing of the organism [1,6,9]. In some instances these procedures may be beneficial, but in other situations they are time-consuming or may fail to yield the desired results. Thus, researchers have begun to devote more time to the development of improved immunological assays which tend to provide a more rapid diagnosis, but problems still exist with regard to the sensitivity and specificity of immunoassays [2,6,9]. For the past several years our laboratory has been involved in the development of novel yeast phase lysate antigens from various isolates of B. dermatitidis and the utilization of these reagents in immunodiagnostic assays for both antibody and antigen detection in blastomycosis [10-13], but these studies have only opened up new avenues of approach with regard to improvement of immunodiagnostic methods in the future.
In recent years other investigators have been approaching the problem of immunodiagnosis and have produced encouraging results with antigen detection assays as second-generation assays have been developed [7,14-18]. Our laboratory has been focusing on the use of the competitive enzyme-linked immunosorbent assay (ELISA) in B. dermatitidis antigen detection studies in urine specimens from dogs with diagnosed blastomycosis [11]. The purpose of this present study was to compare different anti-blastomyces antibodies for their ability to react with the antigen when admixed with urine specimens from dogs with blastomycosis and subsequently assayed in the competitive ELISA (a standard indirect ELISA: STD and a biotin-streptavidin: B-SA ELISA using different B. dermatitidis lysate antigens. In the standard ELISA an anti-rabbit antibody conjugated to horseradish peroxidase that reacts with the primary rabbit antibody. In the B-SA ELISA a streptavidin protein that is covalently conjugated to horseradish peroxidase which binds to biotin that has been conjugated to an anti-rabbit antibody which reacts with the primary rabbit antibody, as stated below in Sections 2.3 and 2.4. This study allowed us to evaluate the different antibody-antigen combinations/ELISA methods and to determine optimal reagents to use in the competitive ELISA for antigen detection in urine specimens from dogs with blastomycosis.
2. Materials & Methods
2.1. Antigens
Yeast phase lysate antigens were prepared from various B. dermatitidis isolates. Mycelial phase cultures (B-5896 Minnesota human isolate or WI-R Wisconsin dog isolate) were converted to yeast cells by culturing on brain heart infusion agar at 37˚C. The yeast phase reagents were prepared by a previously described method [19,20] modified by Johnson and Scalarone [10]. Yeast cells were grown for 7 days at 37˚C with shaking in a chemically defined, nutritionally lean medium. The cells were harvested and washed five times by centrifugation for 5 min at 700 × g in sterile distilled water. The cells were lysed by incubation in sterile water for 7 days at 37˚C with shaking. The suspension was centrifuged (5 min at 700 × g) to remove debris and filter sterilized through a 0.2 um Nalgene filter (Nalge Company, Rochester, NY). Merthiolate (1:10,000) was added as a preservative to the lysate reagents. Protein determinations were made on the lysate antigen preparations using the Pierce BCA (Thermo Fisher Scientific, Rockford, IL) method according to manufacturer directions. The lysate reagents were stored at 4˚C for future use.
2.2. Antibodies
The antibodies were obtained from rabbits immunized with the T-58 (Tennessee dog) and B5896 (Minnesota human) B. dermatitidisyeast phase lysate antigens. The rabbits were housed in accordance with the NIH guide for Care and Use of Laboratory Animals with approval from the Idaho State University IACUC. Each of the 36 urine specimens (400 µL) were added to 400 µL of each antibody and incubated for 30 minutes at 37˚C. The 36 dog urine samples, all of which were previously diagnosed with blastomycosis, were obtained from Dr. A. M. Legendre (College of Veterinary Medicine of the University of Tennessee, Knoxville, TN). No competitive binding was observed in urine specimens from uninfected dogs.
2.3. Competitive STD ELISA Method
Detection of antigen in the urine specimens was determined using the competitive standard enzyme-linked immunosorbent assay (STD ELISA). Each lysate antigen (WI-R or B5896) was diluted (2000 ng of protein/ml) in a carbonate-bicarbonate coating buffer (pH 9.6) and then added to triplicate wells (100 µL) of a Costar 96 well Easy Wash plate(Corning, Thermo-Fisher) and incubated overnight at 4˚C. Upon rinsing the plates three times with phosphate buffered saline containing 0.15% Tween 20 (PBS-T), the dog urine and antibody mixture (100 µL) was added in triplicate and incubated for 30 min at 37˚C. The plates were washed as above. Anti-rabbit horseradish peroxidase conjugate (KPL, Gaithersburg, MD) at a 1:2000 dilution in PBS-T was added (100 µL per well), incubated for 30 min at 37˚C, and the plates were washed as above. One-step Ultra TMB substrate (Pierce: ThermoFisher) was added (100 µL per well) and incubated for 3 min at room temperature. The reaction was stopped by the addition of 100 µL of 2N sulfuric acid to each well. The absorbance was read at 450 nm using the BIO-RAD EIA reader (BIO-RAD, Hercules, CA).
2.4. Biotin-Streptavidin (B-SA) ELISA Method
The ability of each yeast lysate reagent to detect antigen in the above serum specimens was determined using the competitive biotin-streptavidin enzyme-linked immunosorbent assay (B-SA ELISA). Each lysate antigen was diluted (2000 ng of protein/ml) in the coating buffer and then added to triplicate wells (100 µl) of a Costar 96-well microdilution plate (Thermo-Fisher) and incubated overnight at 4˚C followed by washing three times with PBS-T. The urine and antibody mixture (100 µl) were added to the micro-plate wells and incubated for 30 min at 37˚C.
