Pain Studies and Treatment
Vol.2 No.2(2014), Article ID:44850,8 pages DOI:10.4236/pst.2014.22015
Analysis of the Efficacy of the Lidocaine Patch 5% in the Treatment of Neuropathic Pain: Our Feedback
P. Hernández-Puiggròs1, R. Pélaez1, A. Morell1, A. Yañez2, J. L. Aguilar1
1Hospital Son Llàtzer, Palma de Mallorca, Spain
2Fundació d’Investigació Sanitària Illes Balears (FISIB), Palma de Mallorca, Spain
Email: pati000@hotmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 1 February 2014; revised 1 March 2014; accepted 9 April 2014
ABSTRACT
Objective: the objective of this study was to evaluate the efficacy of the lidocaine patch 5% in different types of neuropathic pain. Methods: a prospective, longitudinal, observational study on a sample of 16 patients who consulted for neuropathic pain. A lidocaine patch 5% was applied to the painful area and as primary endpoint, the severity of the pain was studied using the Verbal Numeric Rating Scale (VNRS). Secondary quality of life-related endpoints were sleep during the night, mood and patient global impression of the treatment. Results: demographic data: 62.5% female and 37.5% male; mean age 55.31 ± 13.9 years; time since onset of the pain 8.4 months; and classified into 4 diagnosis groups: post-herpetic neuralgia 18.8%; complex regional pain syndrome 25%; surgical wound 50%; and others 6.3%. There was a reduction of more than 2 points in pain on the VNRS (median 6.5 to 3.5; p = 0.001), an improvement in sleep during the night, mood and relief (p < 0.05), less use of analgesics, no complications and over 30% of subjects reported improvement of over 50%. Conclusions: The lidocaine patch 5% could be a useful tool for the control of neuropathic pain, not only for post-herpetic neuralgia, and it has a good safety and tolerability profile.
Keywords:Chronic Pain, Neuropathic Pain, Lidocaine Patch

1. Introduction
Chronic pain affects around 20% of the adult population in Europe. It is more common in women, elderly people and with certain disabilities [1] .
Over and above the physical and emotional damage, the financial cost is astronomical: in the order of 200 billion euros annually in Europe and some 150 billion dollars in the USA [2] . Detecting potential risk factors is essential if healthcare and complementary strategies are to be devised to control chronic pain [3] . Neuropathic pain is a very particular type of pain. The International Association for the Study of Pain (IASP) defines it as “pain caused by a lesion or disease of the somatosensory nervous system”; according to recent studies, it affects as much as 8% of the UK population [4] .
A number of topical treatments, including capsaicin 0.075% cream, capsaicin patch 8% and the lidocaine patch 5%, are considered as first-line treatment for certain types of neuropathic pain such as post-herpetic neuralgia, particularly when it is associated with allodynia [5] .
Treatment with only one drug is usually ineffective and, in the majority of cases, a combination is given, depending on the severity and qualities of the pain and on any comorbidity [6] .
Post-herpetic neuralgia (PHN) is estimated to occur in 2% - 35% of patients after reactivation of the latent varicella-zoster virus. It is characterized by the presence of pain of varying qualities: constant burning or stinging sensation; intermittent stabbing and a variety of altered sensations, frequently accompanied by touch-induced pain or touch allodynia (in 70% - 90% of cases) [7] .
Allodynia has been studied in many different clinical conditions and there has been found to be increased activity in the basal ganglia and the cerebral cortex [8] [9] . In spite of the numerous treatments for PHN (tricyclic antidepressants, antiepileptic drugs, topical agents, opioids, etc.), it continues to be a difficult condition to manage and since it tends to occur in older people (incidence > 50% in over-60 s and > 70% in over-70 s), the risk of accumulating side effects is a significant problem [10] .
This led to the study of alternatives, such as the lidocaine patch 5%, as a topical first-line treatment for PHN pain [11] .
