World Journal of Cardiovascular Diseases
Vol.3 No.2A(2013), Article ID:30861,7 pages DOI:10.4236/wjcd.2013.32A001
Management of acute heart failure—Is there a paradigm shift around the corner?
![]()
Novartis Pharma AG, Basel, Switzerland
Email: cornel.pater@novartis.com
Copyright © 2013 C. Pater, T. Severin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 17 January 2013; revised 15 March 2013; accepted 18 April 2013
Keywords: Acute Heart Failure; Hemodynamic; Clinical and Residual Congestion; Vasodilators; Diuretics; Management Strategies
ABSTRACT
It has become increasingly apparent that the looming epidemic of heart failure calls for systematic treatment approaches tailored to the needs of individual patient phenotypes. Although chronic heart failure (CHF) therapies are continuously evolving based on the increasing understanding of the involved etiology, acute heart failure (AHF) therapies are still based on hemodynamic improvements and symptom alleviation. Guidelines on AHF management have highlighted that the currently administered AHF therapies lack evidence and have raised concerns on the safety and efficacy of some of the hitherto accepted treatment modalities. Additionally, the high mortality and morbidity rates associated with the current AHF therapies also add to the imperative need to re-visit AHF management. The last decade has witnessed a paradigm shift in the way we define and diagnose AHF. Apart from it being recognized as a distinct clinical entity, research has also led to new data on the pathophysiological changes associated with AHF. These developments along with the limited shortand long-term effects of currently used therapies may herald a paradigm shift in the way we plan and deliver management strategies to treat the pathological progression of heart failure.
1. EPIDEMIOLOGIC CONSIDERATIONS
Clinical and epidemiological evidence derived from studies carried out in the United States, Canada, Japan, Western and Eastern Europe indicate that presentation of the AHF patients to the emergency department (ED), their background etiologies, precipitating factors, and existing co-morbidities are marked by large heterogenity.
This heterogeneity is also observed in the treatment strategies and the overall management of these patients from the time of ED admission to their long-term followup. Diuretics, vasodilators, and positive inotropes comprise the mainstay of AHF management, with wide variations noted in their usage. For example, nitroglycerine is used in 32.8% of patients in Eastern Europe, 24.4% in Western Europe, and 2.5% in the United States; on the other hand carperitide—a recombinant form of alphahuman atrial natriuretic peptide—is used in 69.4% of patients in Japan [1,2]. A common denominator, however, is the extensive use of loop diuretics in up to 90% of patients in all regions. In this respect, a one-size-fits-all pattern seems to be deeply embedded in the ED routines in an almost universal manner, assumedly, to the benefit of the patients receiving these. Apart from management therapies, resource utilization also varies dramatically amongst the regions, with the length of hospital stay varying from 21 days on an average in the Asia-Pacific region to 4 - 9 days in the United States and 8 - 12 days in Europe [1,3,4].
Of more concern is the increasing prevalence of heart failure in parallel with the increase in the aging population worldwide and the improving number of patients with coronary heart disease surviving acute events (there by running a greater risk for developing heart failure). The huge economic and public health burden resulting from heart failure-related morbidity and mortality is common to all healthcare systems. For example, in 2010 alone the total costs associated with heart failure was estimated to be about $39.2 billion in the United States, £1.4 billion in United Kingdom, and EUR2.4 billion in France. The estimated projected cost for heart failure in 2030 for the United States alone is US $97.0 billion [5-7]. In heart failure patients, the in-hospital mortality can vary from 4% - 11% [1,3,8-10], whereas the re-hospitalization rate at Day 30 post-discharge is about 25% [11]. Sixmonth readmission rates are even higher, trending near 50% in most reports [12]. Overall, hospitalizations account for 75% of the total cost for heart failure within the first 48 hours post-admission in the United States, whereas hospitalization in Europe constitute up to 70% of the total heart failure costs [13-17].
Notably, despite these alarming figures, general awareness about heart failure is far from the average knowledge in the general population compared to other cardiovascular entities like myocardial infarctionand stroke. A survey in nine European countries (SHAPE, 2005) exploring general awareness about heart failure has shown that out of almost 8000 inhabitants only 3% could identify heart failure from a description of the symptoms and signs; although, 31% of subjects could identify angina and 51% stroke [18].
