Journal of Water Resource and Protection
Vol.2 No.8(2010), Article ID:2449,5 pages DOI:10.4236/jwarp.2010.28080
Biosorption of Ni(II), Cu(II) and Pb(II) by Punica geranatum from Aqueous Solutions
Department of Phytochemistry, Medicinal Plants and Drugs Research Institute,
Shahid Beheshti University, Tehran, Iran
E-mail: p-salehi@sbu.ac.ir
Received April 7, 2010; revised May 31, 2010; accepted July 15, 2010
Keywords: Biosorption, Punica geranatum, Langmuir, Freundlich, Kinetic Models
ABSTRACT
In this paper removal of Ni(II), Cu(II) and Pb(II) ions from aqueous solutions by leaves of Punica geranatum were investigated. The biosorption was found to be pH dependent and the highest uptake of all the mentioned metal ions occurred at pH 4. Furthermore, the influence of other parameters such as initial metal ions concentration and contact time of biosorbent and sorbents were evaluated. Equilibrium data fitted very well to Langmuir model for all studied metals. It was also concluded that the Freundlich isotherm cannot be enough appropriate for the equilibrium data of all three metals. Biosorption of Ni(II), Cu(II) and Pb(II), reached equilibrium in 60, 60 and 30 min, respectively. Moreover, the adsorption rate of the metals can be best described by the second order model.
1. Introduction
Development of industrialization and human activities culminated in the increased discharge of dreg and wastewater containing heavy metals into environment. The toxic effects of some metals, such as zinc, and the tendency of other metals, such as lead and cadmium, to accumulate within the food chain, make their presence in nature a severe threat to aquatic life [1].
The conventional techniques are commonly applied for the removal of heavy metals from wastewater including chemical (precipitation, neutralization, oxidation or reduction) or physical (ion exchange, membrane separation, reverse osmosis and adsorption processes) methods [2,3]. However, most of these methods are complicated and expensive. Furthermore, when these processes are applied to diluted metal wastes or lower concentrations of metal ions, they are ineffective [4,5].
Consequently, attempts have been made in order to find new straightforward and efficient techniques. Biosorption, the use of microorganisms (such as bacteria and fungi) and photosynthetic life (such as algae, aquatic and emergent plants), for the removal of metal ions, is one emerging technology that holds promise in this regard [6,7]. Considerable potential exists for these naturally existing, abundant and cheap sources of biomass [8]. The biosorption process is effective even if the concentration is as low as 200 μg/ml [9].
There are two general mechanisms for the uptake of dissolved metal ions from water by biological biomass. The first one, metabolism-independent surface reactions, encompasses surface precipitation and surface complexation. This step is very rapid and occurs in a short time after the biomass comes into contact with the metals. The second one, however, is metabolism-dependent uptake step, in which the metal is transported in to the cells. This stage is slower than the first one [6,10].
Several mathematical models have been proposed in literature in order to describe the equilibrium isotherms of adsorption process. Langmuir and Freundlich isotherms, which are very well-known models in describing heavy metal biosorption, have been extensively in this work.
A general expression of the Langmuir model is [11,12]:
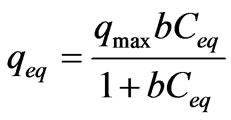 (1)
(1)
where qeq is the amount of metal adsorbed per gram of biomass weight at the equilibrium state, Ceq is the residual metal concentration left in solution after binding, qmax is the maximum possible amount of metallic ion, adsorbed per unit of weight of adsorbent and b is a constant related to the affinity of the binding sites for the metals.
The Freundlich model is expressed as [13]:
 (2)
(2)
where qeq is the amount of solute per weight unit of dry sorbent, KF and n are empirically determined constants and Ceq is the solute equilibrium concentration. The Freundlich equation is often linearized by logarithmic transfer of Equation (2).
 (3)
(3)
The amount of metal ions adsorbed per unit of empty sorbent was obtained by using the following equation:
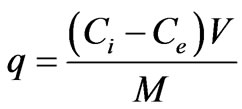 (4)
(4)
where q is the amount of metal ions, adsorbed onto the mass unit of the adsorbent, Ci and Ce are the concentrations of the metal ions before and after biosorption, V is the volume of the aqueous phase, and M is the amount of the adsorbent.
The kinetics of heavy metal adsorption can be modeled by the Lagergren pseudo-first order and second order reaction rate equations.