Following incubation the wells were washed as above and 100 µl of anti-rabbit biotin labeled antibody (KPL) was added to each well and incubated for 30 min at 37˚C. The plates were washed as above and 100 µl of peroxidase labeled streptavidin was added to each well and incubated for 30 min at 37˚C. The plates were again washed as above and 100 µl of 1-step Ultra TMB-ELISA peroxidase substrate (Pierce: Thermo-Fisher) was added to each well and incubated for approximately 2 min at room temperature. The reaction was stopped by the addition of 2N sulfuric acid and the absorbance read at 450 nm using a BIO-RAD 2550 EIA reader.
3. Results
3.1. B5896 Ab-WI-R Ag Combination
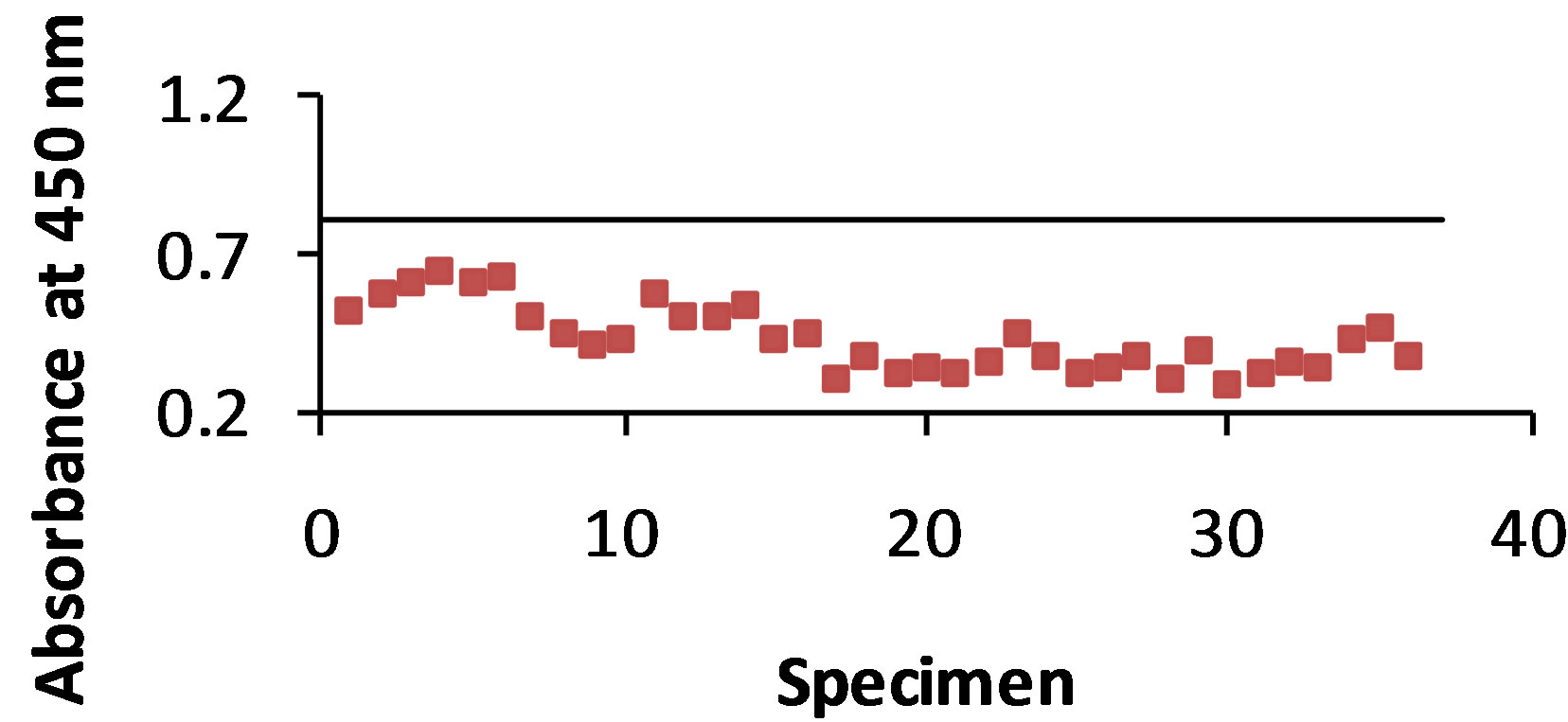
As shown in Figure 1(a) antigen was detected with the STD ELISA in 100% of the 36 dog urine specimens. The control value was 0.806 and the absorbance value range of antigen detection was 0.289 - 0.635. The B-SA ELISA (Figure 1(b)) detected antigen in 100% of the urine specimens with a control value of 1.302 and an absorbance value range of 0.400 - 1.006.
3.2. T-58 Ab-WI-R Ag Combination
As shown in Figure 2(a) antigen was detected with the STD ELISA in 94% of the 36 dog urine specimens. The control value was 1.363 and the absorbance value range of antigen detection was 0.913 - 1.465. The B-SA ELISA (Figure 2(b)) detected antigen in 81% of the urine specimens with a control value of 2.667 and an absorbance value range of 1.419 - 3.077.
