Journal of Biosciences and Medicines
Vol.05 No.03(2017), Article ID:74902,14 pages
10.4236/jbm.2017.53014
Inhibition of the Na+/Ca2+ Exchanger NCX1 Expressed in Xenopus Oocyte by Glycyrrhizic Acid and Cyclophylin A
Jan Laudenbach1,2,3, Yu Wang1,4, Beibei Xing1,5, Silvia Schwarz1,5, Yinfang Xu1,5, Quanbao Gu1, Wolfgang Schwarz1,2,3,5*
1Shanghai Research Center for Acupuncture & Meridians, Shanghai, China
2Max-Planck-Institute for Biophysics and Institute for Biophysics, Frankfurt am Main, Germany
3Institute for Biophysics, Goethe University, Frankfurt am Main, Germany
4Shanghai Research Institute of Acupuncture & Moxibustion and Meridian, Shanghai, China
5Shanghai Key Laboratory for Acupuncture Mechanism and Acupoint Function, Shanghai, China

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 23, 2016; Accepted: March 24, 2017; Published: March 27, 2017
ABSTRACT
The Na+/Ca2+ exchanger plays an important role in regulation of airway smooth muscle contraction by regulating intracellular calcium, and is a potential target for treatment of asthma. To test modulation of exchanger activity, we used Xenopus oocytes as model system. Na+/Ca2+ exchanger was expressed in the cells by microinjection of cRNA of the exchanger isoform NCX1. The activity of NCX1 was determined as Ni2+-sensitive current under voltage clamp in low Cl− medium and in the presence of the Cl−-channel inhibitor niflumic acid. Only this composition of solution allowed determining NCX1-mediated current with sufficient accuracy. Among a few tested Chinese herbal drugs, glycyrrhizic acid turned out to be a potent inhibitor of NCX1 with an apparent IC50 value of 40 μM. Previous work had revealed elevated cyclophylin A concentration in serum of asthmatic rats after receiving acupuncture treatment. Extracellular incubation of the oocytes in cyclophylin A for one day led to significant inhibition with an apparent IC50 value of about 1 μM. We suggest that effects of acupuncture and application of glycyrrhizic acid as an active constituent of Chinese medicine for treatment of asthma symptoms may partially be attributed to inhibition of the reversed mode of NCX1 and that these compounds may stimulate the search for new anti-asthmatic drugs.
Keywords:
Sodium-Calcium Exchanger, Asthma, Voltage Clamp, Glycyrrhizic Acid, Cyclophylin A

1. Introduction
Asthma is a disease characterised by reversible contraction of airway smooth muscle (ASM). Several signalling pathways are now known to be related to the process of ASM contraction, and almost all of them involve Ca2+ handling [1] . Cytoplasmic Ca2+ activity (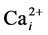 ) homeostasis is controlled by several ionic signalling mechanisms, one of which is the reversed mode of the Na+/Ca2+ exchanger (NCX) of the plasma membrane [2] [3] . Although little is currently known about NCX in the airways and its involvement in asthma, some investigators have shown that expression and function of the isoform 1 of the exchanger (named NCX1) in ASM is up-regulated in asthmatic animals and inhibition of NCX may ameliorate the symptoms of asthma. This observation makes NCX a potential target for asthma treatment [2] [4] . The aim of the present study was to examine effects of various chemicals that might act as inhibitors of NCX.
) homeostasis is controlled by several ionic signalling mechanisms, one of which is the reversed mode of the Na+/Ca2+ exchanger (NCX) of the plasma membrane [2] [3] . Although little is currently known about NCX in the airways and its involvement in asthma, some investigators have shown that expression and function of the isoform 1 of the exchanger (named NCX1) in ASM is up-regulated in asthmatic animals and inhibition of NCX may ameliorate the symptoms of asthma. This observation makes NCX a potential target for asthma treatment [2] [4] . The aim of the present study was to examine effects of various chemicals that might act as inhibitors of NCX.
