American Journal of Analytical Chemistry
Vol.07 No.01(2016), Article ID:62996,8 pages
10.4236/ajac.2016.71010
Synthesis, Characterization and X-Ray Structure of a Ba(II)/Ag(I)/Cr(III)-Oxalate Salt with Water-Filled Nanochannels
Clémence Eboga Tanke1, Bridget N. Ndosiri1, Yves A. Mbiangué2, Gouet Bebga3*, Justin Nenwa1
1Inorganic Chemistry Department, Faculty of Science, University of Yaounde 1, Yaounde, Cameroon
2Chemistry Department, Higher Teacher Training College, University of Maroua, Maroua, Cameroon
3Chemistry Department, Higher Teacher Training College, University of Yaounde 1, Yaounde, Cameroon

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 11 December 2015; accepted 22 January 2016; published 25 January 2016
ABSTRACT
A novel mixed barium(II)/silver(I)/chromium(III) oxalate salt, Ba0.5Ag2[Cr(C2O4)3]・5H2O (1), with open architecture has been synthesized in water and characterized by elemental analysis, vibrational and electronic spectra, and single crystal X-ray structure determination. Compound 1 crystallizes in a monoclinic space group C2/c, with unit cell parameters a = 18.179(3), b = 14.743(2), c = 12.278(2) Å, β = 113.821(3)˚, V = 3010.34(90) Å3, Z = 8. The structure is characterized by a network of anionic [Cr(C2O4)3]3− units connected through the O atoms of the oxalates to Ba2+ and Ag+ sites, forming a three-dimensional coordination polymer with one-dimensional isolated nanochannels parallel to the c axis, and encapsulating hydrogen-bonded guest water molecules. The bulk structure is consolidated by O−H・・・O bridgings within the nanochannels and by coulombic interactions.
Keywords:
Crystal Structure, Chromium(III) Complex, Nanochannels, Coordination Polymer, Guest-Water Molecules

1. Introduction
Open-framework materials have drawn increasing attention over the past few years not only because of the wide diversity in structures, but also for their potential applications as functional materials in the areas of catalysis, magnetism, non-linear optics, gas storage and separation processes [1] -[6] . Amongst multidentate ligands usually engaged in the construction of this class of materials, the oxalate(2−) dianion (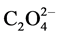 ) occupies an important place, since it is one of the simplest imaginable connectors potentially able to bridge metals ions in the bidentate chelating manner. Major emphasis in studies employing this highly versatile ligand is associated with the formation of exotic network structures. Transition metal ion complexes provide useful building blocks for stereochemistry control. In this process, the well-documented tris(oxalato)chromate(III) ion, [Cr(C2O4)3]3−, plays a crucial role based on: 1) its remarkable nature as a chiral and paramagnetic building block, 2) its ability to function as a metalloligand (or auxillary ligand) that can mediate electronic effects between paramagnetic centers (e.g. magnetic interactions), and 3) its ability to form interesting long-range hydrogen-bonded solid-state networks.
) occupies an important place, since it is one of the simplest imaginable connectors potentially able to bridge metals ions in the bidentate chelating manner. Major emphasis in studies employing this highly versatile ligand is associated with the formation of exotic network structures. Transition metal ion complexes provide useful building blocks for stereochemistry control. In this process, the well-documented tris(oxalato)chromate(III) ion, [Cr(C2O4)3]3−, plays a crucial role based on: 1) its remarkable nature as a chiral and paramagnetic building block, 2) its ability to function as a metalloligand (or auxillary ligand) that can mediate electronic effects between paramagnetic centers (e.g. magnetic interactions), and 3) its ability to form interesting long-range hydrogen-bonded solid-state networks.
Over the last decade, the family of materials with a variable Ag-Cr-oxalate channel lattice, formulated generally as [(MxAg0.50−x)(H2O)3]@[Ag2.50Cr(C2O4)3] (0 ≤ x ≤ 0.50; M = K, Cs, Ag), has been at the focus of interest in contemporary research and development [7] . These silver salts generally crystallize in a nonmolecular polymer framework preferentially with flexible deficiencies x of Ag+ ions, thus generating a negatively charged lattice grid. They share in their crystal structures a one-dimensional channel lattice as their characteristic feature.