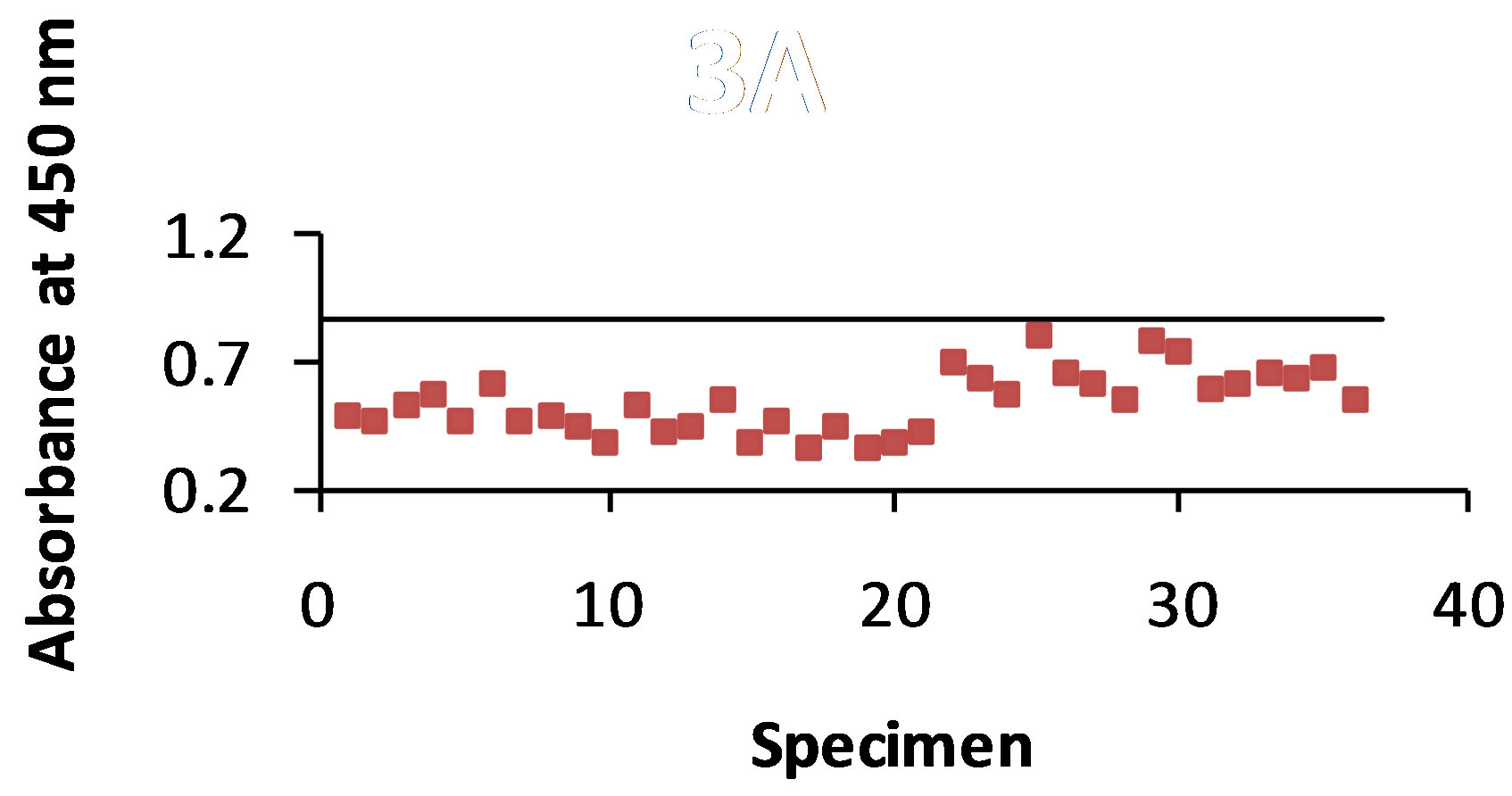
3.3. B5896 Ab-B5896 Ag Combination
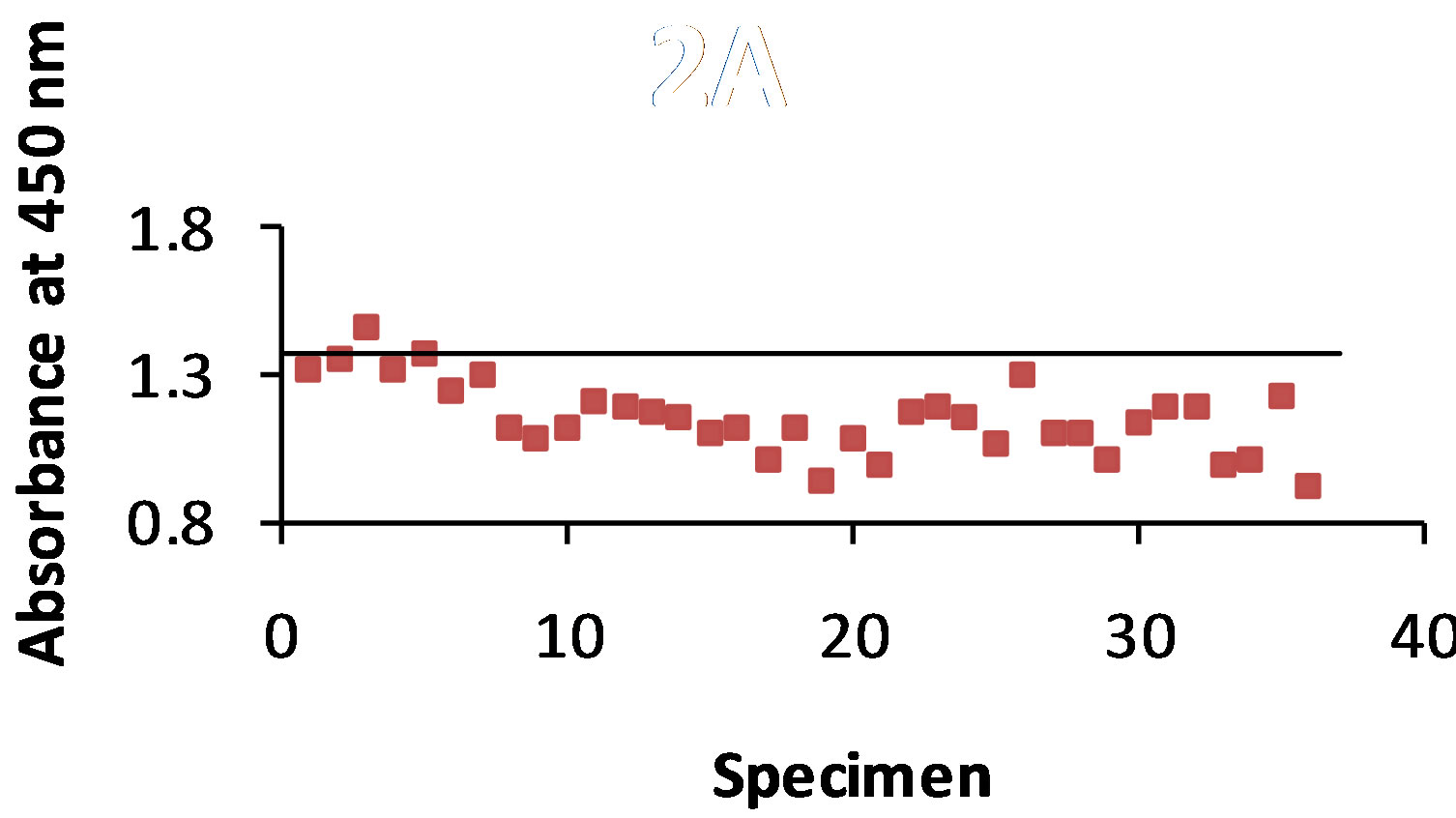
As shown in Figure 3(a) antigen was detected with the STD ELISA in 100% of the 36 dog urine specimens. The control value was 0.858 and the absorbance value range of antigen detection was 0.350 - 0.793. The B-SA ELISA (Figure 3(b)) detected antigen in 100% of the urine specimens with a control value of 1.246 and an absorbance value range of 0.596 - 0.903.