The mechanism of action of the lidocaine patch 5% is through stabilization of the neuronal membranes, which causes down-regulation of sodium channels in damaged nociceptors with increased expression, leading to a reduction in pain.
Since there is minimal systemic absorption, its mechanism of action is defined as dual: on the one hand, secondary to local diffusion of the lidocaine and on the other, the mechanical protection effect of the patch [12] . The lidocaine patch 5% interferes with spontaneous pain afferent excitability, thereby reducing the perception of pain, but does not reduce allodynia, probably because it is more related to a central phenomenon where topical treatment has no effect [13] .
2. Method
2.1. Design
Prospective, longitudinal, observational study in a sample of patients with chronic focal neuropathic pain. The patients were recruited in the Chronic Pain Unit and agreed to take part in the study.
2.2. Participants
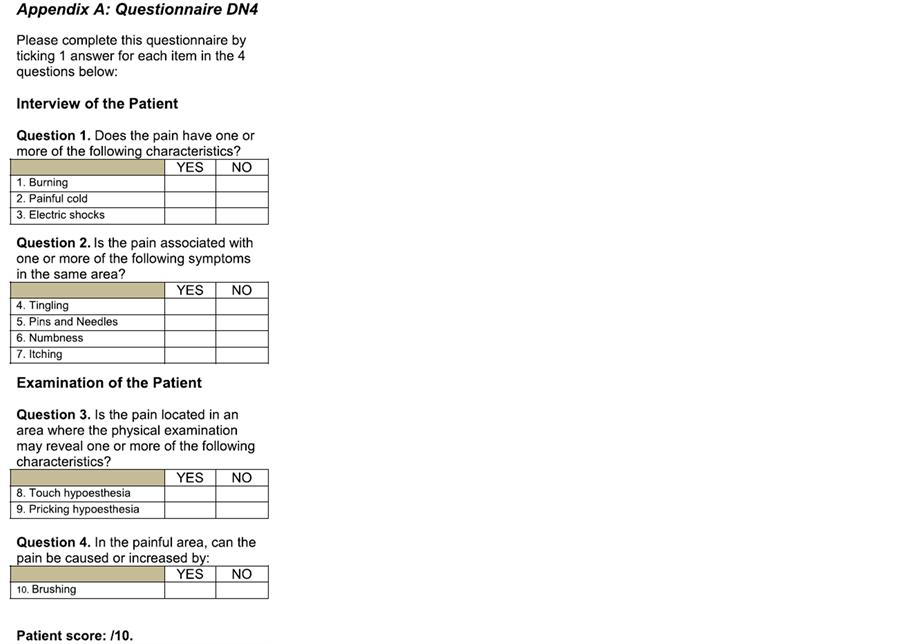
Patients at our Chronic Pain Unit with neuropathic-type pain (according to the DN4 Questionnaire*) for over 3 months in whom lidocaine patch 5% was applied (a maximum of 3) to cover the painful area.
2.3. Inclusion and Exclusion Criteria: Table 1
*DN4 Questionnaire: Spanish Version (Spain): Pérez C, et al. EFIC 2006. APPENDIX 1.
2.4. Follow-Up
Monitoring of pain intensity in each patient was performed in three periods through the primary efficacy endpoint Verbal Numeric Rating Scale at rest and in motion (VNRS of 0 = no pain, to 10 = unbearable pain).
Pain was assessed using the VNRS at baseline and at two consecutive visits separated by at least one month after application of the lidocaine patch 5%; baseline medication was initially continued, changing the dose, number of rescue doses and/or withdrawing it depending on how the pain evolved and on the patient’s needs, at the discretion of the attending anesthetist.
The following were studied as secondary endpoints:
• Sleep quality: 0 = I don’t sleep at all, to 10 = I sleep through the night
• Mood: 0 = depressed, to 10 = in great spirits
• Perception of relief: 0 = no relief, to 10 = maximum relief
• Use of analgesics before and after treatment, describing it as less than, equal to or greater than prior to application of the lidocaine patch 5%
• Patient global impression of improvement was studied using the 7-point Likert Scale Table 2.
