Journal of Water Resource and Protection
Vol. 3 No. 6 (2011) , Article ID: 5726 , 7 pages DOI:10.4236/jwarp.2011.36044
Cylindrospermopsin in Water Supply Reservoirs in Brazil Determined by Immunochemical and Molecular Methods
1Departamento de Ciências Biológicas, Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, São Paulo, Brasil
2Programa de Pós-Graduação em Ciências Biológicas, Universidade Estadual Paulista, Rio Claro, Brasil
3 Departamento de Análises Clínicas e Toxicológicas, Universidade Federal do Rio Grande do Norte, Natal, Brasil
4Departamento de Biologia, Universidade Federal Rural de Pernambuco, Recife, Brasil
E-mail: mbitt@esalq.usp.br, vipiccin@yahoo.com.br, paulakujbida@gmail.com, ariadne_moura@hotmail.com
Received April 11, 2011; revised May 12, 2011; accepted June 10, 2011
Keywords: Aphanizomenon, Cyanobacteria, Cylindrospermopsis raciborskii, CYN, Sphaerospermopsis aphanizomenoides, Toxin
ABSTRACT
It is reported for the first time in Brazil and South America the presence of cylindrospermopsin (CYN) in water supply reservoirs. CYN is a powerful hepatotoxic alkaloid implicated in outbreaks of human sicknesses. We detected CYN in different sources of water in Northeastern Brazil using molecular and immunological techniques. The highest concentrations of toxin occurred in the Jucazinho reservoir with the phytoplankton containing the potentially CYN-producing Cylindrospermopsis raciborskii and Sphaerospermopsis aphanizomenoides (previously known as Aphanizomenon aphanizomenoides). The polyketide synthase (PKS) and peptide synthetase (PS), which are directly related to the ability to produce CYN, were found in all the analyzed samples. The result of the present study emphasizes the need to improve monitoring of CYN in water bodies used for drinking and recreation, in order to avoid exposure of human populations to this toxin.
1. Introduction
CYN, a powerful hepatotoxin, was responsible for a human intoxication occurred in Australia in 1979 after water ingestion from a reservoir. This Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju bloom was treated with an algaecide releasing the intracellular toxin [1,2].
CYN also affects kidneys, intestinal tract, thymic system, vascular system and muscles [3-6]. At low doses, CYN suppresses protein synthesis, perhaps through the inhibition of the ribosomal translation via binding to protein associated with the eukaryotic protein synthesis system [7]. There is also evidence that this toxin has genotoxic, carcinogenic and mutagenic effects [8]. Moreover, there are reports of its accumulation in organisms indicating a possible pathway for human exposure to the cyanotoxin [9-14].
Brazil has a history of human and mammal contamination by microcystin, another hepatotoxin, with tenths of patients deaths at a dialysis center [15,16]. Carmichael et al. [10] has pointed out a possible CYN presence in liver samples of patients exposed in this outbreak [16]. However, because of the limited sample availability such a possibility has never been confirmed. On the other hand, there are not to date records for CYN occurrence in water bodies.
Brazilian legislation for water supply monitoring to the human population only recommends an upper limit of 15 μg·L–1 for CYN [17]. Little attention was paid to this issue because CYN has never been recorded before in the country and South America. CYN production has been reported in others cyanobacteria [18-21]. When toxic, isolated Brazilian strains of C. raciborskii and Aphanizomenon spp, have been found to produce saxitoxins (SAX) [22-25].
No other previous study found CYN in public water supplies in Brazil. In view of this, it was our goal to investigate if CYN occurs in Brazil. The present paper reports the presence of CYN in public water supplies for the first time in Brazil and South America. A molecular method, based on detection of genes encoding polyketide synthase (PKS) and peptide synthetase (PS) enzymes, plus immunoassay analysis (ELISA), were used to confirm the presence of this toxin. This finding highlights the importance of monitoring water quality with respect to CYN in public water supplies.
2. Material and Methods
2.1. Study Area
The Jucazinho, Duas Unas and Arcoverde reservoirs are located in Northeastern Brazil, supplying water for more than one million inhabitants. The climate of the region is low-latitude, semi-arid, with a mean annual temperature of 25˚C, mean wind speed of 5.0 m·s–1 and mean annual precipitation of 599 mm. There are two distinct seasons – one rainy (March to August) and the other dry (September to February).