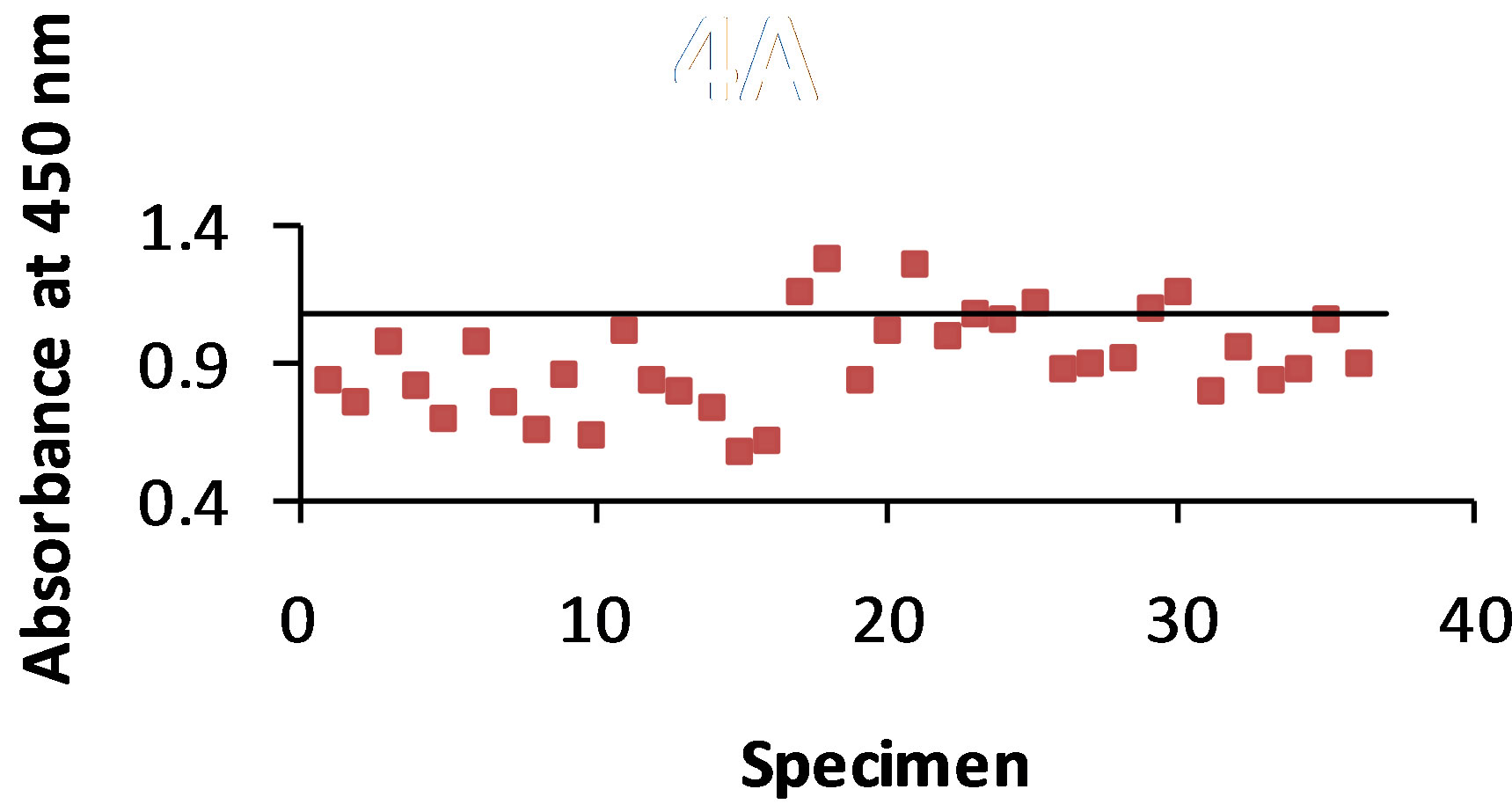
3.4. T-58 Ab-B5896 Ag Combination
As shown in Figure 4(a) antigen was detected with the STD ELISA in 86% of the 36 dog urine specimens. The control value was 1.081 and the absorbance value range of antigen detection was 0.572 - 1.266. The B-SA ELISA (Figure 4(b)) detected antigen in 83% of the urine specimens with a control value of 1.432 and an absorbance value range of 0.570 - 1.713.