Lagergren developed a model for adsorption kinetics based on the first order reaction [14]. The equation is expressed as follows:
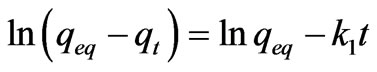 (5)
(5)
where k1 is Lagergren rate constant for adsorption, qeq is the amount of adsorbate, adsorbed at equilibrium state and qt is the amount of adsorbate adsorbed at any given time t.
A second order, reaction rate equation was proposed by Ho and Mckay to study the kinetics of adsorption [15]. The equation is expressed as follows:
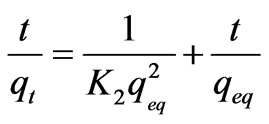 (6)
(6)
where K2 is the second order adsorption rate constant.
In the present study the potential of Punica geranatum leaves for removal of Ni(II), Cu(II) and Pb(II) ions from aqueous solutions, and the effect of various parameters such as contact time of biosorbent and sorbents, pH of metal solutions and initial metal ions concentration have been investigated. Equilibrium modeling of biosorption was carried out using Langmuir and Freundlich, adsorption models equations. In addition, the biosorption Lagergren pseudo-first order and second order models were used to elucidate the kinetic characteristics of this process.
2. Materials and Methods
2.1. Preparation of Biomass and Metal Solutions
The leaves of Punica geranatum were collected in May 2006 from Yazd, Iran. The leaves were extensively washed with deionized water and then dried at room temperature. Dried biomass was powdered in a laboratory blender and sorted by sieving technique using the standard test sieves. The batch of biomass with suitable particle size (35-60 mesh) was selected for further experiments. The stock solutions (1000 mg/L) of Ni(II), Cu(II) and Pb(II) were prepared from analytical grade nitrate salts (Merck). The working solutions were made by diluting the stock solutions to appropriate volumes. The pH of each solution was adjusted, with diluted or concentrated HCl and NaOH solutions before mixing of biomass.
2.2. Experimental Conditions
Batch biosorption studies were conducted in 100 ml flasks by transferring 50 ml of metal solutions with the appropriate pH and concentration, and 0.5 gr of the biomass. The flasks were agitated on a shaker at 100 rpm for a predetermined sorption time period. After the incubation time, solutions were filtered and the metal ions concentrations were measured by shimadzu, AA-6800 model flame atomic absorption spectrophotometer equipped with Hallow Cathode Lamp and air acetylene burner.
3. Results and Discussion
3.1. The Effect of pH on Biosorption
The solution pH is one of the most important parameters affecting the biosorption process [16]. The effect of pH on metal uptake is shown in Figure 1. It was observed that the biosorption of Ni(II), Cu(II) and Pb(II) on P. granatum leaves increased with pH up to 4.0. Solution pH affects both cell surface metal binding sites and metal chemistry in water [17]. At low pH values, cell wall ligands are totally involved in H+ and H3O+ ions, so repulsive forces make binding sites inaccessible for the metal ions. With an increase in pH, the negative charge density on the biosorbent rises which results in attraction between these negative charges and the metal ions and therefore in increasing the biosorption [18,19].
It was revealed that biosorption was very nominal in pH 2 (12.6%, 11.7% and 21.4%, for Ni(II), Cu(II) and Pb(II), respectively). It was also found that maximum uptake percent of all metal ions was in pH 4. An insignificant decline in biosorption could be observed in pH 6 due to the decrease in the metal solubility and precipitation of metal hydroxides.