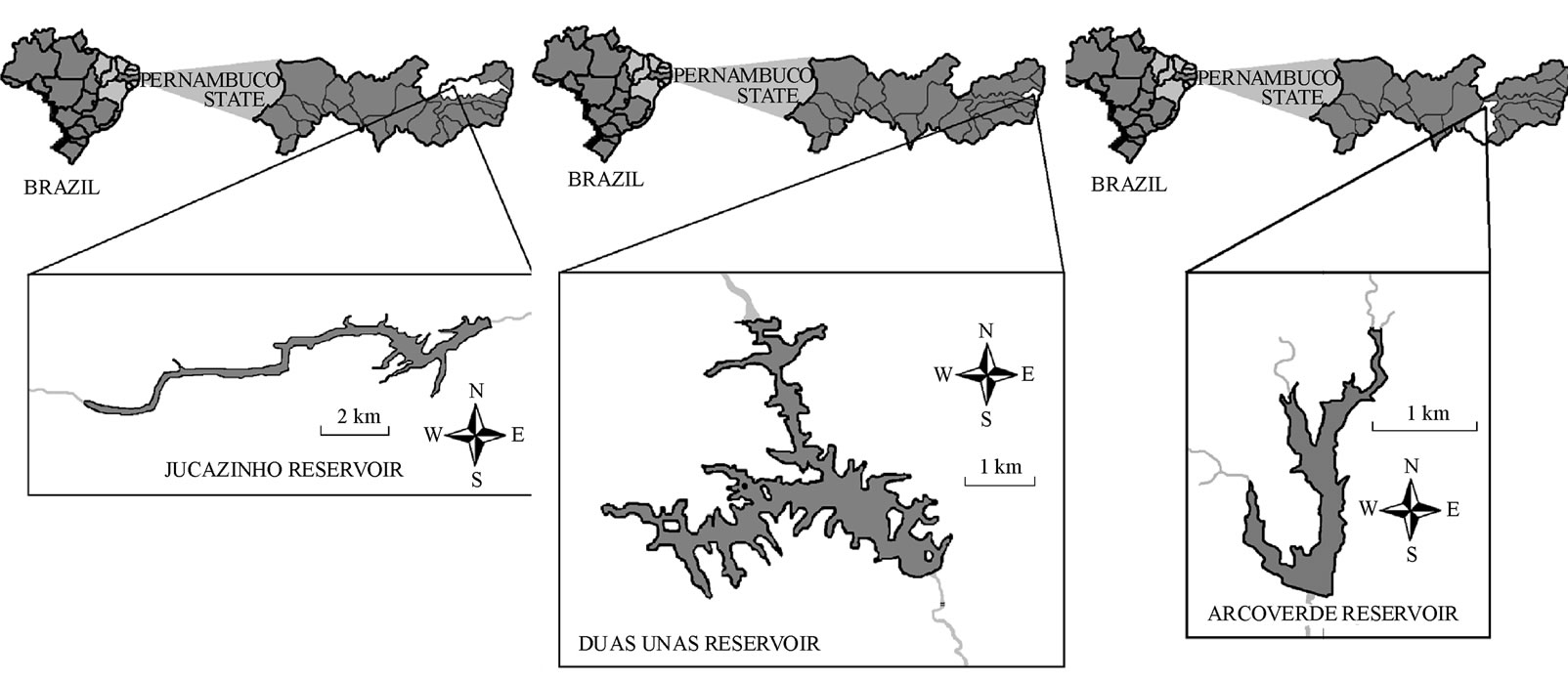
The Jucazinho reservoir belongs to the Agreste region (transition between the moist coastal/rainforest zone and the semi-arid deep interior of the state) (08˚56'41.1''S and 36˚29'32.20''W) (Figure 1(a)), with a volume of 327,035,818 m3. Intensive breeding of “tilapia” (Oreochromis niloticus Linnaeus) has been carried out at this location, increasing thus cyanobacterial blooms. The Duas Unas reservoir (Figure 1(b)) (08˚05'31''S and 35˚02'19''W) is located in the coastal/rainforest zone and has an accumulation capacity of 28,548,500 m3. There are vast areas of sugarcane cultivation in its hydrographic basin, along with strips of native preserved Atlantic rainforest. The Arcoverde reservoir (08˚33'33''S and 36˚59'07''W) (Figure 1(c)) is located in the semi-arid region and has a maximal capacity of 16,800,000 m3 in an area of 200 hectares.
2.2. Environmental Samples
Collections were carried out in 2009 at the Jucazinho (Feb 17, Mar 24, Apr 28 and Oct 27), Duas Unas (May 4) and Arcoverde (May 12) reservoirs, thereby totaling six samples. Samples for toxicological analyses and taxonomic identification were gathered from the surface of the reservoirs using a 20 µm mesh plankton net in the littoral zone. For quantitative analyses, water aliquots were collected using a van Dorn bottle and preserved in acetic Lugol’s solution. Environmental samples were immediately concentrated by centrifugation, lyophilized and stored at –20˚C until processing.