In traditional medicine, herbal extracts are often applied in treatment of asthma, and the search for natural components has become promising to discover new anti-asthmatic drugs. Extracts of roots of licorice (Glycyrrhiza glabra) have been applied in treatment of a large variety of diseases (see e.g. [5] ). The triterpene glycoside glycyrrhizic acid (GA) is one of the major active constituents of licorice:

GA has been used as a hepatoprotective drug [5] , and recent studies also revealed its anti-asthmatic effects [6] [7] . Though modulation of various pathways has been discussed (see [8] [9] ), it is unclear whether GA can interfere with NCX1, and hence exert its anti-asthmatic effects.
In a recent investigation on rats, we found significantly elevated cyclophilin A (CyPA) level in the serum of acupuncture-treated asthmatic rats compare to that of untreated rats [10] . CyPA is a member of the cyclophilin (CyP) family, which possesses peptidyl-prolyl isomerase (PPIase) activity. CyPs are involved in diverse cellular processes including cell-cycle regulation, receptor signalling, protein folding, and they form cellular targets for immune-suppressant drugs such as cyclosporine A (CsA) [11] . CyPA has multiple intracellular functions (see e.g. [12] [13] ), but can also be secreted [14] and act extracellularly as an inflammatory mediator that may be involved in inflammatory diseases such as atherosclerosis [15] [16] and rheumatoid arthritis [17] . In our investigation we consider extracellular CyPA as a drug for treatment of asthma by inhibiting NCX, which might be a molecular mechanism of asthma therapy by acupuncture. In this study, we choose the human cyclophilin A (hCyPA), one of 7 major cyclophilins in humans [18] .
To monitor changes of transport activity of the exchanger, we used the Xeno- pus oocyte for heterologous expression of NCX1. NCX operates at a 3:1 or 4:1 Na+:Ca2+ stoichiometry [19] [20] , the transporter is electrogenic, therefore, the activities can be monitored by measuring current using two-electrode voltage clamp (TEVC). Since the oocytes have functionally expressed only a limited number of endogenous membrane proteins, the application of this model system allows investigating effects on NCX with low background signals and restricted functional interference from other membrane proteins.
2. Materials and Methods
2.1. NCX1-cRNA and hCyPA Preparation
The construct with NCX1 of dog was kindly provided by Dr. Luis Beauge (Laboratorio de Biophisica, Cordoba, Argentina) and linearised with XbaI, then transcribed into cRNA in vitro using mMESSAGEmMACHINESP6 kit (Ambion, USA). The final concentration of cRNA was adjusted to 0.2 ng/nL.
The plasmid pQE30-CyPA was kindly provided by Dr. Xu Sheng (Drug Discovery and Design Center and State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences). Expression and purification of the hCyPA protein was performed as described elsewhere [21] [22] . Gels and buffers used for native PAGE were made according to the standard Laemmli SDS protocol omitting the SDS. Native gels (12% polyacrylamide) were run at 12 mA and 45 min and stained with Coomassie Brilliant Blue R-250. The molecular weight of hCyPA was slightly less than 20 kDa (compare Figure 1) in line with the reported weight of 18 kDa. Unstained protein-molecular-weight marker was from Fermentas Life Science (USA).
2.2. Xenopus Oocytes Preparation and Microinjection
Xenopus oocytes were used as expression for NCX1 and as a model system to test the effects of herbal extracts and CyPA. This expression system is particularly suited because endogenous ion channels and transporters are functionally expressed only to a low extend, and hence, exogenous current components can easily be extracted. Females of the clawed toad Xenopus laevis (purchased from Maosheng Bio-Technology Com., Shanghai, China) were anaesthetised in a bath medium containing 1 g/L tricaine (Sandoz, Basel, Switzerland) and kept on ice. Parts of ovary were removed and treated with 0.5 or 0.25 mg/mL collagenase (Sigma) for 2 - 4 h, or overnight, respectively. Full-grown prophase-arrested
Figure 1. SDS-PAGE analysis of hCyPA. Lane 1: hCyPA- antibody marked band at slightly less than 20 kDa. Lane 2: marker.
oocytes of Dumont stages V and VI [23] were selected for cRNA injection, and cultured with daily changed G-ORi solution or C-ORi solution (ORi plus 0.07 μg/mL gentamycin (Sigma) or ciprofloxacin hydrochloride (Sigma), respectively) at 20˚C. For expression of NCX1 about 1.5 ng NCX1-cRNA was microinjected into an ooctye at a flow-rate of 8 nL/s. Uninjected oocytes served as controls.