3.2. Effect of Initial Metal Concentrations
The metal uptake mechanism is remarkably dependent
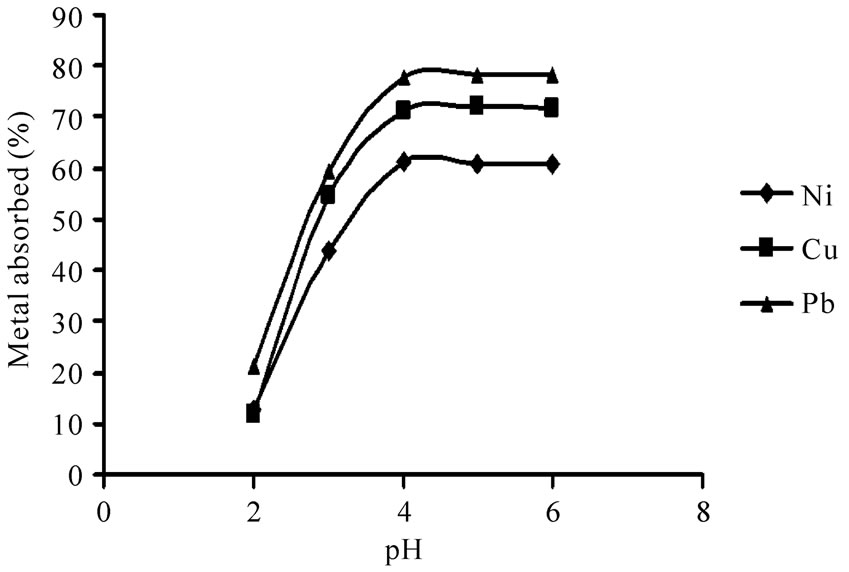
Figure 1. The effect of pH on the biosorption of Ni(II), Cu(II) and Pb(II) from 10 mg/L metal ion solution and 10 g/L P. granatum under equilibrium times and shake flask at 100 rpm at room temperature.
on the initial metal ions concentrations [20]. Increasing the initial metal ion concentration causes an increase in the biosorption capacity of the biosorbent which stems from the fact that the probability of collusion between metal ions and biosorbent increases in this condition which enhances the biosorption ability [18,21].
Sorption of heavy metals on P. geranatum biomass was carried out at diverse initial metal ion concentrations (5-1000 ppm) at pH 4. According to Figure 2, the slopes of the curves (qeq) were enhanced in lower concentrations with increasing Ceq (equilibrium concentration) for all the examined metals and with further increment in concentration, qeq values exhibited negligible variations due to the saturation of the binding sites.
3.3. Adsorption Isotherms
An adsorption isotherm is characterized by special constants which express the surface properties and the affinity of the sorbate to the sorbent [22]. Out of several isotherm equations, Langmuir and Freundlich models have been applied for this study, which were expressed previously in detail.
The linearized Langmuir and Freundlich adsorption isotherms of each metal ion are exhibited in Figures 3 and 4.
The Langmuir and Freundlich adsorption constants which were mentioned in Equations (1) and (2), together with the correlation coefficients (r2) are listed in Table 1.
The r2 values are respected as a measure of fitness of experimental data on the isotherm models [23], which for all the metals in the Langmuir model were very close to 1. The experimental maximum uptake values were 11.4, 15.5 and 18.4 for Ni(II) Cu(II), and Pb(II), respectively which showed acceptable accordance to theoretical values from the Langmuir equation. The qmax (maximum uptake) for Pb(II) was higher than other metals which

Figure 2. The effect of initial metal ion concentration (5-1000 mg/L), on the biosorption of Ni(II), Cu(II) and Pb(II), pH 4, 10 g/L P. granatum under equilibrium times in shake flask at 100 rpm at room temperature.
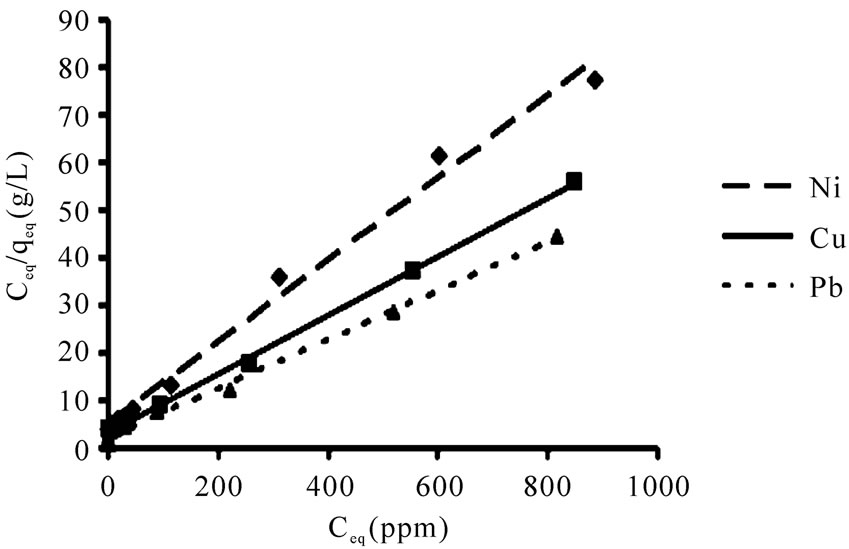
Figure 3. The Langmuir adsorption isotherms for Ni(II), Cu(II) and Pb(II) biosorption by P. granatum (10 g/L). Conditions: initial metal concentration of 5-1000 mg/L, pH 4, flask shaking at 100 rpm at room temperature under equilibrium time for each metal.