2.5. Statistical Analysis
A descriptive analysis was made of the demographic data recorded and the Wilcoxon Signed-Rank Test was used to compare the medians of the VNRS scores over the course of the study period; statistical significance was considered when p < 0.05.
3. Results
A prospective, longitudinal, observational study was conducted on a sample of 16 patients, applying the lidocaine patch 5% over the painful area in order to control their neuropathic pain. The sample consisted of 10 women and 6 men, with a mean age of 55.31 ± 13.89 and an average time from onset to being investigated at the Chronic Pain Unit of 8.4 months (3 - 16 months).
According to the WHO analgesic ladder, 9 patients were treated with step 1 drugs, 5 with step 2 and 2 with step 3. The diagnoses treated are shown in Table 3.
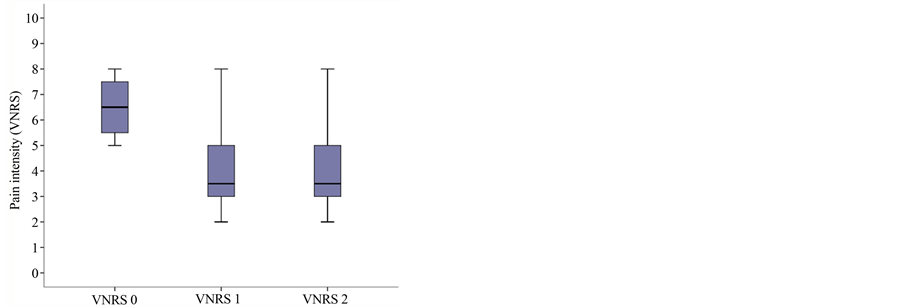
The mean intensity of pain at the baseline visit at rest on the VNRS (Verbal Numeric Rating Scale at time 0 at rest = VNRS0r) was 6.5 (range 5.25 - 7.75) and in motion (VNRS0m) was 7.5 (range 7 - 8.25). At the second visit, the scores were lower, at 3.5 (range 3 - 5) and 5 (range 4 - 6), respectively, and at the third, 3.5 (range 3 - 5) and 4 (range 3 - 5), respectively; with statistical significance of p = 0.001 (Figure 1(a)).
Quality of sleep was assessed before and after application of the lidocaine patch 5% using an 11-item numeric scale (0 = I don’t sleep at all, to 10 = I sleep through the night); initial median sleep quality was 3.5 (range: 2 - 6), with 6.5 (5 - 8) subsequently and 6.5 (5.25 - 8) in the final visit. Mood was similarly studied (0 = depressed, to 10 = in great spirits), with the following results: baseline 3 (1.25 - 4.75); and 5 (2.25 - 6) and 5 (3.25 - 6) subsequently. Subjective impression of relief scored as follows: 2.5 (1.5 - 3.5) at baseline and 5 (3.25 - 6) and 5 (3 - 6), respectively, at the subsequent visits; with these differences being statistically significant (p < 0.05) in the three secondary endpoints comparing baseline to the subsequent visits, although there were no differences between those two visits (Figure 1(b) and Figure 1(c) and Figure 1(d)).
Table 3. Demographic data.