2. DIAGNOSTIC CHALLENGES
Data from two of the largest heart failure databases (ADHERE and OPTIMIZE) indicate that majority of AHF patients present with clinical picture of worsening of previously diagnosed HF and only 12% - 25% are de novo cases, whereas a minority of the total (1% - 2%) have cardiogenic shock. Furthermore, the most often encountered physical signs are dyspnea (61% - 89%), rales (62% - 68%), and peripheral edema (66%) [3,8]. Notably, in the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE), rales were present in 20% and edema in 40% of AHF patients, suggesting again the great variability in the findings from different research sources [19].
With the great majority of AHF patients actually being chronic heart failure patients (and thereby known to the local health care system) and with physical signs that are easily identifiable even by junior physicians, it may come as a surprise to often hear that accuracy of HF diagnosis overall is quite poor [20,21]. In one study, the combination of clinical signs had only a 58% rate of sensitivity in detecting patients with elevated pulmonary capillary wedge pressure (PCWP) [22], whereas radiographic pulmonary congestion was absent in 53% of patients with a PCWP of 16 to 29 mmHg and in 39% of patients with a PCWP of 30 mmHg [23]. Thus, the low diagnostic sensitivity of clinical assessments is recognized; on the other hand, the role of biomarkers (such as B-type natriuretic peptide [BNP] and N-terminal pro B-type natriuretic peptide [NT-proBNP]) in improving the same is also acknowledged [24,25]. In highly specialized and well-resourced tertiary centers, diagnostic accuracy can accordingly be close to 90% as well [24]. The guidelines for diagnosis and treatment of heart failure from American College of Cardiology Foundation/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) have delineated the current methodology to be adopted to standardize the diagnostic workup. Clinical assessments are recommended for supplementation by objective evidence of cardiac dysfunction and, if doubt persists, by response to initial treatment. Objective assessments such as electrocardiogram (ECG), chest x-ray, biomarkers, and echocardiography are required for confirmation of diagnosis [12,26]. Diagnostic and monitoring aspects of AHF patient care continuum are further exquisitely covered in this issue by J. Cleland et al. [Acute Heart Failure: Initial Diagnosis and Subsequent Evaluation with Traditional and Novel Technologies].
At closer scrutiny, diagnostic challenges seem to be compounded by a universal inertia by which acute heart failure is a 2nd tier urgency for which a 50-year-old management pattern (including oxygen supplementation, furosemide, eventually nitrovasodilators, and morphine) is used to relieve dyspnea and to allow for patient’s early discharge or transfer to a ward. As a matter of fact, a relatively large proportion of patients seem to be discharged within hours after the acute event subsided or the day after. In a study by Richter et al., only 55% of 448 randomly selected patients presenting to an ED were admitted. Of those not admitted, nearly 20% were either re-hospitalized or died in the following 30 days [27]. Not to much surprise, the early discharged patients leave with a persistent high degree of pulmonary and/or systemic congestion. To make things even worse, data indicate that often congestion may not be adequately addressed during hospitalization, which results in patients being discharged with improved symptoms yet with persistently elevated left ventricular (LV) filling pressures. This ultimately leads to early readmission when symptoms of congestion recur [28].
3. THE CURRENT ER PARADIGM
Sequential pathophysiological changes, commonly triggered by patient-related factors such as excessive salt and water intake, nonadherence to medication regimens, concurrent medication, acute infection, etc., are leading to a number of successive events with recognizable pattern regardless of geographical location.
Gradually increasing LV filling pressure, present in a majority of patients with AHF, is more or less silent until a sudden increase in the PCWP causes an overt, dramatic clinical picture of acute pulmonary edema (or alike) that compels the patient to seek urgent care (Figure 1).