For investigating the effect of intracellular hCyPA, oocytes were first microinjected with NCX1-cRNA and divided into 4 groups. The cells of each group were additionally microinjected with hCyPA (5, 10, 20 or 40 nl per oocytes, respectively, at a concentration of 4.27 µg/µL). For investigating extracellular effect of hCyPA, oocytes were first microinjected with NCX1-cRNA and divided into 4 groups. The cells of each group were then incubated with different amounts of hCyPA; 200 µL of incubation medium contained 1, 2, 4 or 8 µL of hCyPA, respectively (4.27 µg/µL). Thereafter, oocytes were cultured in G-ORi or C-ORi at 20˚C for up to 2 days.
2.3. Electrophysiological Recording
Since NCX is electrogenic, NCX-mediated current is a measure for transporter activities. To investigate the function of NCX1, membrane currents were measured by conventional two-electrode voltage clamp (TEVC) using Turbo TEC-03 with Cell Works software (NPI electronic, Tamm, Germany). Glass microelectrodes were filled with
2.4. Solutions and Drugs
The composition of ORi was (in mM): 90 NaCl, 2 KCl, 2 CaCl2 and 5 MOPS (adjusted to pH 7.4 with Tris). To elevate intracellular Na+, cells were incubated for 30 min in “Na-loading solution” consisting of (in mM): 110 NaCl, 2.5 Na- citrate, 5 MOPS (adjusted to pH 7.4 with Tris) and stored thereafter for at least another 30 min in “Post-loading solution” consisting of (in mM): 100 NaCl, 5 BaCl2, 20 TEA, 5 MOPS (adjusted to pH 7.4 with Tris). Standard test solutions contained (in mM): 100 Na-gluconate, 2 CaCl2, 0.1 niflumic acid, 5 MOPS, and 0 or
Herbal extracts were kindly provided from Shanghai Institute Materia Medica (CAS) by Drs. CG Huang and CH Ma (supercritical fluid extraction of root of Acorus tatainowii Schott) and by Dr. LJ Xuan (dried ethanol extracts of Ilex pubescence and Gossampinus malabarica), and were dissolved in DMSO. Final concentration of the herbal extracts in test solution was 40 mg/L.
GA (CAS 1405-86-3, purity ≥95%) and α-asarone (CAS 2883-98-9, purity 98%) were purchased from SIGMA. Stock solutions of 1 or 100 mM were prepared in DMSO and diluted to the final concentration in the test solution. DMSO concentrations in all test solutions were below 1%, which was without effect on the membrane currents.
2.5. Data Analysis
Analysed data were represented as means (±SEM) from N experiments. Means were considered as significantly different by Student’s t test on the basis of p < 0.05.
3. Results
The Na+/Ca2+ exchanger is considered to transport 3 or 4 Na+ against 1 Ca2+, and hence generating in its forward mode (Ca2+ extrusion) an inward-directed current, and in its reversed mode (Ca2+ uptake) an outward-directed current (for a review see [25] ). To determine this current, we used
3.1. Ni2+-Sensitive Currents Represent only in Part NCX1-Mediated Current
In control oocytes not injected with cRNA of NCX1 Ni2+-sensitive could never be detected (Figure 2(a)), confirming that the cells do not express significant endogenous Na+/Ca2+ exchanger on the plasma membrane [26] . On the contrary,
Figure 2. Ni2+-sensitive current in un-injected and NCX1- cRNA-injected oocytes. (a) Only injected oocytes show Ni2+- sensitive current. (b) Addition of 100 nM niflumic acid strongly blocked the outward-directed current component, while inward current became enhanced. (c) Reduction of external Cl− from 100 to
oocytes being injected with cRNA of NCX1 exhibited huge currents, in particular at positive potentials in outward direction. Interestingly, the NCX-dependent current could even exceed 10 µA, which can hardly be mediated by a carrier protein even at high density in the cell membrane. Nevertheless, such large currents had been considered to be mediated by NCX (see e.g. [27] ). On the other hand, Xenopus oocytes exhibit Ca2+-activated Cl− current (see [28] ). Therefore, an alternative interpretation would be that the transporter operates at these potentials in reversed mode accumulating Ca2+ at the intracellular membrane surface. This accumulated Ca2+ would activate the Cl− channels. After blocking NCX1 by Ni2+ also the Cl− channels will no longer be activated.