 (a)
(a)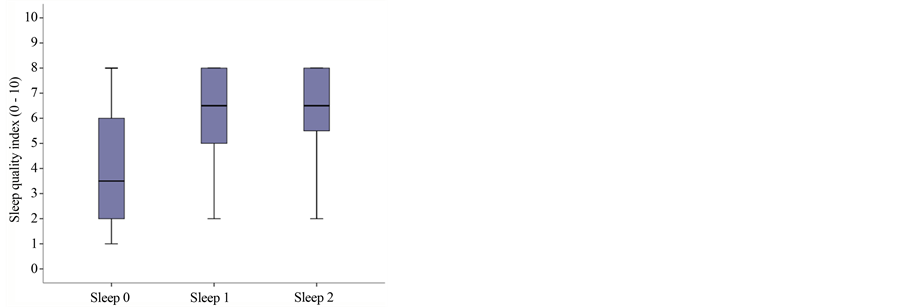 (b)
(b)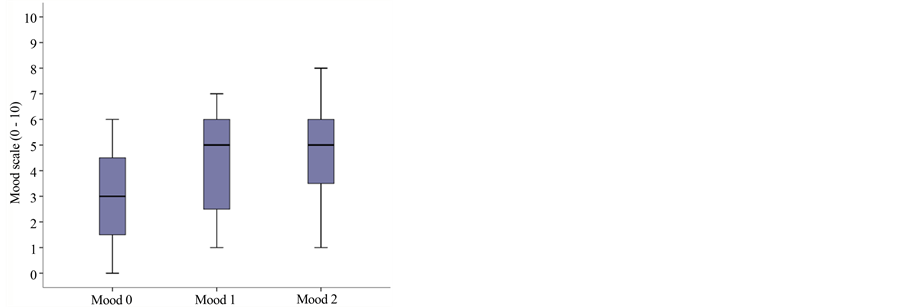 (c)
(c)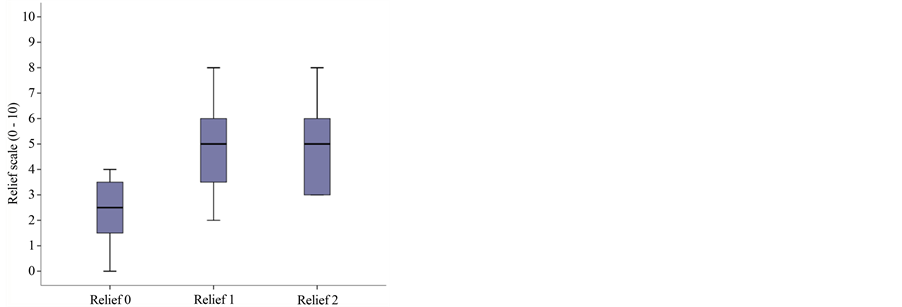 (d)
(d)
Figure 1. Boxplot graph of median and percentiles for: (a) Pain intensity at baseline and subsequent visits: VNRS0r = 6.5 (percentiles 25 - 75: 5.25 - 7.75); VNRS1r = 3.5 (3 - 5); VNRS2r = 3.5 (3 - 5); p = 0.01; (b) Sleep quality at baseline and subsequent visits: 3.5 (range: 2 - 6), subsequently 6.5 (5 - 8) and 6.5 (5.25 - 8); p = 0.003; (c) Mood at baseline and subsequent visits: 3 (range 1.25 - 4.75); 5 (2.25 - 6) and 5 (3.25 - 6); p = 0.002; (d) Relief at baseline and subsequent visits: 2.5 (1.5 - 3.5) at baseline and 5 (3.25 - 6) and 5 (3 - 6), respectively, at the subsequent visits; p = 0.005.
On the use of analgesics, at the end of the registering period, it was found that 25% remained on the same medication, 6.3% had had to increase the dose of a medication that was already prescribed and 68.8% had been able to reduce the amount of analgesics they took.
Analysis of the percent improvement according to the Likert Scale is shown in Table 4 and Figure 2. Only one patient reported worsening of his pain. This was a 60-year-old male with a 6-month history of frontal/parietal post-herpetic neuralgia who had tried a multitude of systemic treatments with little improvement, with initial and final VNRS scores of 8; this patient had been resistant to treatment with the patch from the start because of the fact that it had to be stuck on his head. Two patients reported no improvement in their pain; one was a 70-year-old male with neuropathic pain at the anterior aspect of his knee following total knee replacement surgery and the other was a 90-year-old woman who had suffered post-herpetic neuralgia in her left arm for over a year. Interestingly, although both subjectively rated the treatment as providing “no improvement”, they were both found to have a reduction of 2 points on the VNRS and improvement in their mood and sleep quality. Of the total, 50% reported an improvement of over 25% and 31.3%, over 50% improvement.