2.3. Identification and Quantification of Phytoplankton
Taxonomic identification was performed using a light microscope (Nikon YS100, Melville, NY, USA). Cyanobacteria were photographed with a light microscope (Nikon E200, Melville, NY, USA) equipped with a video camera system (Samsung SCC833, Tokyo, Japan) using the software Imagelab (Softium, São Paulo, SP, Brazil). Cell densities were determined using the Utermöhl method [26] expressed in cell·mL–1 and in percentage. In order to express results for the species as percentage the number of cells of each taxon was multiplied by 100 and then divided by the total number
 (a) (b) (c)
(a) (b) (c)
Figure 1. Location of reservoirs sampled in northeastern Brazil; (a) Jucazinho reservoir; (b) Duas Unas reservoir; (c) Arcoverde reservoir.
of cells in the sample.
2.4. DNA Extraction, Amplification and Sequencing
DNA was extracted from living cells from the environmental samples, according with [27]. For PCR amplification of partial PKS and PS genetic determinants, the oligonucleotide primer pairs (M4/M5 and M13/M14) described in [20] respectively, were used. Amplification of PKS (aoaC gene) and PS (aoaB gene) (650 to 725 bp) was performed using a GeneAmp PCR System 9700 thermocycler (Applied Biosystems, Foster City, CA, USA) and the pureTaq Ready-To-Go PCR Beads kit (GE Healthcare, Fairfield, CT, USA) according with [20]. Negative controls were carried out by using the same reaction conditions and primers, but without DNA. PCR reactions were performed at least in duplicate. Amplification products were visualized through electrophoresis on 1% agarose gels stained with ethidium bromide (0.2 mg·mL–1). PCR products were purified using the Purelink Kit (Invitrogen, Carlsbad, CA, USA), following manufacturer’s instructions.
Amplified fragments from the environmental samples were directly sequenced using the forward and reverse primers with the DYEnamic ET Terminator Cycle Sequencing Kit (Amersham Biosciences, Pittsburgh, PA, USA) in the 3100 ABI Sequencer (Applied Biosystems, Foster City, CA, USA), following the manufacturer’s instructions. The sequences were checked by visual inspection using the BioEdit routine version 7.0.9.0 [28], analyzed by a similarity search using BLAST (Basic Local Alignment Search Tool) and stored for nucleotide sequences at the GenBank database.
2.5. Cylindrospermopsin Analysis by Immunoassay Method
The lyophilized cells from environmental samples were broken by sonication and used directly for analysis of CYN. Toxin quantification was carried out using a commercial ELISA kit (Beacon Analytical Systems Inc., Portland, ME, USA), following the manufacturer’s protocols. The detection limit for CYN by ELISA was 0.1 ppb. The absorbance was measured using an ELISA plate reader (Asys Expert Plus, Cambs, UK) at a wavelength of 450 nm. The negative and positive controls of ELISA analysis are included in the commercial kit.
3. Results
Our results demonstrated the presence of CYN in environmental samples from Jucazinho, Duas Unas and Arcoverde reservoirs (Table 1). The highest concentrations of CYN occurred in the Jucazinho reservoir (2,718.0 ng.g-1 freeze-dried cells). The phytoplankton community contains the potentially CYN-producing species Sphaerospermopsis aphanizomenoides (previously denominated Aphanizomenon aphanizomenoides Forti) (Figure 2 (a)-(b)) and C. raciborskii (straight and coiled) (Figure 2 (c)-(e)). It was not possible to determine which was the CYN-producing cyanobacteria.
In all of the three studied reservoirs the phytoplankton community was constituted nearly exclusively by potentially toxin-producing cyanobacteria (Table 2, Figure 2), with percentages ranging from 99.2 to 100%.
All samples were positive for the PKS (aoaC gene), and PS (aoaB gene), which are directly related to the ability to produce CYN (Figure 3). The partial PKS (457 - 521 bp) and PS (406 - 505 bp) nucleotide sequences of three environmental samples from the Jucazinho reservoir (Feb 17, 2009, Mar 24, 2009 and Apr 28, 2009) were identical. The partial sequences of the PS shared 100% identity with published PS from Aphanizomenon ovalisporum Forti (accession number EU076460 and AF395828), and PKS (aoaC gene) shared 100% identity with Aph. ovalisporum (EU076461 and AF395828) in the GenBank database.
