World Journal of Cardiovascular Diseases
Vol.3 No.2(2013), Article ID:30393,6 pages DOI:10.4236/wjcd.2013.32035
Safety and efficacy of Amplatzer duct occluder for percutaneous closure of ventricular septal defects with tunnel shape aneurysm: Medium term follow up
![]()
1Hamad General Hospital, Doha, Qatar
2Southampton University Hospital, Southampton, UK
Email: *dilawarmd@yahoo.com
Copyright © 2013 Muhammad Dilawar, Zaheer Ahmad. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 16 January 2013; revised 11 March 2013; accepted 15 April 2013
Keywords: Ventricular Septal Defect; Amplatzer Duct Occluder; Aneurysm
ABSTRACT
Objectives: Different devices including Amplatzer duct occluder has been used for percutaneous closure of ventricular septal defects. This study reports our medium term follow up of perimembranous and muscular ventricular septal defects with tunnel shape aneurysm closure using the Amplatzer duct occluder. Materials and Methods: From May 2006-December 2012, we used Amplatzer duct occluder in seven ventricular septal defect patients here at Hamad General Hospital, Doha, Qatar. There were 4 male and 3 female patients with an age range of 4 - 32 years with a median of 8 years and weight range of 16 - 63 kg with a median of 33 kg. In this group, 6 were perimembranous and 1 muscular and all these ventricular septal defects had a tunnel shape aneurysm. Transesophageal echocardiographic diameter ranged from 4 - 8 mm and Qp/Qs was 1 - 1.6. Angiographically, the diameter on the left ventricular side measured 3.5 - 10 mm and on right ventricular side 2.4 - 5 mm. 8/6 mm Amplatzer duct occluder was used to close these ventricular septal defects. Results: There were no major complications and immediately after the procedure there was no residual shunt in any of these patients and all the patients remained in normal sinus rhythm. One patient was expatriate and no further follow up was available. The rest of the 6 patients had 1 - 80 months with a median of 54 months follow up and none of these patients had any residual shunt and all remained in normal sinus rhythm. Two patients developed trivial aortic valve regurgitation immediate post procedure, one remained unchanged and the 2nd has progressed to mild at this latest follow up. Conclusion: Amplatzer duct occluder is feasible and a safe device for percutaneous closure of selective tunnel shape aneurysmal perimembranous and muscular ventricular septal defects.
1. INTRODUCTION
Ventricular septal defects (VSDs) are the commonest congenital cardiac malformation accounting for almost one fifth of all defects [1] and can be located in membranous or muscular septum. Eighty percent of these defects are perimembranous involving membranous and adjacent muscular septum. Acquired ventricular septal defects are rare and can be seen after myocardial infarction or as residual defects subsequent to surgical closure [2]. In 1954, Lillehei and associates performed the first surgical closure of a congenital ventricular septal defect [3]. Since then, surgical closure has been regarded as the gold standard treatment, albeit it remains associated with morbidity and mortality, postoperative discomfort and the need for sternotomy and a residual scar [4,5]. Because of the complications associated with surgical closure, percutaneous closure has been advocated and in 1988 Lock et al. [6] reported the first human experience of transcatheter closure of muscular defects. The introduction of Amplatzer family of devices has widened the application of transcatheter techniques for closure of these defects [7,8]. Successful closure of these defects by the device hinges on many factors, specifically on the location/type and the size of the VSD. Recently, a new Amplatzer device was designed with an eccentric shape for the use of perimembranous defects. However, the presence of aneurysmal tissue in perimembranous VSDs can make it difficult and challenging to be closed with the conventional membranous Amplatzer device [9].
We have previously reported our initial experience of Amplatzer duct occluder (ADO) to close the VSDs with tunnel shape aneurysm [10]. In this article, we report our medium term follow up of transcatheter closure of perimembranous and muscular VSDs with tunnel shape aneurysm using ADO.