There were no cases of any complications deriving from the lidocaine patch 5%.
At the end of the study period, each patient was asked to use one phrase to define the effect of the lidocaine patch 5% on their everyday quality of life Table 5.
4. Discussion
Despite the limitations of this study as a result of the small sample of patients and the possible placebo effect in the absence of double-blinding or prior randomization, our feedback with the lidocaine patch 5%, not only in
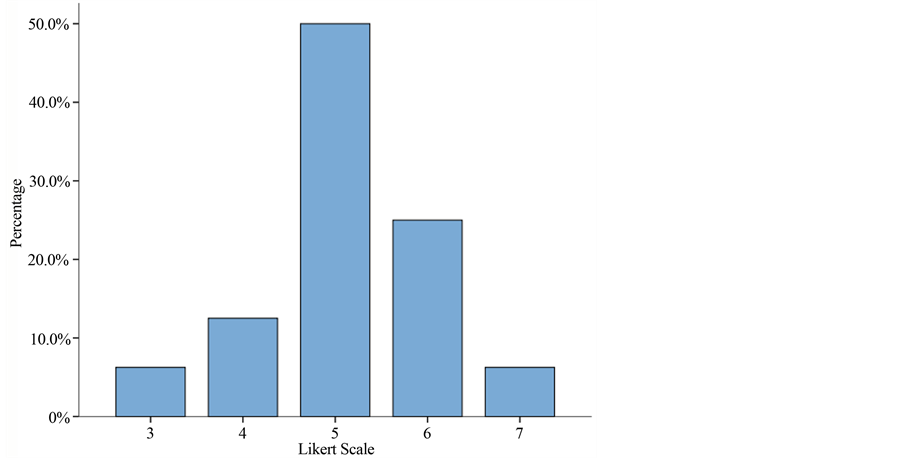
Figure 2. Histogram graph of the Likert Scale’s percentage. Over 80% of the patients treated with the lidocaine patch 5% showed an improvement (Likert > 5). Of these, over 30% (Likert 6 + 7) felt an improvement of over 50%.
Table 4. Improvement in number of patients according to the likert scale.
Table 5. Subjective comments at the end of the study period.
post-herpetic neuralgia, but also in other types of neuropathic pain, is that it could be a useful tool for reducing pain, with excellent tolerability and no detected side effects.
Studying other secondary variables that can affect the quality of everyday life, such as mood, sleep quality and perceived pain relief, we also observed an improvement after applying the lidocaine patch 5%.
The Likert Scale allows us to obtain a subjective assessment of each patient in relation to the applied treatment, so while being aware that pain is a “subjective” feeling, we can conclude that over 80% of our patients treated with the lidocaine patch 5% showed an improvement. Of these, 50% only experienced 25% improvement, which therefore rules out any placebo effect, but over 30% felt an improvement of over 50%.
In our study, interestingly, one of the patients who found the patch not to be effective was a case of post-herpetic neuralgia, which is the only indication currently approved by the FDA. However, it has to be said that the site was difficult and highly visible and the patient did state this as the reason he did not like that type of treatment; perhaps this provoked a phenomenon of aversion towards the patch. Furthermore, the site of application of the patch could have influenced its action; PHN can occur in any part of the body but very often affects the trigeminal nerve and dermatomes of the brachial plexus; Nalamachu et al. looked at whether or not the anatomical location of the lidocaine patch 5% was associated with variations in efficacy and tolerability. They concluded that the patch was effective and generally well tolerated in all areas assessed, but application to the head was less well tolerated compared with the torso and limbs [14] .
In the review of Wolff et al. [15] they concluded that, compared with other treatments, the lidocaine patch 5% was generally associated with a greater reduction in pain. The LP and pregabalin showed equivalence in different measurements of pain, but the LP was associated with fewer side effects than the systemic drugs. As the LP seems to have equal efficacy and better tolerability, it could be considered as first-line treatment in PHN.