Along the lines of our progressing research program, we recently reported some closely related channel lattice networks with chemical compositions [Ag3−xCr(C2O4)3]x−, where the negative charge (−x) is offset by an equivalent fractional charge from protons [8] , monoatomic cations (M+) from group 1 elements [9] [10] or from metal(III) ions [11] . In connection with these results, we have been investigating other silver/chromium-oxalate compounds with a view not only to produce new materials but also to unravel the subtle structural features that characterize these solids. During the course of these investigations, we have now isolated a novel Ag-Cr-oxalate channel lattice compound of the formula Ba0.5Ag2[Cr(C2O4)3]∙5H2O (1), in which the negative charge of the lattice grid with chemical composition [Ag2Cr(C2O4)3]− is counterbalanced by a half Ba2+ cation. Herein, we describe the title compound as a useful precursor for the forthcoming metathetic syntheses of new compounds containing the negative lattice grid [Ag2Cr(C2O4)3]−.
2. Experimental Procedure
2.1. Chemical Preparation
The purchased salts, silver nitrate (Riedel-de Haën, 99.5%) and barium nitrate (Riedel-de Haën, 99.5%) were used as received; the precursor salt K3[Cr(C2O4)3]∙3H2O was prepared using a literature method [12] . A mixture of AgNO3 (1.36 g, 8 mmol) and Ba(NO3)2 (0.53 g, 2 mmol) was dissolved in water (50 mL). The precursor salt K3[Cr(C2O4)3]∙3H2O (1.95 g, 4 mmol) was dissolved in water (60 mL). This latter solution was added dropwise into the former. The mixture was stirred at 60°C for 2 h. The resulting violet solution was filtered and left to stand undisturbed in the hood at room temperature. After five days, elongated dark-violet crystals were harvested. Yield: 0.77 g (55.9%) based on K3[Cr(C2O4)3]∙3H2O salt. M.P.: >250°C. Elemental analysis: Calc. for C6H10Ag2Ba0.50CrO17 (690.55 g∙mol−1) C, 10.44; H, 1.46. Found: C, 10.42; H, 1.41. These crystals are readily soluble in water. IR (KBr, cm−1): 3417 (broad), 1623 (sharp), 1375 (sharp), 1258 (sharp), 901 (sharp), 800 (sharp), 543 (sharp) and 409 (sharp). UV-Vis (H2O solution, nm): 416; 568.
2.2. Investigation Techniques
2.2.1. Elemental Analysis
Elemental analysis (C, H) was performed on a VARIO EL (Heraeus) CHNS analyzer.
2.2.2. Infrared Spectroscopy
The infrared spectrum was recorded using a Nicolet Avater 360 FT-IR spectrometer in the range 4000 - 400 cm−1 with KBr (500 mg KBr/0.5 mg sample).
2.2.3. Ultraviolet-Visible Spectroscopy
The UV-Vis spectrum was recorded on an AQUALYTIC spectrophotometer, in water solution, in the range 300 - 800 nm.
2.2.4. X-Ray Single Crystal Structural Analysis
A shining violet crystal with approximate dimensions 0.20 × 0.18 × 0.12 mm3 was carefully selected in oil at room conditions and attached to the tip of a nylon loop. It was mounted in a stream of cold nitrogen and centered in X-ray beam by using a video camera. Crystal evaluation and X-ray data collection were performed on a model Xcalibur 3 diffractometer, equipped with Mo Kα (λ = 0.71073 Å) radiation. The reflections obtained were indexed by an automated indexing routine built in the SMART program [13] . The highly redundant data sets were corrected for Lorentz and polarization effects. The collected data were reduced with SAINT [14] and an empirical absorption correction was carried out by using the SADABS program [15] . The crystal structure was solved by direct methods and refined by full-matrix least squares refinement [16] , based on F2. Crystal data and structure refinement parameters for 1 are given in Table 1, and selected bond lengths and angles in Table 2.
Table 1. Crystal data and structure refinement parameters for 1.
3. Results and Discussion
3.1. Elemental Analysis of Compound 1
Elemental and full X-ray structure analyses converge to support the formulation of compound 1 as Ba0.5Ag2 [Cr(C2O4)3]∙5H2O. This fact of matter unambiguously demonstrates that the single crystal used for the X-ray structure determination is representative of the entire complex synthesized.
3.2. IR Spectrum of 1
The FTIR spectrum of compound 1 (Figure 1) shows a broad absorption band around 3417 cm−1 which is assigned to water molecules within the channels. The bands centered at 1623 cm−1 and 1375 cm−1 are attributed to
Table 2. Selected bond distances (Å) and angles (˚) of the anionic complex [Cr(C2O4)3]3− in 1.