A variable dose of loop diuretic combined (or not) with peroral nitrate agent or low dose morphine and supplemental oxygen will be administered usually before a positive diagnostic is established and, for most part, before common investigations are even started. Early vasoactive treatment has been reported to improve heart failure outcome and reduce in-hospital mortality [29,30]. Such
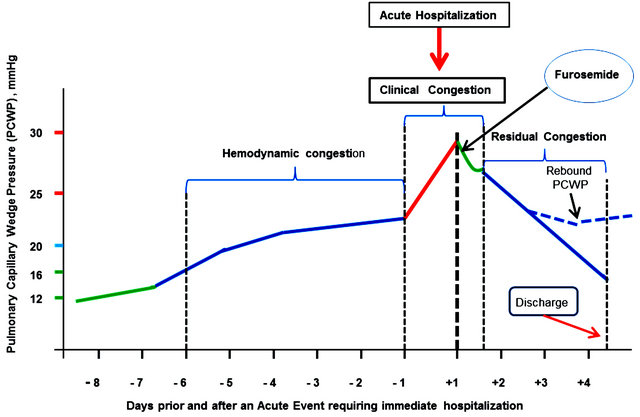
Figure 1. Hemodynamic, clinical and residual in AHF—An inbisible continuum.
treatment is likely to relieve dyspnea and confer the patient with a relative general well-being within the next 15 - 60 minutes as a result of quick decrease in the PCWP (the green descending line in Figure 1).
This short-term improvement is very much in line with the prerogatives of an emergency department, i.e., to stabilize the patient’s hemodynamic and clinical status; it justifiably pleases the patient and the medical staff alike and may pave the path for the decision of early discharge.
Patients deemed to need more comprehensive investigations or who are having refractory heart failure or cardiogenic shock are transferred to an intensive or coronary care unit and may benefit from mechanical ventricular support, nowadays with good long-term prognosis.
The fate of the patients discharged should, however, attract particular attention, as in these patients the apparent clinical improvement leading to their early discharge may be masking residual high level LV filling pressure despite intensive diuretic treatment. A multitude of extraneous factors (e.g., excessive salt and water intake, nonadherence to medication regimens, etc.) may easily trigger a rebound of peak increase in the PCWP, acute readmission and a down spiraling with disease progression, and further increased morbidity and mortality.
Figure 1 is a schematic representation of basic pathophysiological changes occurring as a result of fluid retention or fluid redistribution. Fluid redistribution is induced by vascular mechanisms (vasoconstriction) as well as neurohormonal and inflammatory activation, renal dysfunction, and possibly inappropriate use of some medications. Fluid redistribution in particular causes elevated LV filling pressure with consequent gradual increase in the PCWP [31-33]. The resulting congestion is an obvious pathophysiological continuum consisting of three distinct stages: hemodynamic congestion [34], clinical congestion [34], and residual congestion. Calling this an “invisible continuum” would very much reflect the fact that clinically there is a typical discrepancy between the dyspnea severity and PCWP magnitude, especially during the hemodynamic and the residual congestion stage (adding up together to 90% of the time under observation) [34].
Overall, however, during the hemodynamic congestion stage [8] (1 - 2 weeks long), patients are minimally symptomatic. Quantifying congestion during this stage or at least qualitatively diagnosing it is a difficult task.
The clinical congestion, termed the “tip of the iceberg” by Gheorghiade [32], usually lasts a few hours and culminates with dramatically worsened dyspnea, which compels the patient to seek acute care. This is when management in ED seems to be carried out according to oldfashion patterns, embedded in known routines, rather than aligned to current guidelines and applied to the particular individual needs.
The third stage, residual congestion, is probably the most challenging one. Patients might be discharged early, which is highly advisable if reasonable decongestion has been attained. However, if substantial residual congestion persists at discharge, there is a great risk for shortand medium-term poor prognosis implying the need for sooner or later acute readmission.
Same logic applies to the patients who have been hospitalized for a while. If decongestion is inadequately assessed before discharge, as it may be the case when judgment is based mainly on body weight changes known nowadays to be neither sensitive nor specific to allow for identification or monitoring of patients with heart failure, the risk is again great that the patient will have to be readmitted within a short duration [28,35]. Data from the ADHERE registry indicate that up to 45% of patients have incomplete resolution of symptoms at the time of discharge [36,37].
4. MANAGEMENT PRINCIPLES
Figure 2 suggests that overall management of patients with AHF might be structured around three distinct pathophysiological processes occurring sequentially during the course of 3 - 4 weeks.