4. Discussion
Falconer & Humpage [29] and [30] mentioned CYN presence in South America only vaguely in articles abstracts, without proving any kind of published literature, or data set, all along the text. In 2006, Falconer & Humpage [31] stated that (sic) “CYN identified in water
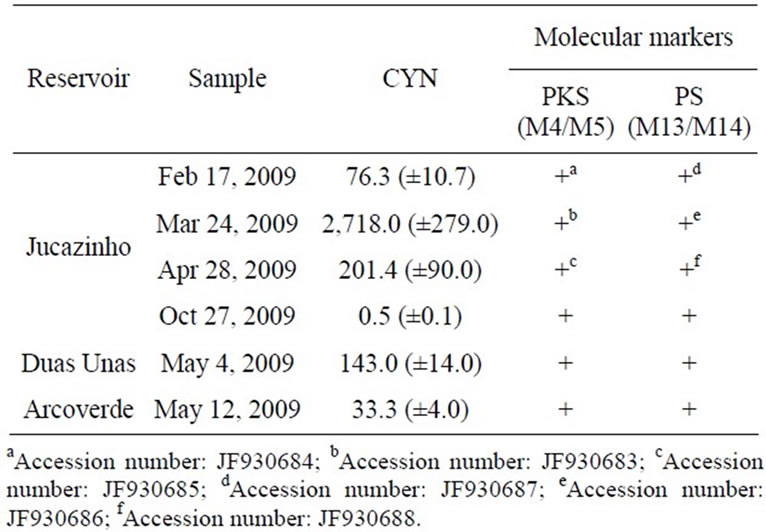
Table 1. Concentrations of CYN (ng·g-1 freeze-dried cells) in the environmental samples obtained by ELISA (mean of three replicates); (+) presence of molecular markers. PS. peptide synthetase. PKS. polyketide synthase.
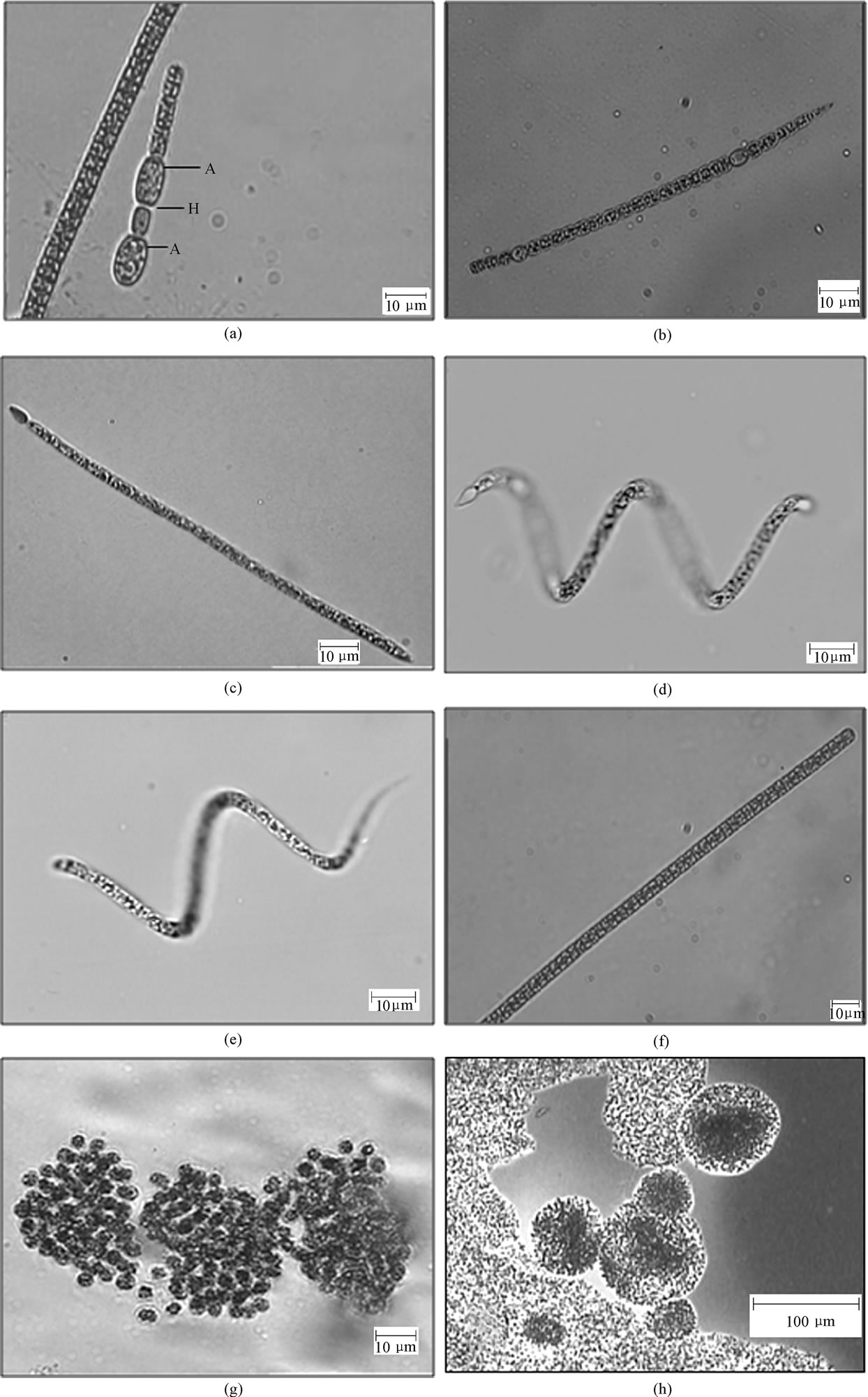
Figure 2. Bloom-forming and potential toxin-producing cyanobacteria found in samples; (a)-(b) S. aphanizomenoides; c. C. raciborskii (straight); d-e. C. raciborskii (coiled); f. Planktothrix agardhii; g. M. novacekii, h. M. panniformis. Jucazinho Reservoir: a-e, Duas Unas reservoir: f-h. A: akinete; H: heterocyte.