Figure 1. Infrared absorption spectrum of compound 1.
ν(C=O) and ν(C-O) stretches respectively [8] -[11] . The bands centered at 900 cm−1 and 800 cm−1 are attributed to C-C stretches. The sharp band observed at 543 cm−1 can be assigned to the Cr-O vibration [8] [9] and the band at 409 cm−1 to the Ba-O vibration [17] .
3.3. Ultraviolet-Visible Spectrum of 1
The UV-Vis spectrum of compound 1 (Figure 2) displays two absorption bands centered at 416 and 568 nm, which are due to d-d electronic transitions within the octahedral complex ions [Cr(C2O4)3]3− contained in 1 [8] [9] [17] . It is obvious that the present electronic absorption spectrum is virtually superimposable with that reported since the spectral informations thus obtained solely relate to the [Cr(C2O4)3]3− species.
3.4. Crystal Structure of Compound 1
The X-ray structure determination of 1 reveals the chemical formulation Ba0.5Ag2[Cr(C2O4)3]∙5H2O. Compound 1 is a nonmolecular material that crystallizes in a nanoporous structure in which infinite one-dimensional channels run parallel to the crystallographic c-axis, each channel (diameter ca. 8.27 Å) being well isolated from its nearest neighbors by thick walls of the three-dimensionally connected network of Ba2+ and Ag+ cations and [Cr(C2O4)3]3− complex anions. Hydrogen-bonded water molecules are found in the channels. Figure 3 is a ball- and-stick representation of the unit cell of 1, projected onto the ab-plane highlighting water molecules within the channels and showing clearly that the host lattice consists exclusively of Ba2+ and Ag+ cations and [Cr(C2O4)3]3− complex anions. Compound 1 represents a new member of the broad family of silver-deficient oxalatometalate(III) salts with water-filled nanochannels generally formulated as [(MxAg0.50−x)(H2O)3]@[Ag2.50Cr(C2O4)3] (0 ≤ x ≤ 0.50; M = K, Cs, Ag) [7] .
The constitutive ionic building blocks of the structure of 1 − the [Cr(C2O4)3]3− complex, the Ag and Ba atoms ―are depicted in Figure 4. The anionic building stone, [Cr(C2O4)3]3−, functions as a metalloligand, or else, as an internetting bridge. Hence, this internetting bridge interconnects, across its O atoms, the three independent sites Cr, Ag1/Ba1 and Ag2/Ba2 into a three-dimensional polymeric neutral host lattice network that surrounds water-filled channels.
The Cr center (Figure 4(a)) is pseudo-octahedrally chelated by three oxalato ligands with helical orientation, coordination to the metal occurring across the six deprotonated “internal” (hydroxylato) O atoms (O1, O3, O4, O6, O7, O8), and giving rise to Cr-O bond lengths and O-Cr-O bond angles which fit well within the ranges of previous results [8] -[11] [17] .
Figure 2. Electronic absorption spectrum of compound 1.
Figure 3. Ball-and-stick representation of the unit cell of 1 projected down [001] highlighting the water molecules within the tubular channel and showing that the host lattice consists exclusively of Ag and Ba cations and [Cr(C2O4)3]3− complex anions.

Figure 4. Coordination polyhedra around the different metallic centers in 1: Cr (a), Ag1/Ba1 (b) and Ag2/Ba2 (c).
The coordination geometry around Ag1/Ba1 (Figure 4(b)) is a severely distorted octahedron involving four carbonyl O atoms (O9, O9i, O17, O17i) in the equatorial plane and two O atoms (O8, O8i) in the axial positions. Ag1 and Ba1 ions are statistically disordered [ratio 0.667(6):0.333(6)] on the same crystallographic site. All the Ag-O lengths of this octahedron are different with the values ranging from 2.328(3) to 2.559(4) Å but are comparable with reported values [8] -[10] .
The Ag2/Ba2 site (Figure 4(c)) experiences pentacoordination―not tetracoordination like in the structures reported by Dean et al. [7] ―due to one chelation via the O atoms (O10, O16), and to three monodentate linkages across O5, O7 and O13. Bond lengths and bond angles for the coordination sphere around Ag2 diverge markedly from one another just as previously reported [9] [10] . Ag2 and Ba2 ions are statistically disordered [ratio 0.667(8):0.333(8)] on the same crystallographic site.