2. METHODS AND MATERIALS
Table 1 shows the demographics, electrocardiographic, echocardiographic and catheterization data of all these cases. Informed consent was obtained and all the cases were done under general anesthesia and transesophageal echocardiographic (TEE) guidance, as previously published [10]. Femoral venous and femoral arterial access was achieved and bolus of 80 units/kg heparin and 25 mg/kg of cefazoline were given. Right and left heart catheterization and left ventricular angiography (Figure 1) was performed using a single plane with left anterior oblique 60˚ and 40˚ cranial projection and VSD size was measured. With the assistance of 0.035 inch Terumo (Radifocus) guide wire, 4 French Judkins right (Cordis) or 4 French Amplatzer right (Cordis J&J) catheter was crossed from the left ventricle (LV) to the right ventricle (RV) and then into main pulmonary artery (MPA) in 4 cases and superior vena cava (SVC) in 3 cases based on the wire and catheter feasibility. Terumo wire was exchanged with 0.035 inch Amplatzer guide wire with 6 cm soft tip (AGA, Medical Corporation, MN, USA). Through the femoral vein, an Ensnare guide catheter (InterV Medical device Technologies, Gainesville, FL) was placed in the MPA or SVC. An Ensnare with 9 - 15
Table 1. Demographic, electrocardiographic, echocardiographic and catheterization data of these patients.
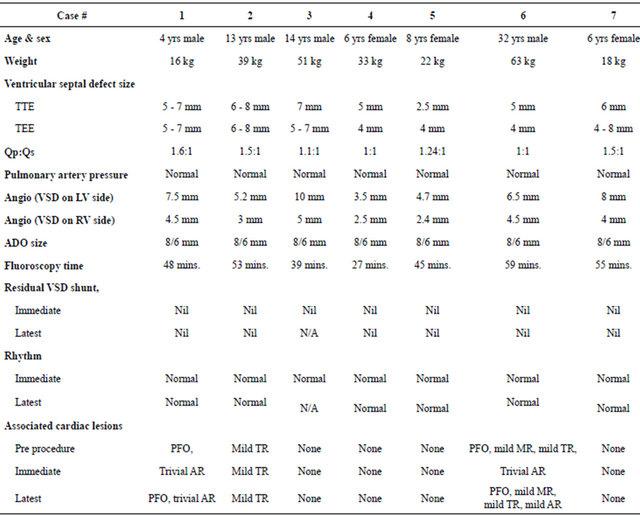
TTE: Transthoracic echocardiogram; TEE: Transesophageal echocardiogram; Qp/Qs: Pulmonary blood flow/systemic blood flow; ADO: Amplatzer duct occluder.
mm wire was used to snare and exteriorize the Amplatzer wire through the femoral vein. Then a 45 degree angle VSD delivery sheath (AGA) was placed in the femoral vein and advanced over the exteriorized wire into ascending aorta and the wire and dilator were removed. Amplatzer duct occluder was loaded on the delivery system and advanced to the tip of the delivery sheath into the ascending aorta (Figure 2). A 4 French Pigtail (Cordis, J&J) catheter was placed retrograde into the LV. Under TEE, fluoroscopy and LV cine angiographic guidance, the retention skirt of ADO was deployed just
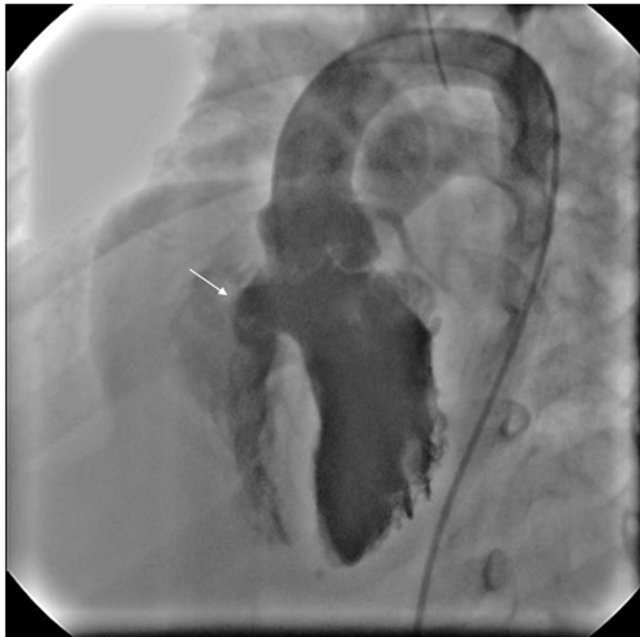
Figure 1. Arrow shows aneurysmal membranous VSD by left ventricular angiography in LAO 60, cranial 40. LAO = Left Anterior Oblique.