To reduce this Ca2+-dependent background current, we used as an inhibitor of the Cl− channels 100 nM niflumic acid [29] in the test solutions, which indeed led to a considerable reduction of the Ni2+-sensitive outward-directed current (Figure 2(b)); in addition the inward-directed current was enhanced. Figure 2(c) illustrates that reduction of external Cl− from
3.2. Drug Effects on NCX1-Dependent Current
For a first rough screening we looked for drug effects on total Ni2+-sensitive current. To determine this current, we used standard external oocyte-Ringer’s-like solution (ORi) in the absence of niflumic acid without and with 2 mM NiCl2. The extracts of Ilex and Gossampinus showed slight, but statistically significant inhibition at 40 mg/L by about 15% or 25%, respectively; the Acorus extract showed slight stimulation by about 15% of the Ni2+-sensitive outwardly directed current at +10 mV (Table 1). We also tested several pure compounds in addition to the Acorus extract α-asarone, which showed slight inhibition by 10% (Table 1). Out of several tested drugs only the GA from Glycyrrhiza galabra exhibited significant and clear inhibition by about 60% at 40 mg/L (Table 1). In the following, therefore, our focus was on the effect of GA. Since the scatter of current measurements under voltage clamp to positive potentials in general is pretty large, we will concentrate on the analysis at negative potentials.
3.3. GA Inhibits NCX1-Mediated Current
In the standard experiment, membrane currents were measured in different solutions with low Cl− and 100 nM niflumic acid that were applied usually in the sequence:
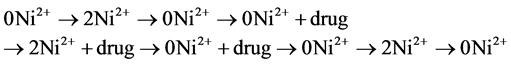
The currents measured in the respective 0Ni2+ solution before and after the
Table 1. Effect of selected drugs on Ni2+-sensitve current measured under voltage clamp at +10 mV. Data represent averages of N measurements (±SEM). p values refer to difference to 1 (one-sample t-test).
application of 2 mM Ni2+ were averaged to partially compensate for small drift with time. The currents in 2 mM Ni2+ were then subtracted to obtain the NCX1- mediated current. In stable experiments, the  sequence could be repeated with another drug concentration. In some experiments an abbreviated protocol was applied with the solution sequence
sequence could be repeated with another drug concentration. In some experiments an abbreviated protocol was applied with the solution sequence
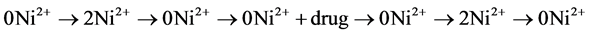
The result of the effect of 20 μM GA on the current-voltage dependence of NCX-mediated current is illustrated in Figure 3(a) showing a significant inhibition of the activity of the exchanger over the entire potential range. A more detailed analysis of the concentration dependency yielded an IC50 value of about 40 μM at −100 mV (Figure 3(b)). The inhibition did not significantly depend on membrane potential.
3.4. The Effect of hCyPA on NCX-Mediated Current
Microinjection of hCyPA up to 86 ng/µL (corresponding to 4.8 µM within the cytoplasm, calculated by assuming an oocyte volume of 1 µL) hardly affected the NCX1-mediated current (Figure 4(a)). Interestingly, extremely high concentration (9.6 µM) obviously stimulated the current by about 30%. Despite the large error bars, the current increase is statistically significant.
Incubation of NCX1-expressing oocytes in 1.2 µM hCyTA resulted in significant inhibition of NCX-mediated current (p < 0.05 compared to untreated cells); higher concentration 4.8 µM produced only insignificantly more inhibition (Figure 4(b)). Despite the considerable scatter of data an IC50 value for 50% inhibition of less than 1 µM could be estimated (see inset of Figure 4(b)). For the experiments described above, the oocytes were incubated in the respective hCyPA solution for 2 days. One hour of incubation already showed some tendency of inhibition (about 10%), but only after one day maximum inhibition could be detected. From 2 batches of oocytes we found that the current at −100 mV was after 1 h of incubation reduced to 0.87 ± 0.16 and after one day to 0.34 ±
Figure 3. Inhibition of NCX-mediated current by GA. (a) Effect of 20 μM GA on current-voltage denendency. The data are normalised to the current at −60 mV in the absence of drug, and represent averages of N = 12 oocytes (±SEM). (b) Dependence of NCX-medi- ated current at −100 mV on GA concentration. Data represent averages of N = 5 to 12 oocytes (±SEM). The solid line is a fit of 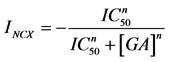 to the data with IC50 = 40 μM (n = 0.5). INCX is the normalised current at the respective drug concentration [GA].
to the data with IC50 = 40 μM (n = 0.5). INCX is the normalised current at the respective drug concentration [GA].