The bulk structure of 1 is consolidated by O-H・・・O bridgings within the nanochannels and by coulombic interactions.
In this new member of open framework silver-oxalatochromate(III) compounds, Ba0.5Ag2[Cr(C2O4)3]∙5H2O (1), cancellation of the negative charge of the lattice grid with chemical composition [Ag2Cr(C2O4)3]− is realized by 0.5 Ba2+ ion. This Ba2+ ion can be easily replaced by other cations and even by protons through metathetic syntheses. Such materials in which Ba2+ cations are solely replaced by protons are expected to exhibit very high proton conductivity provided that the intervening H3O+ ions are incorporated within the nanochannels and not into the Ag-Cr-oxalate host lattice [8] [18] . Work on this theme is now one of our main focuses.
4. Conclusion
The compound, Ba0.5Ag2[Cr(C2O4)3]・5H2O, isolated from aqueous solution as dark-violet needles, is a novel nanostructured silver-deficient salt hosting H2O-guest molecules. Following these results which confirm the great flexibility of synthetic manoeuvres for the construction of silver-oxalatometalate(III) assemblies, our future works will doubtless target the synthesis of other members of this family of open-framework coordination polymers, with the main objective of cancelling the negative charge of the host lattice grid [Ag2CrIII(C2O4)3]− by hydronium (H3O+) ions solely. Evidence of the protonic charge allowed to migrate very fast up and down within the “water-streams” in the channels opens the door into the fascinating family of materials that may be dubbed Nanochannelled One-dimensional Proton Conducting Solids (1D-PCS). Progress in this research line will likely continue in the near future.
Acknowledgements
We thank Prof. Boniface P.T. Fokwa for Single crystal X-ray diffraction measurement and structure determination during his stay at Aachen University (Germany). We are grateful to Prof. Michel M. Bélombé for fruitful discussions.
Cite this paper
Clémence EbogaTanke,Bridget N.Ndosiri,Yves A.Mbiangué,GouetBebga,JustinNenwa, (2016) Synthesis, Characterization and X-Ray Structure of a Ba(II)/Ag(I)/Cr(III)-Oxalate Salt with Water-Filled Nanochannels. American Journal of Analytical Chemistry,07,99-106. doi: 10.4236/ajac.2016.71010
References
- 1. Cheetham, A.K., Férey, G. and Loiseau, T. (1999) Open Framework Inorganic Materials. Angewandte Chemie International Edition, 38, 3268-3292.
http://dx.doi.org/10.1002/(SICI)1521-3773(19991115)38:22<3268::AID-ANIE3268>3.0.CO;2-U - 2. Lee, J.Y., Farha, O.K., Roberts, J., Scheidt, K.A., Nguyen, S.T. and Hupp, J.T. (2009) Metal-Organic Framework Materials as Catalysts. Chemistry Society Reviews, 38, 1450-1459.
http://dx.doi.org/10.1039/b807080f - 3. Zhang, X.M., Wang, Y.Q., Wang, K., Gao, E.Q. and Liu, C.M. (2011) Metamagnetism and Slow Magnetic Dynamics in an Antiferromagnet Composed of Cobalt(II) Chains with Mixed Azide-Carboxylate Bridges. Chemistry Communications, 47, 1815-1817.
http://dx.doi.org/10.1039/C0CC04492J - 4. Coe, B.J., Foxon, S.P., Harper, E.C., Helliwell, M., Raftery, J., Swanson, C.A., Brunschwig, B.S., Clays, K., Franz, E., Garin, J., Orduna, J., Horton, P.N. and Hursthouse, M.B. (2010) Evolution of Linear Absorption and Nonlinear Optical Properties in V-Shaped Ruthenium(II)-Based Chromophores, Journal of the American Chemical Society, 132, 1706-1723.
http://dx.doi.org/10.1021/ja908667p - 5. Yaghi, O.M., O’Keeffe, M., Ockwig, N.W., Chae, H.K., Eddaoudi, M. and Kim, J. (2003) Reticular Synthesis and the Design of New Materials. Nature, 423, 705-714.
http://dx.doi.org/10.1038/nature01650 - 6. Lim, C.S., Schnobrich, J.K., Wong-Foy, A.G. and Matzger, A.J. (2010) Metal-Dependent Phase Selection in Coordination Polymers Derived from a C2v-Symmetric Tricarboxylate. Inorganic Chemistry, 49, 5271-5275.