The ability to identify hemodynamic congestion before its symptoms arise forms a secondary prevention step for a heart failure worsening episode and may help to avoid hospitalizations and reduce disease progression in heart failure patients. Device-based fluid status monitoring (covered in this issue by Cleland et al.) including invasive alternatives (e.g., CRT-D or IDS) or noninvasive impedance monitoring (ICG) appear to develop into novel and innovative modalities for management of heart failure patients [38].
Management of heart failure in the ED might need to be refined; albeit, a simple but thorough bedside evaluation can provide key information about the degree of decompensation and overall prognosis. A simple strategy suggested by Nohria et al. classifies patients into fourspecific hemodynamic profiles based on the absence or presence of congestion (wet or dry) and the adequacy of perfusion (warm or cold), where congestion is defined by a Pulmonary Capillary Wedge Pressure (PCWP) > 18 mmHg [39]. These bedside hemodynamic profiles can be used successfully to guide therapy in most patients who are perfusing well and displaying different degrees of volume overload (includes approximately 70% of AHF patients). These patients are typical candidates for combined early diuretic and vasodilator therapy, with the caveat that therapy should be adjusted to maintain a blood pressure adequate for cerebral perfusion and to avoid postural hypotension [40]. Likewise, special attention should be paid with regard to the risk of some treatments inducing or worsening previous renal dysfunction, as this contributes to a prolonged length of hospital stay as well as increased mortality [26,41,42].
Management of heart failure in the ward (whether cardiology or internal medicine profiled) is multifactorial by definition. Frequently, coexistence of concomitant dis-

Figure 2. A holistic approach to management strategies in acute heart failure.
eases like diabetes, asthma, COPD, renal dysfunction, and variable level of cognitive impairment complicate both diagnosis as well as the management strategy. Obviously, particular attention is given to the underlying precipitants such as acute infections, ischemia, arrhythmia, etc. [43].
Evidence-based therapies should be instituted prior to discharge to improve long-term outcomes. Patients should receive education regarding healthy lifestyles, dietary discretion, medication adherence, and monitoring for and response to changes in fluid status. This can be facilitated by an early follow-up, possibly within 1 - 2 weeks after discharge to ensure adherence and clinical stability. Lastly, heart failure disease management programs have consistently been shown to reduce heart failure hospitalizations and should be optimally utilized, especially for high-risk patients [44,45].
5. ACKNOWLEDGEMENTS
The authors are both Novartis employees and declare that they have no competing interests.
REFERENCES
- Sato, N., Kajimoto, K., Asai, K., et al. (2010) Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: Rationale, design, and preliminary data. American Heart Journal, 159, 949-955. doi:10.1016/j.ahj.2010.03.019
- Collins, S.P., Pang, P.S., Lindsell, C.J., et al. (2010) International variations in the clinical, diagnostic, and treatment characteristics of emergency department patients with acute heart failure syndromes. European Journal of Heart Failure, 12, 1253-1260. doi:10.1093/eurjhf/hfq133
- Adams Jr., K.F., Fonarow, G.C., Emerman, C.L., et al. (2005) Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). American Heart Journal, 149, 209- 216. doi:10.1016/j.ahj.2004.08.005
- Cleland, J.G., Swedberg, K., Follath, F., et al. (2003) The EuroHeart Failure survey programme—A survey on the quality of care among patients with heart failure in Europe. Part 1: Patient characteristics and diagnosis. European Heart Journal, 24, 442-463. doi:10.1016/S0195-668X(02)00823-0