0.06 compared to untreated cells.
4. Discussion
Asthma is a reversible airway restriction based on ASM contraction, which is governed by intracellular calcium. A key role plays the release of Ca2+ from intracellular stores, and the refilling of the stores involves the reversed mode of NCX (for a brief review see [3] ). The aim of the present study was to examine effects of various drugs that inhibit the NCX, and hence might act as antiasthmatic drugs. We used Xenopus oocytes with heterologously expressed NCX1 as a
Figure 4. The effect of hCyPA on NCX1-mediated current. (a) Effect of intracellular hCyPA. Oocytes injected with NCX1- cRNA were additionally microinjected with hCyPA to gain the respective intracellular concentration. Data represent averages of N = 5 to 11 oocytes ± SEM; (b) The effect of extracellular hCyPA. Oocytes were incubated in the respective amounts of hCyPA for two days. The inset shows the dependence of NCX1- mediated normalised current at −100 mV on hCyPA concentration. Data represent averages of N = 7 to 10 oocytes ± SEM. The inhibition of current at all concentrations is significant on the basis of p < 0.05.
model system, and Ni2+ as specific inhibitor of the exchanger. Effects on NCX1- mediated current could only be investigated as Ni2+-sensitive current in the presence of niflumic acid to block Ca2+-activated Cl−-currents and at lowered external Cl− activity. Otherwise the Ni2+-dependent current also included a large component of Ca2+-activated Cl− current (compare [27] ). Since the oocytes have functionally expressed only a limited number of endogenous membrane proteins, the application of this model system allows investigating effects on NCX with low background signals and restricted interference with other membrane proteins.
Our drug screening revealed that GA is a potent inhibitor of NCX1. GA has particularly been used in the treatment of liver diseases [5] , but also seems to have anti-asthmatic effects [6] [7] . Since inhibition of NCX1 in ASM will lead to reduced Ca2+ influx and reduced refilling of the intracellular Ca2+ stores, reduced muscle tone can be expected [2] [3] [4] . Though our measurements with GA were performed on the forward mode of NCX1, the screening experiments with effects on the outward-directed current at +10 mV indicate that also the reversed mode can be inhibited.
Several lines of evidence implicate that intracellular CyPA plays a critical multifunctional role, and interaction with cyclosporine A (CsA) has been shown to be an important step [18] [30] . Our functional analysis revealed that intracellular CyPA also affected the NCX1 protein, and up-regulated NCX1-mediated current was observed though only at very high concentration of about 10 μM. The dissociation constant of CyPA from CsA is in the submicromolar range [31] . The much higher concentration in the micromolar range for intracellular stimulation of NCX1 by CyPA, therefore, makes physiological relevance unlikely. On the other hand, Ca2+ uptake experiments with HEK cells co-transfected with NCX1 and CyPA suggest involvement of CyPA in the regulation of NCX1 expression and transport activity [32] .
In fact CyPA can be secreted [16] via a vesicular pathway [14] . It had been demonstrated previously that acupuncture treatment on asthmatic rats can reduce airway restrictions [33] , and this was associated with the elevation of CyPA in the serum [10] . Our results suggest that extracellular hCyPA down-regulates NCX1-mediated current, which could account for release of the airway restrictions.
The involvement of extracellular CyPA in allergic lung inflammation had been suggested on the basis of the anti-inflammatory effect of an extracellularly applied membrane-impermeable CsA derivative [34] . Whether the effect of hCyPA found in our experiments results from direct interaction with the NCX protein needs further investigation. Our finding that the extracellular inhibition needed several hours of incubation is in favour of an indirect effect.