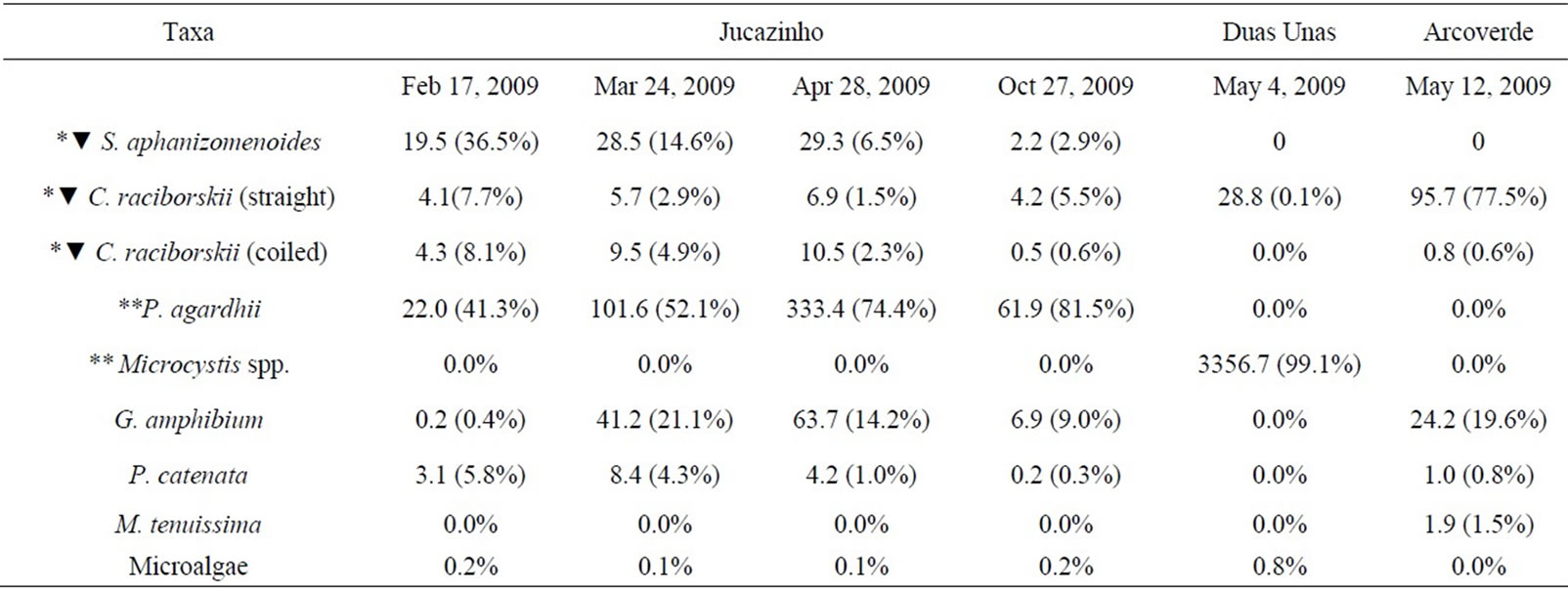
Table 2. Diversity and density of cyanobacteria and microalgae (106cell. mL–1); corresponding percentages of cell. mL–1 in phytoplankton are in parentheses; n: mean number of cells per organism; (*) Potential cylindrospermopsin-producing; (**) Potential microcystin-producing; (▼) Potential saxitoxin-producing; Sphaerospermopsis aphanizomenoides (n = 40); Cylindrospermopsis raciborskii (straight) (n = 20); C. raciborskii (coiled) (n = 25); Geitlerinema amphibium (n = 60); Planktothrix agardhii (n = 100); Pseudanabaena catenata (n = 20); Merismopedia tenuissima (n = 10); Microcystis spp.: M. panniformis and M. novacekii.