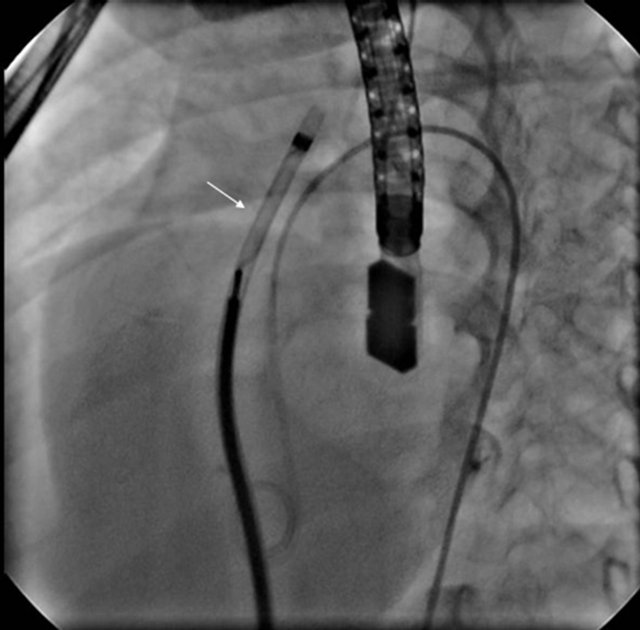
Figure 2. 45˚ angle sheath with Amplatzer duct occluder inside it being pulled down from ascending aorta to VSD.
below the aortic valve. Then the sheath and device were pulled slowly and the device was deployed just as if closing a patent ductus arteriosus. Proper position was confirmed with TEE and LV angiography, device was released and LV angiography was repeated (Figure 3). After achieving femoral hemostasis, heparin infusion was started at 10 units/kg/hour until next morning, two more doses of cefazoline every 8 hourly were given and patient was kept in the hospital for overnight observation. Next morning, electrocardiogram, transthoracic echocardiogram (TTE) (Figure 4) and chest X-ray were done and patients were discharged home on low dose (antiplatelet) aspirin for 6 months and follow up clinic visits at 1, 3, 6, 12 months and then on yearly basis with electrocardiogram and echocardiogram were planned.
During the study period of 79 months, total of 7 cases
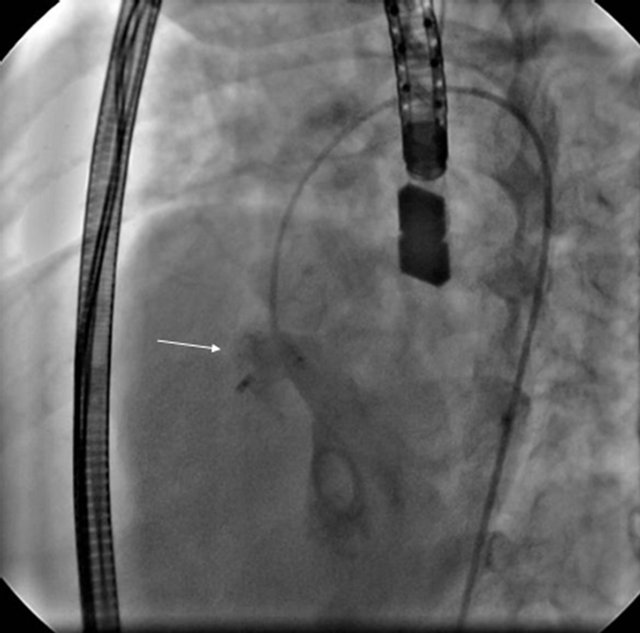
Figure 3. Arrow showing Amplatzer duct occluder deployed in the aneurysmal membranous VSD.