5. Conclusion
In conclusion, our data suggest that GA and acupuncture-induced elevation of hCyPA in the serum may both contribute via inhibition of reversed NCX to reduced refilling of 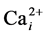 stores, which can promote reduced contraction of ASM, reduced airway restriction, and thus relieve the asthmatic symptoms. Such effects need to be verified in future animal experiments. Nevertheless, derivatives of GA and CyPA may form the basis for development of a new generation of more potent drugs for asthma therapy.
stores, which can promote reduced contraction of ASM, reduced airway restriction, and thus relieve the asthmatic symptoms. Such effects need to be verified in future animal experiments. Nevertheless, derivatives of GA and CyPA may form the basis for development of a new generation of more potent drugs for asthma therapy.
Acknowledgements
We are very grateful to Drs. Luis Beauge and Sheng Xu for providing the plasmids for NCX1 and hCyPA, respectively, and to Drs. CG Huang, CH Ma, and LJ Xuan for the extracted herbal drugs. The excellent technical assistance from Heike Biehl, Guohui Chen, Huiming Du and Heike Fotis is gratefully acknowledged. This work was supported by the National Basic Research Development Program of China (No. 2012CB518502), Shanghai Key Laboratory of Acupuncture Mechanism and Acupoint Function (14DZ2260500), National Natural Science Funds of China (No. 30701123 to YW, No. 81403489 to YFX).
Cite this paper
Laudenbach, J., Wang, Y., Xing, B.B., Schwarz, S., Xu, Y.F., Gu, Q.B. and Schwarz, W. (2017) Inhibition of the Na+/Ca2+ Exchanger NCX1 Expressed in Xenopus Oocyte by Glycyrrhizic Acid and Cyclophylin A. Journal of Bio- sciences and Medicines, 5, 128-141. https://doi.org/10.4236/jbm.2017.53014
References
- 1. Janssen, L.J. and Killian, K. (2006) Airway Smooth Muscle as a Target of Asthma Therapy: History and New Directions. Respiratory Research, 7, 123.
https://doi.org/10.1186/1465-9921-7-123 - 2. Hirota, S., Pertens, E. and Janssen, L.J. (2007) The Reverse Mode of the Na+/Ca2+ Exchanger Provides a Source of Ca2+ for Store Refilling Agonist-Induced Ca2+ Mobilization. American Journal of Physiology Lung Cellular Molecular Physiology, 292, L438-L447.
https://doi.org/10.1152/ajplung.00222.2006 - 3. Janssen, L.J. (2009) Asthma Therapy: How Far Have We Come, Why Did We Fail and Where Should We Go Next. European Respiratory Journal, 33, 11-20.
https://doi.org/10.1183/09031936.00068508 - 4. Hirota, S. and Janssen, L.J. (2007) Store-Refilling Involves Both L-Type Calcium Channels and Reverse-Mode Sodium-Calcium Exchange in Airway Smooth Muscle. European Respiratory Journal, 30, 269-278.
https://doi.org/10.1183/09031936.00008507 - 5. Li, J.Y., Cao, H.Y., Liu, P., Cheng, G.H. and Sun, M.Y. (2014) Glycyrrhizic Acid in the Treatment of Liver Diseases: Literature Review. Glycyrrhizic Acid in the Treatment of Liver Diseases: Literature Review, 2014, Article ID: 872139.
- 6. Ma, C., Ma, Z., Liao, X.L., Liu, J., Fu, Q. and Ma, S. (2013) Immunoregulatory Effects of Glycyrrhizic Acid Exerts Anti-Asthmatic Effects via Modulation of Th1/Th2 Cytokines and Enhancement of CD4(+) CD25(+) Foxp3+ Regulatory T Cells in Ovalbumin-Sensitized Mice. Journal of Ethnopharmacology, 148, 755-762.
- 7. Ram, A., Mabalirajan, U., Das, M., Bhattacharya, I., Dinda, A.K., Gangal, S.V. and Ghosh, B. (2006) Glycyrrhizin Alleviates Experimental Allergic Asthma in Mice. International Immunopharmacology, 6, 1468-1477.
- 8. Kao, T.C., Wu, C.H. and Yen, G.C. (2013) Glycyrrhizic Acid and 18a-Glycyrrhetinic Acid Recover Glucocorticoid Resistance via PI3K-Induced AP1, CRE and NFAT Activation. Phytomedicine, 20, 295-302.