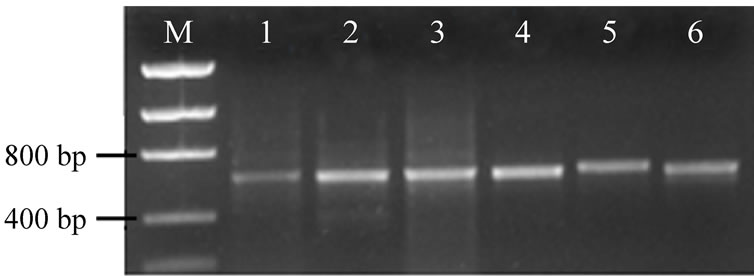
Figure 3. Presence of cylindrospermopsin biosynthesis genes in environmental samples by PCR amplification from Jucazinho reservoir. M. Molecular marker (Low DNA MassTM); Lanes 1, 3 and 5: PCR products for partial PKS genes (M4/M5 primers); Lanes 2, 4 and 6: PCR products for partial PS genes (M13/14 primers); Lanes 1-2: Feb 17, 2009 sample; Lanes 3-4: Mar 24, 2009 sample; Lanes 5-6: Apr 28, 2009 sample. Ethidium bromide-stained 1% agarose gel.
in Brazilian drinking water reservoir has not yet been attributed to any particular species of cyanobacterium”, without mentioning even a single reference of literature, or by simply showing a new finding.
We have detected CYN in cyanobacterial blooms in three reservoirs in Northeastern Brazil belonging to geographic basins located at different phyto-geographic areas (coastal/forest zone, Agreste and semi-arid region). However, these hydrographic basins are not isolated from each other, which can explain the dispersion of the toxin and of its producing organisms.
The PKS and PS genetic determinants are located within the aoaC and aoaB genes, respectively [32].The genes encoding polyketide synthase (PKS) and peptide synthetase (PS) enzymes have been found to be related to the production of CYN. The presence of these genes indicates the ability to produce CYN [20].
The present findings show the 100% homology between sequences of PCR fragments amplified from DNA samples from Brazilian reservoirs and those from in the GenBank database for the aoaB and aoaC genes indicating that molecular method was specific to detect genes which are directly related to the production of CYN.
Our results report detection of CYN in water supply reservoirs in Brazil using molecular and immunological assays. Brazilian legislation imposes the monitoring of water bodies for public supply. This monitoring should involve density of cyanobacteria (cell·mL–1) and an upper microcystin concentration of 1 μg·L–1 in drinking water. This bylaw, on the other hand, only recommends a maximal limit of 15 μg·L–1 for CYN [17], but this is not mandatory.
Because of the occurrence of CYN in water public supply, we stress the monitoring necessity of water bodies used for drinking and leisure, in order to avoid exposure of humans to this toxin.
5. Acknowledgments
This study was supported by grants from CNPQ (Proc. 576890/2008-1, 300794/2004-5) and FAPESP (Proc. 2008/56534-5) – Brazilian agencies for the promotion of Science.
6. Abbreviations
CYN, cylindrospermopsin, PKS, polyketide synthase, PS, Peptide synthetase.
REFERENCES
- P. R. Hawkins, M. T. C. Runnegar, A. R. B. Jackson and I. R. Falconer, “Severe Hepatotoxicity Caused by the Tropical Cyanobacterium (Blue-Green Alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya et Subba Raju Isolated from a Domestic Water Supply Reservoir,” Applied and Environmental Microbiology, Vol. 50, No. 5, 1985, pp. 1292-1295.
- I. Ohtani, R. E. Moore and M. T. C. Runnegar, “Cylindrospermopsin: A Potent Hepatotoxin from the BlueGreen-Alga Cylindrospermopsis raciborskii,” Journal of American Chemical Society, Vol. 114, No. 20, 1992, pp. 7941-7942. doi:10.1021/ja00046a067
- S. M. Froscio, E. Cannon, H. M. Lau and A. R. Humpage, “Limitad Uptake of the Cyanobacterial Toxin Cylindrospermopsin by Vero Cells,” Toxicon, Vol. 54, No. 4, 2006, pp. 299-304.
- K. Terao, S. Ohmori, K. Igarashi, I. Ohtani, M. F. Watanabe, K. I. Harada, E. Ito and M. Watanabe, “Electron Microscopic Studies on Experimental Poisoning in Mice Induced by Cylindrospermopsin Isolated from Blue-Green Alga Umezakia natans,” Toxicon, Vol. 32, No. 7, 1994, pp. 833-843. doi:10.1016/0041-0101(94)90008-6
- L. R. Falconer, S. J. Hardy, A. R. Humpage, S. M. Froscio, G. J. Tozer and P. R. Hawkins, “Hepatic and Renal Toxicity of the Blue-Green Alga (Cyanobacterium) Cylindrospermopsis raciborskii in a Male Swiss Albino Mice,” Environmental Toxicology, Vol. 14, No. 1, 1999, pp. 143-150. doi:10.1002/(SICI)1522-7278(199902)14:1<143::AID-TOX18>3.0.CO;2-H
- S. M. Froscio, S. Fanok, and A. R. Humpage, “Cytotoxicity Screening for the Cyanobacterial Toxin Cylindrospermopsin,” Journal of Toxicology and Environmental Health Part A, Vol. 72, No. 5, 2009, pp. 345-349. doi:10.1080/15287390802529906
- S. M. Froscio, A. R. Humpage, W. Wickramasinghe, G. Shaw and I. R. Falconer, “Interaction of the Cyanobacterial Toxin Cylindrospermopsin with the Eukaryotic Protein Synthesis System,” Toxicon, Vol. 51, No. 2, 2008, pp. 191-198. doi:10.1016/j.toxicon.2007.09.001
- A. R. Humpage, M. Fenech, P. Thomas and I. R. Falconer, “Micronucleus Induction and Chromosome Loss in Transformed Human White Cells Indicate Clastogenic and Aneugenic Action of the Cyanobacterial Toxin, Cylindrospermopsin,” Mutation Research, Vol. 472, No. 1-2, 2000, pp.155-161.