 (a)
(a)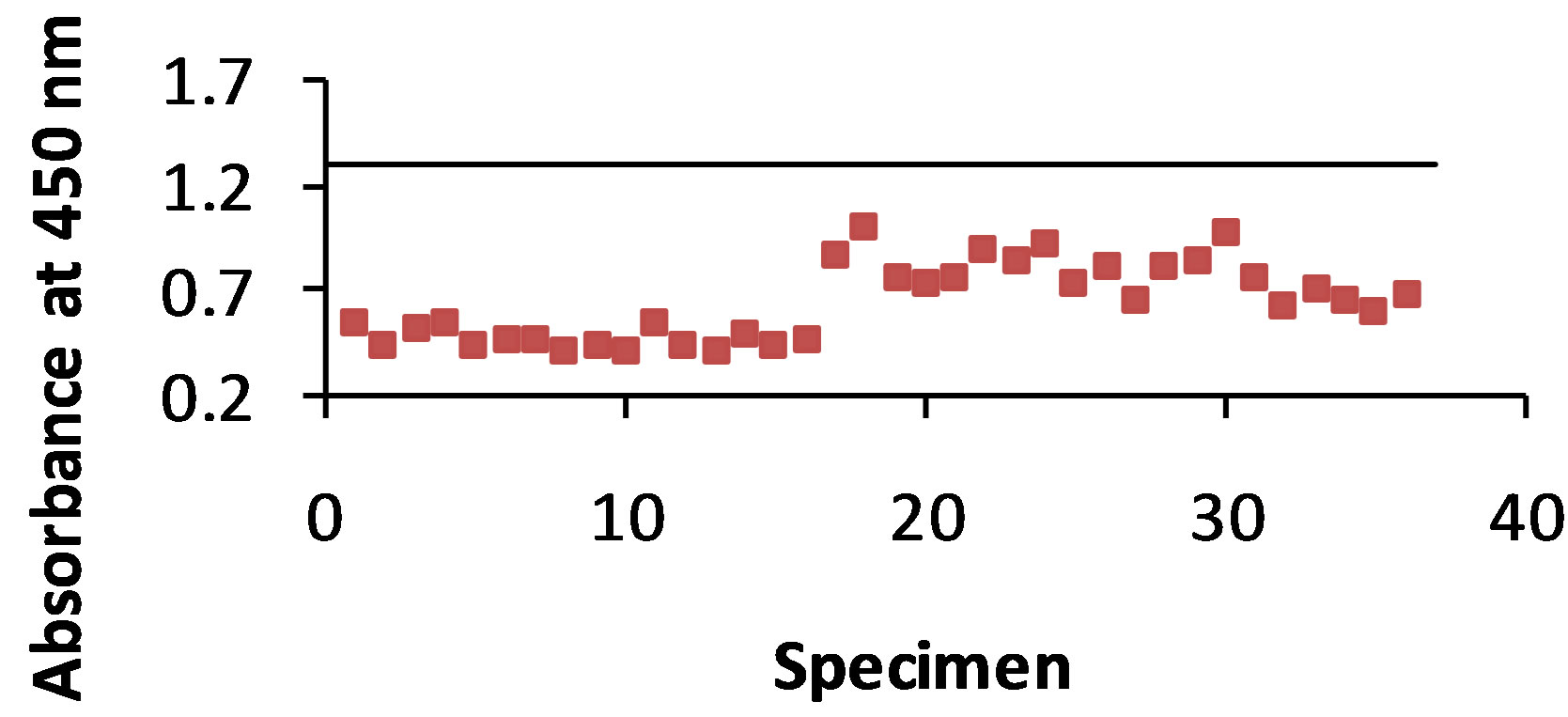 (b)
(b)
Figure 1. (a) and (b): The specimens were from 36 dogs that were infected with B. dermatitidis. The antibody B5896 was mixed with each of the 36 urine samples, and then added to the plate containing the WI-R antigen. The horizontal line represents the control. Any result that is above the control line shows that no antigen was detected. Figure 1(a) shows 100% antigen detection with the STD ELISA and Figure 1(b) shows 100% antigen detection with the B-SA ELISA.