- 9. Wu, Q., Tang, Y., Hu, X., Wang, Q., Lei, W., Zhou, L. and Huang, J. (2016) Regulation of Th1/Th2 Balance through OX40/OX40L Signalling by Glycyrrhizic Acid in a Murine Model of Asthma. Respirology, 21, 102-111.
https://doi.org/10.1111/resp.12655 - 10. Wang, Y., Cui, J.M., Ma, S.L., Liu, Y.Y., Yin, L.M. and Yang, Y.Q. (2009) Proteomics Analysis of Component in Serum with Anti-Asthma Activity Derived from Rats Treated by Acupuncture. Journal of Acupuncture and Tuina Science, 7, 326-331.
https://doi.org/10.1007/s11726-009-0326-y - 11. Göthel, S.F. and Marahiel, M.A. (1999) Peptidy-Propyl Cis-Trans Isomerases, a Superfaliy of Ubiquitous Folding Catalysts. Cellular and Molecular Life Sciences, 55, 423-436.
https://doi.org/10.1007/s000180050299 - 12. Liu, J., Farmer, J.D., Lane, W.S., Frieman, J. and Schreiber, S.L. (1991) Calcineurin Is a Common Target of Cyclophilin-Cyclosporin A and FKBP-FK506 Complexes. Cell, 66, 807-815.
- 13. Bukrinsky, M.I. (2002) Cyclophilins: Unexpected Messengers in Intercellular Communications. Trends in Immunology, 23, 323-324.
- 14. Suzuki, J., Jin, Z.G., Meoli, D.F., Matoba, T. and Berk, B.C. (2006) Cyclophilin A Is Secreted by a Vesicular Pathway in Vascular Smooth Muscle Cells. Circulation Research, 98, 811-817.
https://doi.org/10.1161/01.RES.0000216405.85080.a6 - 15. Jin, Z.G., Lungu, A.O., Xie, L., Wang, M., Wong, C. and Berk, B.C. (2004) Cyclophilin A Is a Proinflammatory Cytokine That Activates Endothelial Cells. Arteriosclerosis Thrombosis and Vascular Biology, 24, 1186-1191.
https://doi.org/10.1161/01.ATV.0000130664.51010.28 - 16. Jin, Z.G., Melaragno, M.G., Liao, D.F., Yan, C., Haendeler, J., Suh, Y.A., Lambeth, D. and Berk, B.C. (2000) Cyclophilin A Is a Secreted Growth Factor Induced by Oxidative Stress. Circulation Research, 87, 789-796.
https://doi.org/10.1161/01.RES.87.9.789 - 17. Arora, K., Gwinn, W.M., Bower, M.A., Watson, A., Okwumabua, I., MacDonald, H.R., Bukrinsky, M.I. and Constant, S.L. (2005) Extracellular Cyclophilins Contribute to the Regulation of Inflammatory Responses. The Journal of Immunology, 175, 517-522.
https://doi.org/10.4049/jimmunol.175.1.517 - 18. Wang, P. and Heitman, J. (2005) Protein Family Review: The Cyclophilins. Genome Biology, 6, 226.
https://doi.org/10.1186/gb-2005-6-7-226 - 19. Dong, H., Dunn, J. and Lytton, J. (2002) Stoichiometry of the Cardiac Na+/Ca2+ Exchanger NCX1.1 Measured in Transfected HEK Cells. Biophysical Journal, 82, 1943-1952.
- 20. Bers, D.M. and Ginsburg, K.S. (2007) Na: Ca Stoichiometry and Cytosolic Ca-Dependent Activation of NCX in Intact Cardiomyocytes. Annals of the New York Academy of Sciences, 1099, 326-338.
https://doi.org/10.1196/annals.1387.060 - 21. Luo, C., Luo, H., Zheng, S., Gui, C., Yue, L., Yu, C., Sun, T., He, P., Chen, J., Shen, J., Luo, X., Li, Y., Liu, H., Bai, D., Shen, J., Yang, Y., Li, F., Zuo, J., Hilgenfeld, R., Pei, G., Chen, K., Shen, X. and Jiang, H. (2004) Nucleocapsid Protein of SARS Corona Virus Tightly Binds to Human Cyclophilin A. Biochemical and Biophysical Research Communications, 321, 557-565.