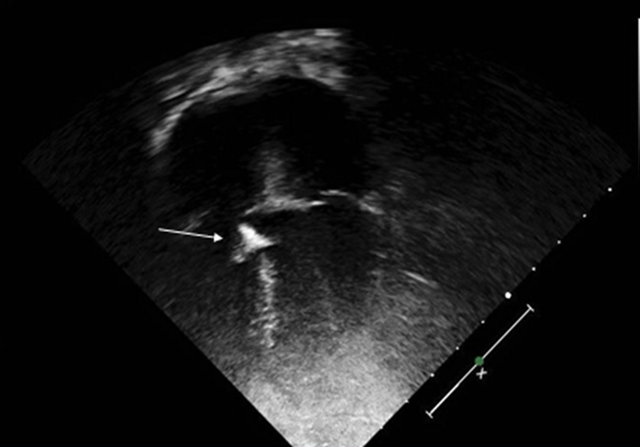
Figure 4. TTE (2-D) 4 chamber view showing ADO device closing the VSD. ADO = Amplatzer Duct Occluder; TTE = Transthoracic Echocardiogram.
(6 perimembranous and 1 muscular) VSD’s with tunnel type aneurysmal tissue were closed using 8/6 mm ADO. There were 4 male and 3 females patients with age range of 4 - 32 years with median of 8 years and weight range of 16 - 63 kg with median of 33 kg. Transthoracic echocardiographic VSD size ranged from 2.5 - 8 mm and TEE diameters ranged from 4 - 8 mm. Cardiac catheterization data revealed Qp/Qs with range of 1 - 1.6 and normal pulmonary artery pressure in all the cases. Left ventricular angiography showed VSD measurements of 3 - 10 mm on LV side and 2.4 - 5 mm on RV side. 8/6 mm ADO devices were used to close these VSD’s and right end of this device turn out to be an average of 2.4 mm bigger than the smallest diameter of the VSD. Fluoroscopy time ranged from 27 - 59 minutes with median of 48 minutes.
One (case # 1) patient was a case of Down syndrome with patent foramen of Ovale, 1 (case # 2) had mild tricuspid valve regurgitation and 1 (case # 6) patient had patent foramen of Ovale and mild mitral and mild tricuspid valves regurgitation before attempting the procedure.
In I (case # 6) patient, initially a 6 mm Amplatzer muscular device was deployed which got embolized to abdominal aorta with in 10 minutes of its release. The device was snared and removed easily through femoral artery approach. After one month, patient was recatheterized and 8/6 mm ADO was used successfully to close this VSD. It is noteworthy that this VSD was relatively difficult to cross from LV to RV side probably due to significant RV muscle bundles and this technical difficulty might have led to aortic valve injury leading to trivial regurgitation.
3. RESULTS
We closed six perimembranous and one muscular ventricular septal defect with tunnel shape aneurysmal tissue using ADO (Table 1). Immediately post procedure, TEE and LV angiography showed excellent device position without device disfigurement or impingement of aortic valve and there was no residual shunt in any of these patients, however trivial aortic valve regurgitation was noticed in 2 cases (cases # 1, 6). Immediate post procedure, electrocardiogram revealed normal sinus rhythm without any arrhythmia, bundle branch block or heart block in any of these patients.
All the patients except 1 (case # 3) who was an expatriate and had left the country were followed up both clinically and by TTE and ECG. The latest follow up in these 6 patients ranging from 1 - 80 months with median of 54 months showed that only 1 (case # 6) patient had progression of aortic valve regurgitation from trivial immediately post procedure to mild at this follow up and all the other patients had unchanged pre/immediate post procedure cardiac findings. All the patients at this follow up remained clinically asymptomatic with normal clinical examination and electrocardiographically were in normal sinus rhythm without any arrhythmia, bundle branch block or heart block.