 (a)
(a)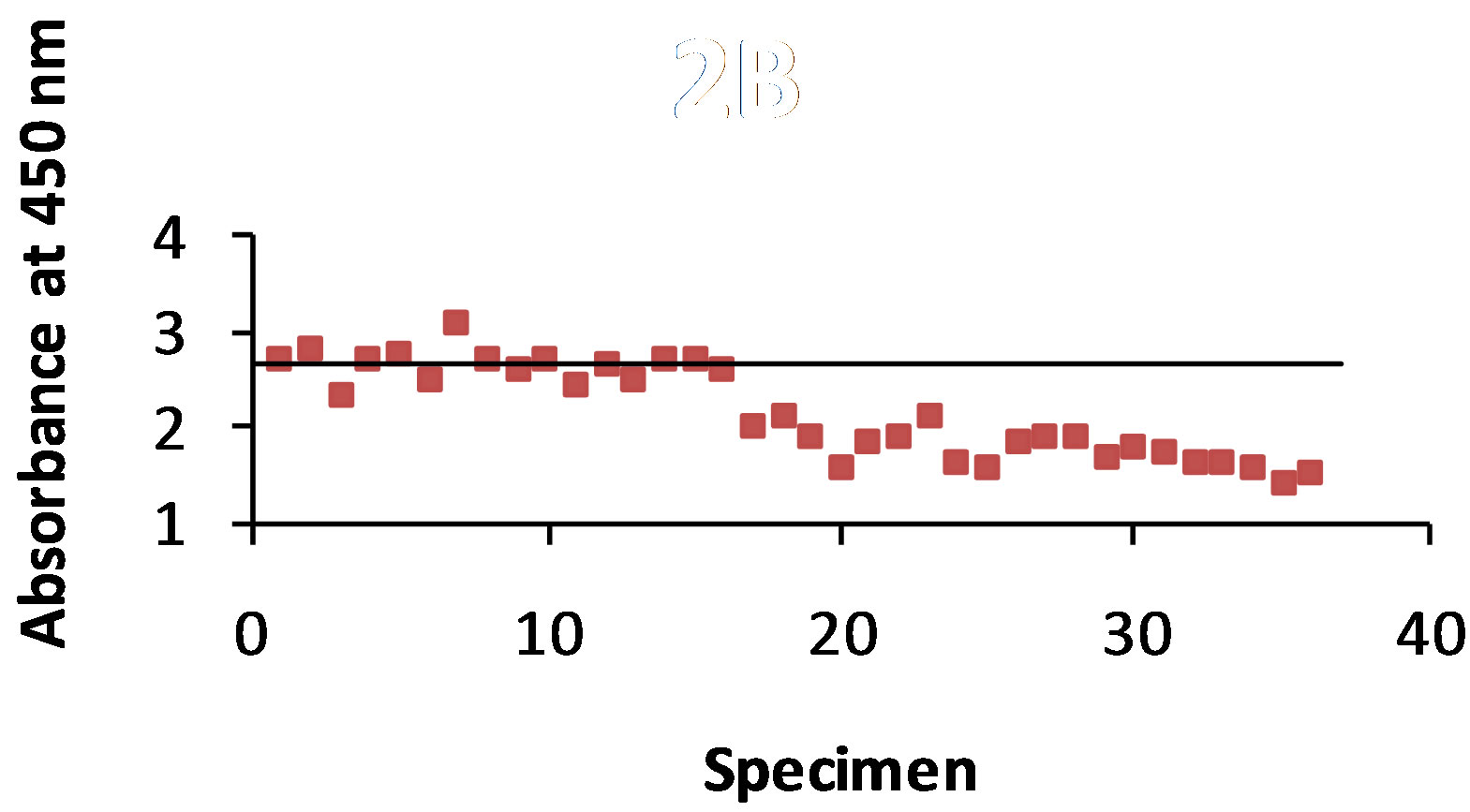 (b)
(b)
Figure 2. (a) and (b): The specimens were from 36 dogs that were infected with B. dermatitidis. The antibody T-58 was mixed with each of the 36 urine samples, and then added to the plate containing the WI-R antigen. The horizontal line represents the control. Any result that is above the control line shows that no antigen was detected. Figure 2(a) shows a 94% antigen detection with the STD ELISA and Figure 2(b) shows 81% antigen detection with the B-SA ELISA.
 (a)
(a) (b)
(b)
Figure 3. (a) and (b): The specimens were from 36 dogs that were infected with B. dermatitidis. The antibody B5896 was mixed with each of the 36 urine samples, and then added to the plate containing the B5896 antigen. The horizontal line represents the control. Any result that is above the control line shows that no antigen was detected. Figure 3(a) shows 100% antigen detection with the STD ELISA and Figure 3(b) shows 100% antigen detection with the B-SA ELISA.
 (a)
(a)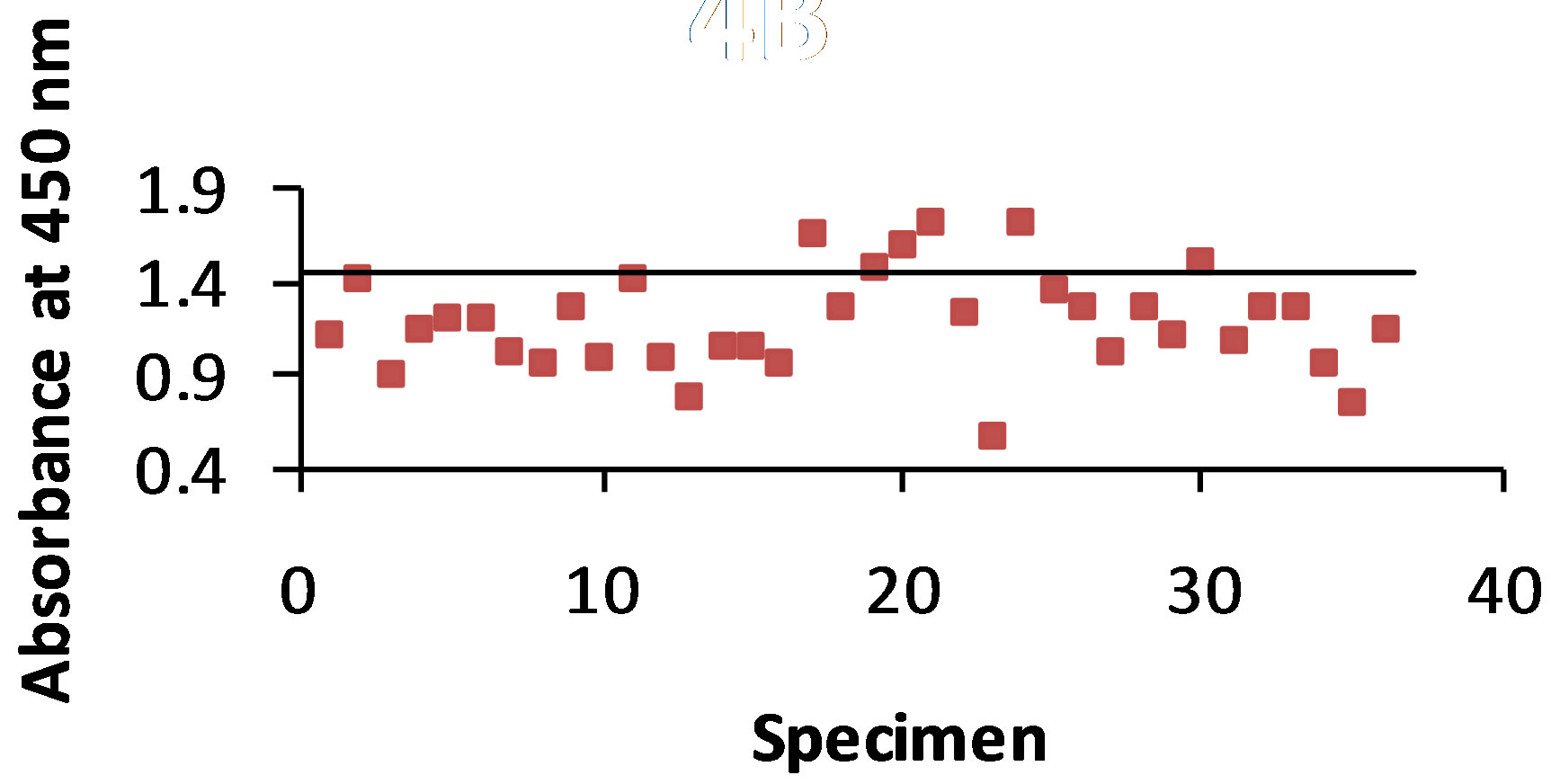 (b)
(b)
Figure 4. (a) and (b): The specimens were from 36 dogs that were infected with B. dermatitidis. The antibody T-58 was mixed with each of the 36 urine samples, and then added to the plate containing the B5896 antigen. The horizontal line represents the control. Any result that is above the control line shows that no antigen was detected. Figure 4(a) shows a 86% antigen detection with the STD ELISA and Figure 4(b) shows 83% antigen detection with the B-SA ELISA.
4. Discussion
For the past several years our laboratory has been involved inusing enzyme immunoassays for the detection of antibody in serum specimens from immunized or infected animals with B. dermatitidis yeast phase lysate antigens prepared from various isolates of the organism. These assays allow for antibody detection in individuals with “normal” immune systems, but of limited value in immunocompromised patients. Therefore we have begun to evaluate antibodies and antigens produced by various B. dermatitidis isolates from human, animal or environmental sources in order to assess the potential of these immunodiagnostic reagents for the detection of antigen in urine specimens. The switch of focus from antibody to antigen detection shows promise as a potential alternative diagnostic method. The primary objective of this present study was to compare different antibody-antigen combinations for the detection of antigen in urine specimens from dogs with diagnosed blastomycosis.