http://dx.doi.org/10.1021/ic100378p - 7. Dean, P.A.W., Craig, D., Dance, I., Russell, V. and Scudder, M. (2004) A Variable Ag-Cr-Oxalate Channel Lattice: [MxAg0.5-x(H2O)3]@[Ag2.5Cr(C2O4)3], M = K, Cs, Ag. Inorganic Chemistry, 43, 443-449.
http://dx.doi.org/10.1021/ic034988k - 8. Bélombé, M.M., Nenwa, J., Mbiangué, Y.A., Gouet, B., Majoumo, F., Hey-Hawkins, E. and Lonnecke, P. (2009) Water-Filled Pseudo-Nanotubes in Ag11.60H0.40[Cr(C2O4)3]4·15H2O: Synthesis, Characterization and X-Ray Structure. Inorganica Chimica Acta, 362, 1-4.
http://dx.doi.org/10.1016/j.ica.2007.03.003 - 9. Nenwa, J., Gouet, B., Djonwouo, P.L. and Fokwa, B.P.T. (2012) Synthesis, X-Ray Structure and Spectroscopic Characterization of a Non-Stoichiometric Nanostructured Silver Salt Hosting Water-Guest Molecules. Research Journal of Chemistry and Environment, 16, 111-115.
- 10. Gouet, B., Signé, M., Nenwa, J. and Fokwa, B.P.T. (2013) Synthesis, Crystal Structure and Spectroscopic Characterization of an Oxalato Bridged Silver-Deficient Chromium(III) Salt with Water-Filled Nanochannels. Research Journal of Chemistry and Environment, 17, 57-63.
- 11. Bélombé, M.M., Nenwa, J., Tene, O.T. and Fokwa, B.P.T. (2010) Synthesis, X-Ray Structure and Thermal Behavior of Isomorphous Silver-Deficient Channel Lattice Frameworks with General Formula
[(Ag0.25/M0.25)(H2O)]@[Ag2M(C2O4)3]·4H2O (M = CoIII, CrIII). Global Journal of Inorganic Chemistry, 1, 34-41. - 12. Bailar Jr, J.C. and Jones, E.M. (1939) Trioxalato Salts. Inorganic Syntheses, 1, 35-38.
http://dx.doi.org/10.1002/9780470132326.ch13 - 13. SMART & SAINT (2003) Software Reference Manual, Version 6.45. Bruker Analytical X-Ray Systems, Inc., Madison, USA.
- 14. Siemens Inc., SAINT (1997) Area-Detector Integration Software, Version 6.01, Siemens. Industrial automation, Inc., Madison, Wisconsin, USA.
- 15. Sheldrick, G.M. (2010) SADABS, Program for Empirical Absorption Correction of Area Detector Data. University of Gottingen, Gottingen, Germany.
- 16. Sheldrick, G.M. (2008) A Short History of SHELX. Acta Crystallographica Section A, 64, 112-122.
http://dx.doi.org/10.1107/S0108767307043930 - 17. Bélombé, M.M., Nenwa, J., Mbiangué, Y.A., Evina-Nnanga, G., Mbomékallé, I.M., Hey-Hawkins, E., Lonnecke, P. and Majoumo, F. (2003) Unusual Aquation of Ba2+ Ions in the Solid State: Synthesis and X-Ray Structural and Spectroscopic Characterization of the Novel Polymeric Complex Salt of Empirical Formula {Ba6(H2O)17[Cr(ox)3]4}·7H2O (ox = Oxalate Dianion). Dalton Transactions, 2117-2118.
http://dx.doi.org/10.1039/b302489j - 18. Mbiangué, Y.A., Nenwa, J., Bélombé, M.M., Ngoune, J. and álvarez, E. (2012) Hydrogen-Bonded Pillars of Alternating Chiral Complex Cations and Anions: 2. Synthesis, Characterization and X-Ray Structure of Isomorphous
catena-{(H3O)[K(H2O)3]@[Ni(H2oxado)3]2[Cr(C2O4)3]2·3H2O} and
catena-{(H3O)[Li(H2O)3]@[Ni(H2oxado)3]2[Cr(C2O4)3]2·3H2O}. ScienceJet, 1, 1-9.
NOTES

*Corresponding author.