- 22. Chen, Z., Mi, L., Xu, J., Yu, J., Wang, X., Jiang, J., Xing, J., Shang, P., Qian, A., Li, Y., Shaw, P.X., Wang, J., Duan, S., Ding, J., Fan, C., Zhang, Y., Yang, Y., Yu, X., Feng, Q., Li, B., Yao, X, Zhang, Z., Xue, X. and Zhu, P. (2005) Function of HAb18G/CD 147 in Invasion of Host Cells by Severe Acute Respiratory Syndrome Coronavirus. The Journal of Infectious Diseases, 191, 755-760.
https://doi.org/10.1086/427811 - 23. Dumont, J.N. (1972) Oogenesis in Xenopus Laevis (Daudin): I. Stages of Oocyte Development in Laboratory Maintained Animals. Journal of Morphology, 136, 153-180.
https://doi.org/10.1002/jmor.1051360203 - 24. Rakowski, R.F., Vasilets, L.A., LaTona, J. and Schwarz, W. (1991) A Negative Slope in the Current-Voltage Relationship of the Na+/K+ Pump in Xenopus Oocytes Produced by Reduction of External [K+]. The Journal of Membrane Biology, 121, 177-187.
https://doi.org/10.1007/BF01870531 - 25. Dipolo, R. and Beauge, L. (2006) Sodium/Calcium Exchanger: Influence of Metabolic Regulation on Ion Carrier Interactions. Physiological Reviews, 86, 155-203.
https://doi.org/10.1152/physrev.00018.2005 - 26. Supplisson, S., Kado, R.T. and Bergman, C. (1991) A Possible Na/Ca Exchange in the Follicle Cells of Xenopus Oocyte. Developmental Biology, 145, 231-240.
- 27. Ruknudin, A., He, S., Lederer, W.J. and Schulze, D.H. (2000) Functional Differences between Cardiac and Renal Isoforms of the Rat Na+-Ca2+ Exchanger NCX1 Expressed in Xenopus Oocytes. The Journal of Physiology, 529, 599-610.
https://doi.org/10.1111/j.1469-7793.2000.00599.x - 28. Weber, W.M. (1999) Ion Currents of Xenopus Laevis Oocytes: State of the Art. Biochimica et Biophysica Acta, 1421, 213-233.
- 29. White, M.M. and Aylwin, M. (1990) Niflumic and Flufenamic Acids Are Potent Reversible Blockers of Ca-2+-Activated Cl-Channels in Xenopus Oocytes. Molecular Pharmacology, 37, 720-724.
- 30. Satoh, K., Shimokawa, H. and Berk, B.C. (2010) Cyclophilin A—Promising New Target in Cardiovascular Therapy .Circulation Journal, 74, 2249-2256.
- 31. Handschumacher, R.E., Harding, M.W., Rice, J., Drugge, R.J. and Speicher, D.W. (1984) Cyclophilin: A Specific Cytosolic Binding Protein for Cyclosporin A. Science, 226, 544-547.
https://doi.org/10.1126/science.6238408 - 32. Rahamimoff, H., Elbaz, B., Kimchi-Sarfaty, C., Gottesman, M.M., Lichtenstein, Y., Eskin-Shwartz, M. and Kasir, J. (2007) Cyclosporin A-Dependent Downregulation of the Na+/Ca2+ Exchanger Expression. Annals of the New York Academy of Sciences, 1099, 204-214.
https://doi.org/10.1196/annals.1387.046 - 33. Xu, Y.D., Cui, J.M., Wang, Y., Yin, L.M., Gao, C.K., Liu, Y.Y. and Yang, Y.Q. (2010) The Early Asthmatic Response Is Associated with Glycolysis, Calcium Binding and Mitochondria Activity as Revealed by Proteomic Analysis in Rats. Respiratory Research, 11, 107.
https://doi.org/10.1186/1465-9921-11-107 - 34. Balsley, M.A., Malesivic, M., Stemmy, E.J., Gigley, J., Jurjus, R.A., Herzog, D., Bukrinsky, M.I., Fischer, G. and Constant, S. (2010) A Cell-Impermeable Cylosporin a Derivative Reduces Pathology in a Mouse Model of Allergic Lung Inflammation. The Journal of Immunology, 185, 7663-7670.
https://doi.org/10.4049/jimmunol.1001707