Several recent studies have focused on antigen detection in blastomycosis in humans and dogs [7,15,16]. In one study, detectable antigenuria was achieved in approximately 85% of the subjects with newly diagnosed blastomycosis, but B. dermatitidis antigen was also detected in 15 patients with histoplasmosis [15]. In another human subject study, antigenuria was detected in approximately 90% of patients with proven blastomycosis. Specificity was 99% in healthy subjects or patients with nonfungal infections, but cross-reactions occurred in approximately 96% of patients with histoplasmosis [16]. An earlier study on antigen detection in dogs with blastomycosis revealed a sensitivity value of 94% in urine specimens and a value of 87% in serum specimens [7]. A multicenter evaluation on histoplasmosis antigen detection revealed 91.8% of 158 patients with disseminated histoplasmosis, 83.3% of 6 patients with acute histoplamosis, 30.4% of 46 patients with sub-acute hisotplasmosis, and 87.5% of 8 patients with chronic pulmonary histoplasmosis; antigenemia was present in 100% of 31 tested cases of disseminated histoplasmosis [18]. In another study on Coccidioides antigen detection, antigenuria was detected in 50% of patients. Specificity of 100% was obtained in healthy subjects, but cross-reactions were seen in 22.2% of patients with histoplasmosis or blastomycosis [14].
Our present study focused on the detection of antigen using different antibody-antigen combinations, as well as a comparison between the STD ELISA and the B-SA ELISA methods. The least sensitive combination was T-58 antibody and B5896 antigen when using either the STD ELISA or the B-SA ELISA at 86% and 83%, respectively. The T-58 antibody and WI-R antigen combination had the overall lowest antigen detection at 81% using the B-SA ELISA. When B5896 antibody was used 100% antigen detection was observed when either B5896 or WI-R antigen were used. However, the optimal combination was the B5896 antibody and B5896 antigen using the B-SA ELISA with an absorbance range of 0.596 - 0.903 which was well below the control value of 1.246. Additional studies are continuing to evaluate additional antigen-antibody combinations for the detection of antigen.
5. Acknowledgements
This research was supported by the Department of Biological Sciences, Idaho State University.
REFERENCES
- A. F. DiSalvo, “Blastomycosis, in Topley and Wilson’s Microbiology and Microbial Infections,” 9th Edition, Arnold Publishers, London, 1998, pp. 337-355.
- J. A. McKinnell and P. G. Pappas, “Blastomycosis: New Insights into Diagnosis, Prevention, and Treatment,” Clinical Chest Medicine, Vol. 30, No. 2, 2009, pp. 227- 239. doi:10.1016/j.ccm.2009.02.003
- J. A. Smith and C. A. Kauffman, “Pulmonary Fungal Infections,” Respirology, Vol. 17, No. 6, 2012, pp. 913-926.