- M. L. Saker and G. E. Eaglesham, “The Accumulation of Cylindrospermopsin fom the Cyanobacterium Cylindrospermopsis raciborskii in Tissues of the Redclaw Crayfish Cherax quadricarinatus,” Toxicon, Vol. 37, No. 7, 1999, pp. 1065-1077. doi:10.1016/S0041-0101(98)00240-2
- W. W. Carmichael, S. M. F. O. Azevedo, J. S. An, R J. R. Molica, E. M. Jochimsen, S. Lau, K. L. Rinehart, G. R. Shaw and G. K. Eaglesham, “Human Fatalities from Cyanobacteria: Chemical and Biological Evidence for Cyanotoxins,” Environmental Health Perspectives, Vol. 109, No. 7, 2001, pp. 663-668. doi:10.1289/ehp.01109663
- I. C. G. Nogueira, M. L. Saker and V. M. Vasconcelos, “Toxicity of the Cyanobacterium Cylindrospermopsis raciborskii to Daphnia magna,” Environmental Toxicology, Vol. 19, No. 5, 2004, pp. 453-459. doi:10.1002/tox.20050
- J. A. Leonard and H. W. Paerl, “Zooplankton Community Structure, Micro-Zooplankton Grazing Impact, and Seston Energy Content in the St. Johns River System, Florida as Influenced by The Toxic Cyanobacterium Cylindrospermopsis raciborskii,” Hydrobiologia, Vol. 537, No. 1-3, 2005, pp. 89-97. doi:10.1007/s10750-004-2483-9
- J. P. Berry and O. Lind, “First Evidence of ‘Paralytic Shellfish Toxins’ and Cylindrospermopsin in a Mexican Freshwater System, Lago Catemaco, and Apparent Bioaccumulation of the Toxins in ‘Tegogolo’ Snails (Pomacea patula catemacensis),” Toxicon, Vol. 55, No. 5, 2010, pp. 930-938. doi:10.1016/j.toxicon.2009.07.035
- V. Messineo, S. Melchiorre, A. Di Corcia, P. Gallo and M. Bruno, “Seasonal Succession of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum Blooms with Cylindrospermopsin Occurrence in the Volcanic Lake Albano, Central Italy,” Environmental Toxicology, Vol. 25, No. 2, 2010, pp. 18-27.
- M. G. L. C. Teixeira, M. C. N. Costa, V. L. P. Carvalho, M. S. Pereira and E. Hage, “Gastroenteritis Epidemic in the Area of the Itaparica, Bahia, Brazil,” Bulletin of PAHO, Vol. 27, No. 3, 1993, pp. 244-253.
- E. M. Jochimsen, W. W. Carmichael, J. An, D. M. Cardo, S. T. Cookson, C. E. M. Holmes, B. C. Antunes, D. A. Melo Filho, T. M. Lyra, V. S. T. Barreto, S. M. F. O. Azevedo and W. R. Jarvis, “Liver Failure and Death after Exposure to Microcystin at a Hemodialysis Center in Brazil,” The New England Journal of Medicine, Vol. 338, No. 13, 1998, pp. 873-878. doi:10.1056/NEJM199803263381304
- Regulation MS N. 518/2004, “Guidelines for Drinking Water Quality,” Official Law Report’s, 26 March 2004, p. 266.