Other indications in which the lidocaine patch has been used, but for which there are currently no randomized, placebo-controlled studies and existing studies have a limited number of participants, are post-traumatic neuralgia, complex regional pain syndrome, cancer-related neuropathic pain, carpal tunnel syndrome, myofascial pain, low-back pain and post-surgical pain [16] .
Among the diagnoses included in our study, one of the most predominant indications is neuropathic pain over surgical wounds. One of the potential risks after surgery is the development of chronic pain: the concept of chronic post-surgical pain (CPSP). There have been several studies on the pathophysiological risk factors and predictors of CPSP in order to prevent it and many questions remain unresolved. It has been observed that some surgical procedures carry a higher risk of chronic pain developing [17] .
The generation/genesis of chronic pain involves many mechanisms and each is subject to the expression of neuronal plasticity, which is the ability of neurons to change their function, chemical profile or structure. These changes, called neuroplasticity modulation, modification or central sensitization, lead a harmful stimulus to evoke a far greater and more prolonged pain response; this is the concept of hyperalgesia. This same response can also be generated after a non-painful stimulus or even spontaneously; this is the concept of allodynia [18] .
This study has not defined the area of allodynia or hyperalgesia in patients with CPSP in relation to the surgical wound, but positive signs, such as burning, stinging, unpleasant to the touch, etc., were observed during the questioning. In this group of patients, the pain reduction after applying the lidocaine patch 5% was important and CPSP was one of the most common diagnoses in which the topical analgesic treatment was indicated.
Worth highlighting is the study by Hashmi et al. in which the placebo patch was found to be equally effective in reducing chronic back pain [19] . The authors describe the patch as a “potent agent for inducing placebo analgesia”. It could be that instead of a placebo effect, the patch itself is acting as an analgesic on the basis of the Gate Theory, which can essentially be summarized as the fact that the Aβ fibers inhibit the Aδ and C fibers [20] ; in the same way as considered in acupuncture, transcutaneous stimulation or even neuromuscular dressing or kinesiotape [21] .
It would be interesting to investigate whether the patch’s effect at the level of the mechanoreceptors in the skin could act as inhibitory in pain transmission as far as the level of the spinal dorsal horn based on the Gate Theory, in which case, it would not, as such, be a placebo effect.
To confirm that the lidocaine patch 5% is effective in other indications apart from post-herpetic neuralgia, prospective, randomized, double-blind studies need to be conducted in each of the different conditions with neuropathic-type pain with prolongation of the study treatment in order to rule out a possible effect of long-term tolerance to the patch in relation to possible tachyphylaxis to the local anesthetic.
References
- Breivik, H., et al. (2006) Survey of Chronic Pain in Europe: Prevalence, Impact on Daily Life, and Treatment. European Journal of Pain, 10, 287-333. http://dx.doi.org/10.1016/j.ejpain.2005.06.009
- Elliott, A., et al. (2002) The Course of Chronic Pain in the Community: Results of a 4-Year Follow-Up Study. Pain, 99, 299-307. http://dx.doi.org/10.1016/S0304-3959(02)00138-0
- Smith, B., et al. (2007) Epidemiology of Chronic Pain, from the Laboratory to the Bus Stop: Time to Add Understanding of Biological Mechanism to the Study of Risk Factors in Population-Based Research. Pain, 127, 5-10. http://dx.doi.org/10.1016/j.pain.2006.11.001