- J. A. Smith and C. A. Kauffman, “Blastomycosis,” Proceedings of the American Thoracic Society, Vol. 7, No. 3, 2010, pp. 173-180. doi:10.1513/pats.200906-040AL
- J. R. Bariola and K. S. Vyas, “Pulmonary Blastomycosis,” Seminars in Respiratory Critical Care Medicine, Vol. 32, No. 6, 2011, pp. 745-753. doi:10.1055/s-0031-1295722
- M. Saccente and G. L. Woods, “Clinical and Laboratory Update on Blastomycosis,” Clinical Microbiology Reviews, Vol. 23, No. 2, 2010, pp. 367-381. doi:10.1128/CMR.00056-09
- D. Spector, A. M. Legendre, J. Wheat, D. Bemis, B. Rohrbach, J. Taboada and M. Durkin, “Antigen and Antibody Testing for the Diagnosis of Blastomycosis in Dogs,” Journal of Veterinary Internal Medicine, Vol. 22, No. 4, 2008, pp. 839-843. doi:10.1111/j.1939-1676.2008.0107.x
- B. S. Klein, R. A. Squires, J. K. Lloyd, D. R. Ruge and A. M. Legendre, “Canine Antibody Response to Blastomyces dermatitidis WI-1 Antigen,” American Journal of Veterinary Research, Vol. 61, No. 5, 2000, pp. 554-558.
- K. S. Vyas, J. R. Bariola and R. W. Bradsher, “Advances in the Serodiagnosis of Blastomycosis,” Current Fungal Infection Reports, Vol. 2, No. 4, 2008, pp. 227-231. doi:10.1007/s12281-008-0033-z
- S. M. Johnson and G. M. Scalarone, “Preparation and ELISA Evaluation of Blastomyces dermatitidis Yeast Phase Lysate Antigens,” Diagnostic Microbiology and Infectious Diseases, Vol. 11, No. 2, 1989, pp. 81-86. doi:10.1016/0732-8893(88)90076-4
- J. F. Shurley, A. M. Legendre and G. M. Scalarone, “Blastomyces dermatitidis Antigen Detection in Urine Specimens from Dogs with Blastomycosis Using a Competitive Binding Inhibition ELISA,” Mycopathologia, Vol. 160, No. 2, 2005, pp. 137-142. doi:10.1007/s11046-005-3153-9
- C. M. Sestero and G. M. Scalarone, “Detection of IgG and IgM in Sera from Canines with Blastomycosis Using Eight Blastomyces dermatitidis Yeast Phase Lysate Antigens,” Mycopathologia, Vol. 162, No. 1, 2006, pp. 33-37. doi:10.1007/s11046-006-0028-7
- W. O. Hatch and G. M. Scalarone, “Comparison of Colorimetric and Chemiluminescent ELISAs for the Detection of Antibodies to Blastomyces dermatitidis,” Journal of Medical and Biological Sciences, Vol. 3, No. 1, 2009, pp. 1-6.
- M. Durkin, L. Estok, D. Hospenthal, N. Crum-Cianflone, S. Swartzentruber, E. Hackett and L. J. Wheat, “Detection of Coccidioides Antigenemia Following Dissociation of Immune Complexes,” Clinical and Vaccine Immunology, Vol. 16, No. 10, 2009, pp. 1453-1456. doi:10.1128/CVI.00227-09
- J. R. Bariola, C. A. Hage, M. Durkin, E. Bensadoun, P. O. Gubbins, L. J. Wheat and R. W. Bradsher, “Detection of Blastomyces dermatitidis Antigen in Patients with Newly Diagnosed Blastomycosis,” Diagnostic Microbiology and Infectious Diseases, Vol. 69, No. 2, 2011, pp. 187-191. doi:10.1016/j.diagmicrobio.2010.09.015
- P. Connolly, C. A. Hage, J. R. Bariola, E. Bensadoun, M. Rodgers, R. W. Bradsher Jr. and L. J. Wheat, “Blastomyces dermatitidis Antigen Detection by Quantitative Enzyme Immunoassay,” Clinical Vaccine Immunology, Vol. 19, No. 1, 2012, pp. 53-56. doi:10.1128/CVI.05248-11
- D. R. Allton, R. G. Rivard, P. A. Connolly, S. McCall, M. M. Durkin, T. M. Boyd, J. P. Flanagan, L. J. Wheat and D. R. Hospenthal, “Detection of Latin American Strains of Histoplasma in a Murine Model by Use of a Commercially Available Antigen Test,” Clinical and Vaccine Immunology, Vol. 17, No. 5, 2010, pp. 802-806. doi:10.1128/CVI.00043-10
- C. A. Hage, J. A. Ribes, N. L. Wengenack, L. M. Baddour, M. Assi, D. S. McKinsey, K. Hammoud, D. Alapat, N. E. Babady, M. Parker, De A. Fuller, A. Noor, T. E. Davis, M. Rodgers, P. A. Connolly, B. El Haddab and L. J. Wheat, “A Multicenter Evaluation of Tests for Diagnosis of Histoplasmosis,” Clinical Infectious Diseases, Vol. 53, No. 5, 2011, pp. 448-454. doi:10.1093/cid/cir435
- H. B. Levine, G. M. Scalarone and S. D. Chaparas, “Preparation of Fungal Antigens and Vaccines: Studies on Coccidioides immitis and Histoplasma capsulatum,” Contributions to Microbiology and Immunology, Vol. 3, 1977, pp. 106-125.
- H. B. Levine, G. M. Scalarone, G. D. Campbell, J. R. Graybill, P. C. Kelly and S. D. Chaparas, “Histoplasmin-CYL, a Yeast Phase Reagent in Skin Test Studies in Humans,” The American Review of Respiratory Diseases, Vol. 119, No. 4, 1979, pp. 629-636.