4. DISCUSSION
Ventricular septal defects are the most common congenital cardiac malformation and perimembranous is the commonest out of all the VSDs. Surgical repair has been the gold standard for VSD closure and although it’s generally a safe procedure, it does have potential risks including heart block in 1% - 5%, significant residual shunt in 1% - 5%, the necessity for reoperation in 2% and death in 0.5% of the patients. Furthermore, infections, tachyarrhythmias and neurologic complications may occur [11,12]. Perimembranous VSDs are located near the conduction tissue and aortic valve, and if there is an aneurysmal tissue around the VSD, it makes its morphology even further complex [13]. Yilmaz and colleagues found 20% incidence of aneurysms in their perimembranous VSDs [14].
Percutaneous closure of the perimembranous VSD remains the biggest challenge because of its close proximity to the aortic and tricuspid valves as well as the conduction system which passes at the posterior border of these defects. The Amplatzer perimembranous ventricular septal defect occluder has been designed specifically with a short (1.5 mm) connecting waist and an asymmetric left ventricular disc to avoid impingement on the aortic valve as an advantage but it also has the disadvantage of having a relatively complicated delivery system.
Studies have shown ventricular arrhythmias, heart block, valvular damage and blood loss as the complications of transcatheter VSDs closure. In one of the earlier experiences of transcatheter closure of congenital and postoperative VSDs using STARFlex device showed that the device is feasible and complications are rare [15]. In another study, successful transcatheter closure of perimembranous VSDs was achieved in 96% of the cases without any mortality but 12% of these patients had complications like device embolization, vascular complications and rhythm abnormalities [16].
Complete atrioventricular heart block after device closure of VSDs is reported to occur in 5% - 22% of subjects [17,18]. It’s difficult to ascertain the exact cause of heart block in such cases but young age, small body weight, device oversizing and procedural trauma are the most probable etiologies and authors have suggested that the presence of an aneurysm of ventricular septum is a risk factor for heart block [19,20].
Percutaneous device closure of ventricular septal defect with aneurysm can be challenging and disfigurement of the device can happen due to the aneurysmal tissue as reported by Pedra et al. In case # 6 of our study, initial attempt with muscular Amplatzer device led to embolization of the device because its right sided disk couldn’t open properly and the device got disfigured and later on it was successfully closed with ADO. Use of ADO for closure of aneurysmal VSD seems promising because of the resemblance of this defect to patent ductus arteriosus (PDA) [21-26]. The left ventricular margin of VSD resembles the aortic margin of PDA while the right ventricular margin and septal aneurysm resembles the narrow end of PDA at the pulmonary artery margin.
In our study, deployment of the ADO device was easy and no specific manoeuvring was required. By keeping the sheath in ascending aorta and then bringing the device and sheath down while keeping the device inside the sheath and opening the left retention skirt just below the aortic valve was easy. We didn’t loose the sheath position in any of our patients probably because the sheath was kept supported by this aneurysmal tunnel. The ADO retention skirt stayed just at the orifice or slightly inside the tunnel depending on the skirt/tunnel size and none of our patients developed device disfigurement as the right end of the ADO expands itself depending on the shape and configuration of the tunnel.
In our group, the VSD closure rate was 100% (7/7) immediately after procedure and medium term follow up of 6/7 patients revealed no residual VSD with 100% success. There were no major complications and 2/7 patients developed trivial aortic valve regurgitation immediately after procedure and at medium term follow up, the aortic regurgitation was unchanged in 1/6 while in the other 1/6 patient, it had progressed to mild. Most importantly, we did not encounter any type of arrhythmia, bundle branch block or heart block in any of our patients neither immediately nor at medium term follow up and this is presumably due to the shape of ADO device which cause less trauma to the conduction tissue comparing other devices.
5. CONCLUSION
Our study has shown that Amplatzer duct occluder is feasible and can be used safely and effectively in selective VSDs especially those with aneurysmal tissue. Medium term follow up has shown excellent device position, no device disfigurement, no residual shunt and most importantly no heart block. Because of its safety, efficacy and cost effectiveness, ADO is an attractive option for percutaneous closure of selective VSDs. However, our study comprises a relatively small group of patients with medium term follow up, hence bigger series of such VSDs closed with ADO and their long term follow up is needed before the safety and efficacy of this device is established.