- K. Harada, I. Ohtani, K. Iwamoto, M. Suzuki, M. F. Watanabe, M. Watanabe and K. Terao, “Isolation of Cylindrospermopsin from a Cyanobacterium Umezakia natans and its Screening Method,” Toxicon, Vol. 32, No. 1, 1994, pp. 73-74. doi:10.1016/0041-0101(94)90023-X
- M. Banker, S. Carmeli, O. Hadas, B. Teltsch, R. Porat and A. Sukenik,“Identification of Cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) Isolated From Lake Kinneret, Israel,” Journal of Phycology, Vol. 33, No. 4, 1997, pp. 613-616. doi:10.1111/j.0022-3646.1997.00613.x
- M. A. Schembri, B. A. Neilan and C. P. Saint, “Identification of Genes Implicated in Toxin Production in the Cyanobacterium Cylindrospermopsis raciborskii,” Environmental Toxicology, Vol. 16, No. 5, 2001, pp. 413-421. doi:10.1002/tox.1051
- M. Seifert, G. McGregor, G. Eaglesham, W. Wickramasinghe and G. Shaw, “First Evidence for the Production of Cylindrospermopsin and Deoxy-Cylindrospermopsin by the Freshwater Benthic Cyanobacterium, Lyngya wollei Farlow ex Gomont Speziale and Dyck,” Harmful Algae, Vol. 6, No. 1, 2007, 73-80. doi:10.1016/j.hal.2006.07.001
- N. Lagos, H. Onodera, P. A. Zagatto, D. Andrinolo, S. M. F. O. Azevedo and Y. Oshima, “The First Evidence of Paralytic Shellfish Toxins in the Freshwater Cyanobacterium Cylindrospermopsis raciborskii Isolated from Brazil,” Toxicon, Vol. 37, No. 10, 1999, pp. 1359-1373. doi:10.1016/S0041-0101(99)00080-X
- R. Molica, H. Onodera, C. Garcia, M. Rivas, D. Andrinolo, S. Nascimento, H. Miguro, Y. Oshima, S. Azevedo and N. Lagos, “Toxins in the Freshwater Cyanobacterium Cylindrospermopsis raciborskii (Cyanophyceae) Isolated from Tabocas Reservoir in Caruaru, Brazil, Including Demonstration of a New Saxitoxin Analogue,” Phycologia, Vol. 41, No. 6, 2002, 606-611. doi:10.2216/i0031-8884-41-6-606.1
- J. R. R. Molica, E. J. A. Oliveira, P. V. V. C. Carvalho, A. N. S. F. Costa, M. C. C. Cunha, G. L. Melo, “Occurrence of Saxitoxins and Anatoxin-A (S)-Like Anticholinesterase in a Brazilian Drinking Water Supply,” Harmful Algae, Vol. 4, No. 4, 2005, 743-753. doi:10.1016/j.hal.2004.11.001
- I. A. S. Costa, S. M. F. O. Azevedo, P.A.C. Senna, R. R. Bernardo, S. M. Costa and N. T. Chellappa, “Occurrence of Toxin-Producing Cyanobacteria Blooms in a Brazilian Semiarid Reservoir,” Brazilian Journal of Biology, Vol. 66, No. 1B, 2006, pp. 211-219. doi:10.1590/S1519-69842006000200005
- H. Utermöhl, “Zur Vervollkommung der Quantitativen Phytoplanktonmethodik,” Internationale Vereinigung fuer Theoretische und Angewandte Limnologie Verhand lungen, Vol. 9, 1958, pp. 1-38.
- M. C. Bittencourt-Oliveira, V. Piccin-Santos and S. Gouvêa-Barros, “Microcystin-Producing Genotypes from Cyanobacteria in Brazilian Reservoirs,” Environmental Toxicology, 2010. http://onlinelibrary.wiley.com/doi/10.1002/tox.20659/full
- T. A. Hall, “Bioedit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 97/98/NT,” Nucleic Acids Symposium Series, Vol. 41, 1999, pp. 95-98.
- I. R. Falconer and A. R. Humpage, “Preliminary Evidence for in Vivo Tumour Initiation by Oral Administration of Extracts of the Blue-Green Alga Cylindrospermopsis raciborskii Containing the Toxin Cylindrospermopsin,” Environmental Toxicology, Vol. 16, No.2, 2001, pp. 192- 195. doi:10.1002/tox.1024
- A. Törökné, M. Asztalos, M. Bánkiné, H. Bickel, G. Borbély, S. Carmeli, G. A. Codd, J. Fastner, Q. Huang, A. Humpage, J. S. Metcalf, E. Rábai, A. Sukenik, G. Surányi, G. Vasas, and V. Weiszfeiler, “Interlaboratory Comparison Trial on Cylindrospermopsin Measurement,” Analytical Biochemistry, Vol. 332, No. 2, 2004, pp. 280-284. doi:10.1016/j.ab.2004.05.036
- I. R. Falconer and A. R. Humpage. “Cyanobacterial (Blue-Green Algal) Toxins in Water Supplies: Cylindrospermopsins,” Environmental Toxicology, Vol. 21, No. 4, 2006, pp. 299-304. doi:10.1002/tox.20194
- J. P. Rasmussen, S. Giglio, P. T. Monis, R. J. Campbell and C. P. Saint, “Development and Field Testing of A Real-Time PCR Assay for Cylindrospermopsin-Producing Cyanobacteria,” Journal of Applied Microbiology, Vol. 104, No. 5, 2008, pp. 1503-1515. doi:10.1111/j.1365-2672.2007.03676.x