- Richard Hobbs, F.D. (2010) Clinical burden and health service challenges of chronic heart failure. British Journal of General Practice, 60, 611-615. doi:10.3399/bjgp10X515133
- Lloyd-Jones, D., Adams, R.J., Brown, T.M., et al. (2010) Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation, 121, e46-e215. doi:10.1161/CIRCULATIONAHA.109.192667
- Roger, V.L., Go, A.S., Lloyd-Jones, D.M., et al. (2012) Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation, 125, e2-e220. doi:10.1161/CIR.0b013e31823ac046
- Gheorghiade, M., Abraham, W.T., Albert, N.M., et al. (2006) Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. Journal of the American Medical Association, 296, 2217-2226. doi:10.1001/jama.296.18.2217
- Nieminen, M.S., Brutsaert, D., Dickstein, K., et al. (2006) EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. European Heart Journal, 27, 2725-2736. doi:10.1093/eurheartj/ehl193
- Parissis, J.T., Ikonomidis, I., Rafouli-Stergiou, P., et al. (2011) Clinical characteristics and predictors of in-hospital mortality in acute heart failure with preserved left ventricular ejection fraction. Journal of the American Medical Association, 107, 79-84. doi:10.1016/j.amjcard.2010.08.044
- Ross, J.S., Chen, J., Lin, Z., et al. (2010) Recent national trends in readmission rates after heart failure hospitalization. Circulation: Heart Failure, 3, 97-103. doi:10.1161/CIRCHEARTFAILURE.109.885210
- Hunt, S.A., Abraham, W.T., Chin, M.H., et al. (2009) 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. Journal of the American College of Cardiology, 53, e1-e90. doi:10.1016/j.jacc.2008.11.013
- Dickstein, K., Cohen-Solal, A., Filippatos, G., et al. (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). European Heart Journal, 29, 2388-2442. doi:10.1093/eurheartj/ehn309
- O’Connell, J.B. (2000) The economic burden of heart failure. Clinical Cardiology, 23, III6-III10. doi:10.1002/clc.4960231503
- Howlett, J.G., Johnstone, D.E., Sketris, I., et al. (2003) Identifying opportunities to address the congestive heart failure burden: The Improving Cardiovascular Outcomes in Nova Scotia (ICONS) study. Canadian Journal of Cardiology, 19, 439-444.
- Howlett, J.G., McKelvie, R.S., Arnold, J.M., et al. (2009) Canadian Cardiovascular Society Consensus Conference guidelines on heart failure, update 2009: Diagnosis and management of right-sided heart failure, myocarditis, device therapy and recent important clinical trials. Canadian Journal of Cardiology, 25, 85-105. doi:10.1016/S0828-282X(09)70477-5
- Jencks, S.F., Williams, M.V. and Coleman, E.A. (2009) Rehospitalizations among patients in the Medicare feefor-service program. The New England Journal of Medicine, 360, 1418-1428. doi:10.1056/NEJMsa0803563
- Remme, W.J., McMurray, J.J., Rauch, B., et al. (2005) Public awareness of heart failure in Europe: First results from SHAPE. European Heart Journal, 26, 2413-2421. doi:10.1093/eurheartj/ehi447
- Drazner, M.H., Hellkamp, A.S., Leier, C.V., et al. (2008) Value of clinician assessment of hemodynamics in advanced heart failure: The ESCAPE trial. Circulation: Heart Failure, 1, 170-177. doi:10.1161/CIRCHEARTFAILURE.108.769778
- Remes, J., Miettinen, H., Reunanen, A. and Pyorala, K. (1991) Validity of clinical diagnosis of heart failure in primary health care. European Heart Journal, 12, 315- 321.
- Marcus, G.M., Gerber, I.L., McKeown, B.H., et al. (2005) Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. Journal of the American Medical Association, 293, 2238-2244. doi:10.1001/jama.293.18.2238
- Stevenson, L.W. and Perloff, J.K. (1989) The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. Journal of the American Medical Association, 261, 884-888. doi:10.1001/jama.1989.03420060100040
- Chakko, S., Woska, D., Martinez, H., et al. (1991) Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: Conflicting results may lead to inappropriate care. American Journal of Medicine, 90, 353-359.