REFERENCES
- Mavroudis, C., Backer, C.L. and Jacobs, J.P. (2003) Ventricular septal defect. 3rd Edition, Pediatric Cardiac Surgery, Mosby, Philadelphia, 298-320.
- Ross-Hesselink, J.W., Mejiboom, F.J., Spitaels, S.E.C., et al. (2004) Outcome of patients after surgical closure of ventricular septal defect at young age: Longitudinal follow-up of 22 - 34 years. European Heart Journal, 25, 1057-1062.
- Lillehei, C.W., Cohen, M., Warden, H.E. and Varco, R.L. (1955) The results of direct vision closure of ventricular septal defects in eight patients by means of controlled cross circulation. Surgery Gynecology & Obstetrics, 101, 446-466.
- Nygren, A., Sunnegardh, J. and Berggren, H. (2000) Preoperative evaluation and surgery in isolated ventricular septal defects: A 21 year perspective. Heart, 83, 198-204. doi:10.1136/heart.83.2.198
- Bol-Raap, G., Weerheim, J., Kappetein, A.P., Witsenburg, M. and Bogers, A.J. (2003) Follow-up after surgical closure of congenital ventricular septal defect. The European Journal of Cardio-Thoracic Surgery, 24, 511-515. doi:10.1016/S1010-7940(03)00430-5
- Lock, J.E., Block, P.C., McKay, R.G., Baim, D.S. and Keane, J.F. (1988) Transcatheter closure of ventricular septal defects. Circulation, 78, 361-368. doi:10.1161/01.CIR.78.2.361
- Fu, Y.C., Bass, J., Amin, Z., Radtke, W., Cheatham, J.P., Hellenbrand, W.E., Balzer, D., Cao, Q.L. and Hijazi, Z.M. (2006) Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: Results of the US phase I trial. Journal of the American College of Cardiology, 47, 319- 325. doi:10.1016/j.jacc.2005.09.028
- Pinto, R.J., Dalvi, B.V. and Sharma, S. (2006) Transcatheter closure of perimembranous ventricular septal defects using Amplatzer asymmetric ventricular septal defect occluder: Preliminary experience with 18-month follow up. Catheterization and Cardiovascular Interventions, 68, 145-152.
- Pedra, C.A., Pedra, S.R., Esteves, C.A., Pontes, S.C., Braga, S.L., Arrieta, S.R., Santana, M.V., Fontes, V.F. and Masura, J. (2004) Percutaneous closure of perimembranous ventricular septal defects with the Amplatzer device. Technical and morphological considerations. Catheterization and Cardiovascular Interventions, 61, 403-410. doi:10.1002/ccd.10797
- Dilawar, M., Numan, M., El-Sisi, A., Gendi, S.M. and Ahmad, Z. (2008) Percutaneous closure of ventricular septal defects associated with tunnel shape aneurysm using Amplatzer duct occluder. Pediatric Cardiology, 29, 366-370. doi:10.1007/s00246-007-9092-0
- Hobbins, S.M., Izukawa, T., Radford, D.J., Williams, W.G. and Trusler, G.A. (1979) Conduction disturbances after surgical correction of ventricular septal defect by the atrial approach. British Heart Journal, 41, 289-293. doi:10.1136/hrt.41.3.289
- Bol-Raap, G., Weerheim, J., Kappetein, A.P., Witsenburg, M. and Bogers, A.J. (2003) Follow-up after surgical closure of congenital ventricular septal defects. European Journal Cardio-Thoracic Surgery, 24, 511-515. doi:10.1016/S1010-7940(03)00430-5
- Bian, C., Ma, J., Wang, J., Xu, G., Jiang, J., Yao, S. and Liu, Y. (2011) Perimembranous ventricular septal defect with aneurysm: Two options for transcatheter closure. Texas Heart Institute Journal, 38, 528-532.