- Torrance, N., et al. (2006) The Epidemiology of Chronic Pain of Predominantly Neuropathic Origin: Results from a General Population Survey. Journal of Pain, 7, 281-289. http://dx.doi.org/10.1016/j.jpain.2005.11.008
- Irving, G., et al. (2012) Capsaicin 8% Dermal Patch, Administered alone or in Combination with Systemic Neuropathic Pain Medications, Reduces Pain in Patients with Post-Herpetic Neuralgia. The Clinical Journal of Pain, 28, 101-107. http://dx.doi.org/10.1097/AJP.0b013e318227403d
- Gilron, et al. (2005) Combination Pharmacotherapy for Neuropathic Pain: Current Evidence and Future Directions. Expert Review of Neurotherapeutics, 5, 823-830. http://dx.doi.org/10.1586/14737175.5.6.823
- Sandkühler, J. (2009) Models and Mechanisms of Hyperalgesia and Allodynia. Physiological Reviews, 89, 707-758. http://dx.doi.org/10.1152/physrev.00025.2008
- Maihofner, C., et al. (2006) Functional Imaging of Allodynia in Complex Regional Pain Syndrome. Neurology, 66, 711-717. http://dx.doi.org/10.1212/01.wnl.0000200961.49114.39
- Maihofner, C., et al. (2003) Cortical Processing of Brush-Evoked Allodynia. Neuroreport, 14, 785-789. http://dx.doi.org/10.1097/00001756-200305060-00002
- Volpi, A., et al. (2005) Current Management of Herpes Zoster: the European View. American Journal of Clinical Dermatology, 6, 317-325. http://dx.doi.org/10.2165/00128071-200506050-00005
- Johnson, R.W., et al. (2008) Herpes Zoster and Postherpetic Neuralgia: Optimizing Management in the Elderly Patient. Drug Aging, 25, 991-1006. http://dx.doi.org/10.2165/0002512-200825120-00002
- Baron, R., et al. (2009) 5% Lidocaine Medicated Plaster versus Pregabalin in Post-Herpetic Neuralgia and Diabetic Poluneuropathy: An Open-Label, Non-Inferiority Two Stage RCT Study. Current Medical Research and Opinion, 25, 1663-1676. http://dx.doi.org/10.1185/03007990903047880
- Geha, P.Y., et al. (2007) Brain Activity for Spontaneous Pain of Postherpetic Neuralgia and Its Modulation by Lidocaine Patch Therapy. Pain, 128, 88-100. http://dx.doi.org/10.1016/j.pain.2006.09.014
- Nalamachu, S., et al. (2013) Influence of Anatomic Location of Lidocaine Patch 5% on Effectiveness and Tolerability for Postherpetic Neuralgia. Patient Preference and Adherence, 7, 551-557.
- Wolff, R.F., et al. (2011) REVIEW ARTICLE: 5% Lidocaine-Medicated Plaster vs Other Relevant Interventions and Placebo for Post-Herpetic Neuralgia: A Systematic Review. Acta Neurologica Scandinavica, 123, 295-309. http://dx.doi.org/10.1111/j.1600-0404.2010.01433.x
- Mick, G., et al. (2012) Topical Pain Management with the 5% Lidocaine Medicated Plaster—A Review. Current Medical Research and Opinion, 28, 937-951. http://dx.doi.org/10.1185/03007995.2012.690339
- Perkins, F.M., et al. (2000) Chronic Pain as an Outcome of Surgery. A Review of Predictive Factors. Anesthesiology, 93, 1123-1133. http://dx.doi.org/10.1097/00000542-200010000-00038
- Woolf, C., et al. (2011) Central Sensitization: Implications for the Diagnosis and Treatment of Pain. Pain, 152, 2-15. http://dx.doi.org/10.1016/j.pain.2010.09.030
- Baron, R., et al. (2010) Neuropathic Pain: Diagnosis, Pathophysiological Mechanisms and Treatment. The Lancet Neurology, 9, 807-819. http://dx.doi.org/10.1016/S1474-4422(10)70143-5
- (1978) The Gate Control Theory of Pain (No Authors Listed). British Medical Journal, 2, 586-587. http://dx.doi.org/10.1136/bmj.2.6137.586-a
- Moayedi, et al. (2013) Theories of Pain: From Specificity to Gate Control. Journal of Neurophysiology, 109, 5-12. http://dx.doi.org/10.1152/jn.00457.2012
Appendix 1
*DN4 Questionnaire: Spanish Version (Spain): Pérez C., et al. EFIC 2006.