- Januzzi Jr., J.L., Camargo, C.A., Anwaruddin, S., et al. (2005) The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Journal of the American Medical Association, 95, 948-954. doi:10.1016/j.amjcard.2004.12.032
- Zaphiriou, A., Robb, S., Murray-Thomas, T., et al. (2005) The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. European Journal of Heart Failure, 7, 537-541. doi:10.1016/j.ejheart.2005.01.022
- McMurray, J.J., Adamopoulos, S., Anker, S.D., et al. (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Heart Journal, 33, 1787-1847. doi:10.1093/eurheartj/ehs104
- Richter, C.A., Kalenga, J.C., Rowe, B.H., et al. (2009) Practice patterns and outcomes in patients presenting to the emergency department with acute heart failure. Canadian Journal of Cardiology, 25, e173-e178. doi:10.1016/S0828-282X(09)70092-3
- Gattis, W.A. and O’Connor, C.M. (2004) Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure. Journal of the American Medical Association, 93, 74B-76B. doi:10.1016/j.amjcard.2004.01.019
- Peacock, W.F., Fonarow, G.C., Emerman, C.L., et al. (2007) Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology, 107, 44-51. doi:10.1159/000093612
- Peacock, W.F., Emerman, C., Costanzo, M.R., et al. (2009) Early vasoactive drugs improve heart failure outcomes. Congestive Heart Failure, 15, 256-264. doi:10.1111/j.1751-7133.2009.00112.x
- Cotter, G., Metra, M., Milo-Cotter, O., et al. (2008) Fluid overload in acute heart failure-re-distribution and other mechanisms beyond fluid accumulation. European Journal of Heart Failure, 10, 165-169. doi:10.1016/j.ejheart.2008.01.007
- Gheorghiade, M., Follath, F., Ponikowski, P., et al. (2010) Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. European Journal of Heart Failure, 12, 423-433. doi:10.1093/eurjhf/hfq045
- Nieminen, M.S., Bohm, M., Cowie, M.R., et al. (2005) Executive summary of the guidelines on the diagnosis and treatment of acute heart failure. Revista Española de Cardiología, 58, 389-429. doi:10.1157/13073896
- Gheorghiade, M., Filippatos, G., De, L.L. and Burnett, J. (2006) Congestion in acute heart failure syndromes: An essential target of evaluation and treatment. American Journal of Medicine, 119, S3-S10. doi:10.1016/j.amjmed.2006.09.011
- Adamson, P.B., Magalski, A., Braunschweig, F., et al. (2003) Ongoing right ventricular hemodynamics in heart failure: Clinical value of measurements derived from an implantable monitoring system. Journal of the American College of Cardiology, 41, 565-571. doi:10.1016/S0735-1097(02)02896-6
- Gheorghiade, M. and Filippatos G. (2005) Reassessing treatment of acute heart failure syndromes: The adhere registry. European Heart Journal, 7, B13-B17. doi:10.1093/eurheartj/sui008
- The ADHERE Registry. (2004) First Quarter 2004 National Benchmark Report. Fremont, Scios Inc., CA.
- Von Lueder, T.G. and Krum, H. (2012) Current modalities for invasive and non-invasive monitoring of volume status in heart failure. Heart, 98, 967-973. doi:10.1136/heartjnl-2011-301330
- Nohria, A., Tsang, S.W., Fang, J.C., et al. (2003) Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. Journal of the American College of Cardiology, 41, 1797-1804. doi:10.1016/S0735-1097(03)00309-7
- Arnold, J.M., Howlett, J.G., Dorian, P., et al. (2007) Canadian Cardiovascular Society Consensus Conference recommendations on heart failure update 2007: Prevention, management during intercurrent illness or acute decompensation, and use of biomarkers. Canadian Journal of Cardiology, 23, 21-45. doi:10.1016/S0828-282X(07)70211-8
- Aronson, D. and Burger, A.J. (2010) The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. Journal of Cardiac Failure, 16, 541-547. doi:10.1016/j.cardfail.2010.02.001
- Logeart, D., Tabet, J.Y., Hittinger, L., et al. (2008) Transient worsening of renal function during hospitalization for acute heart failure alters outcome. International Journal of Cardiology, 127, 228-232. doi:10.1016/j.ijcard.2007.06.007
- Fonarow, G.C., Abraham, W.T., Albert, N.M., et al. (2004) Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): Rationale and design. American Heart Journal, 148, 43-51. doi:10.1016/j.ahj.2004.03.004
- Hernandez, A.F., Greiner, M.A., Fonarow, G.C., et al. (2010) Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. Journal of the American Medical Association, 303, 1716-1722. doi:10.1001/jama.2010.533
- McDonald, K., Conlon, C., and Ledwidge, M. (2007) Disease management programs for heart failure: Not just for the “sick” heart failure population. European Journal of Heart Failure, 9, 113-117. doi:10.1016/j.ejheart.2006.05.005