- Yilmaz, A.T., Ozal, E., Arslan, M., Tatar, H., Ozturk, O.Y. (1997) Aneurysm of the membranous septum in adult patients with perimembranous ventricular septal defect. The European Journal of Cardio-Thoracic Surgery, 11, 307-311. doi:10.1016/S1010-7940(96)01058-5
- Knauth, A.L., Lock, J.E., Perry, S.B., McElhinney, D.B., Gauvreau, K., Landzberg, M.J., Rome, J.J., Hellenbrand, W.E., Ruiz, C.E. and Jenkins, K.J. (2004) Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation, 110, 501. doi:10.1161/01.CIR.0000137116.12176.A6
- Butera, G., Carminati, M., Chessa, M., Piazza, L., Micheletti, A., Negura, D.G., Abella, R., Giamberti, A. and Frigiola, A. (2007) Transcatheter closure of perimembranous ventricular septal defects: Early and long-term results. Journal of the American College of Cardiology, 50, 1189. doi:10.1016/j.jacc.2007.03.068
- Sullivan, I.D. (2007) Transcatheter closure of perimembranous ventricular septal defect: Is the risk of heart block too high a price? Heart, 93, 284-286. doi:10.1136/hrt.2006.103671
- Predescu, D., Chaturvedi, R.R., Friedberg, M.K., Benson, L.N., Ozawa, A. and Lee, K.J. (2008) Complete heart block associated with device closure of perimembranous ventricular septal defects. The Journal of Thoracic and Cardiovascular Surgery, 136, 1223-1228. doi:10.1016/j.jtcvs.2008.02.037
- Walsh, M.A., Bialkowski, J., Szkutnik, M., Pawelec-Wojtalik, M., Bobkowski, W. and Walsh, K.P. (2006) Atrioventricular block after transcatheter closure of perimembranous ventricular septal defects. Heart, 92, 1295-1297. doi:10.1136/hrt.2005.084988
- Yip, W.C., Zimmerman, F. and Hijazi, Z.M. (2005) Heart block and empirical therapy after transcatheter closure of perimembranous ventricular septal defect. Catheterization and Cardiovascular Interventions, 66, 436-441. doi:10.1002/ccd.20512
- Tan, C.A., Levi, D.S. and Moore, J.W. (2005) Percutaneous closure of perimembranous ventricular septal defect associated with a ventricular septal aneurysm using the Amplatzer ductal occluder. Catheterization and Cardiovascular Interventions, 66, 427-431. doi:10.1002/ccd.20499
- Chojnicki, M., Haponiuk, I., Jaworski, R., Juściński, J., Steffek, M., Sroka, M. and Pałkowska, L. (2011) [Intraoperative imaging of hybrid procedure for muscular ventricular septal defects closure with Amplatzer duct occluder II]. Kardiologia Polska, 69, 1280-1281.
- Ramakrishnan, S., Saxena, A. and Choudhary, S.K. (2011) Residual VSD closure with an ADO II device in an infant. Congenital Heart Disease, 6, 60-63. doi:10.1111/j.1747-0803.2010.00468.x
- Ergene, O., Nazli, C., Kocabas, U., Duygu, H., Akyildiz, Z.I. and Hijazi, Z.M. (2011) Percutaneous closure of perimembranous ventricular septal defect with an Amplatzer duct occluder in a dextrocardia patient. The International Journal of Cardiology, 150, e77-e80. doi:10.1016/j.ijcard.2009.11.016
- Stapleton, G.E., Carlson, K.M. and Justino, H. (2006) Transcatheter occlusion of a residual muscular ventricular septal defect using an Amplatzer duct occluder in a child with congenitally corrected transposition of the great arteries. Catheterization and Cardiovascular Interventions, 68, 296-300. doi:10.1002/ccd.20850
- Michel-Behnke, I., Le, T.P., Waldecker, B., Akintuerk, H., Valeske, K. and Schranz, D. (2005) Percutaneous closure of congenital and acquired ventricular septal defects—Considerations on selection of the occlusion device. Journal of Interventional Cardiology, 18, 89-99. doi:10.1111/j.1540-8183.2005.04051.x
NOTES
*Corresponding author.