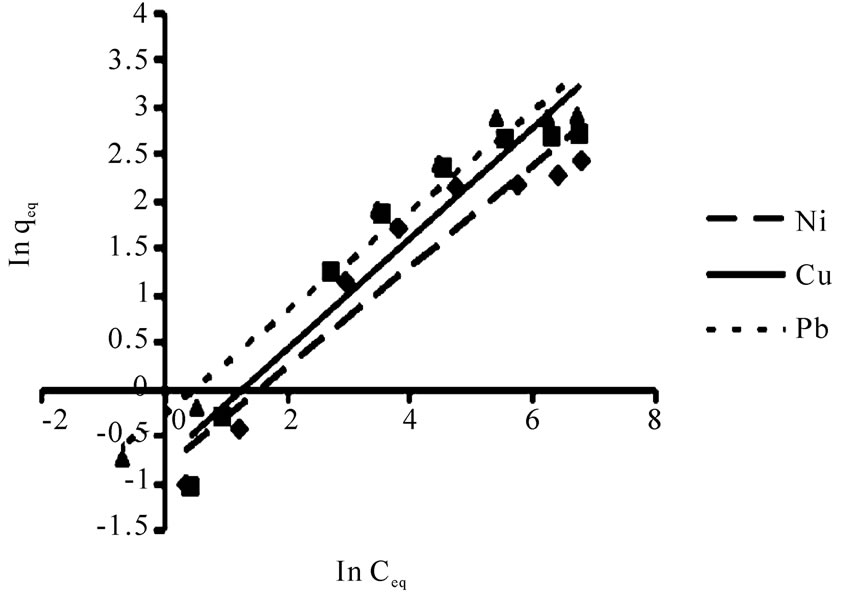
Figure 4. The Freundlich adsorption isotherm for Ni(II), Cu(II) and Pb(II) biosorption by P. granatum (10 g/L). Conditions: initial metal concentration of 5-1000 mg/L, pH 4, flask shaking at 100 rpm at room temperature under equilibrium time for each metal.

Table 1. Langmuir and Freundlich constants for the sorption of each metal with P. granatum biomass.
implied the higher sorption capacity of P. granatum leaves for Pb(II). The relative order of metal uptake affinity of P. granatum leaves was Pb(II) > Cu(II) > Ni(II) based on b amounts.
The r2 values for Freundlich isotherm were lower than Langmuir isotherm which expressed Ni(II), Cu(II) and Pb(II), ions adsorption are relatively conform to the Freundlich model, though not as perfect as to the Langmuir model.
3.4. Biosorption Kinetics
The change in the amount of adsorbed metal with time is shown in Figure 5. Biosorption of heavy metals demonestrated a fast rate during the initial 30 min due to huge amounts of unoccupied sites on the biosorbent. Biosorption of Ni(II), Cu(II) and Pb(II), reached equilibrium in 60, 60 and 30 min, respectively. Beyond these contact times there was no further increase in heavy metal removal because all the active sites had been occupied. The percent of heavy metal removal on the equilibrium times were 61.1%, 70.4% and 78.0% for Ni(II), Cu(II) and Pb(II), respectively.
According to the Equations (5) and (6) which were implied previously, kinetic data could not be described by the pseudo-first order model. Low correlation coefficients (< 0.68) were attained for this model in comparison with coefficients for second order model which were above 0.99 (Figure 6) for all the metals and acceptable fitness of experimental data in this model endorsed this results. It was proved from Table 2 that the theoretical qeq values calculated from Lagergren first order kinetic model for biosorption of Ni(II), Cu(II) and Pb(II), had huge variations with the experimental qeq values, though the experimental and theoretical qeq values for second order kinetic model can interpret more precise description of adsorption kinetic [13].
4. Conclusions
Punica granatum leaves was shown to be significantly effective in uptake of Pb(II) from aqueous solutions
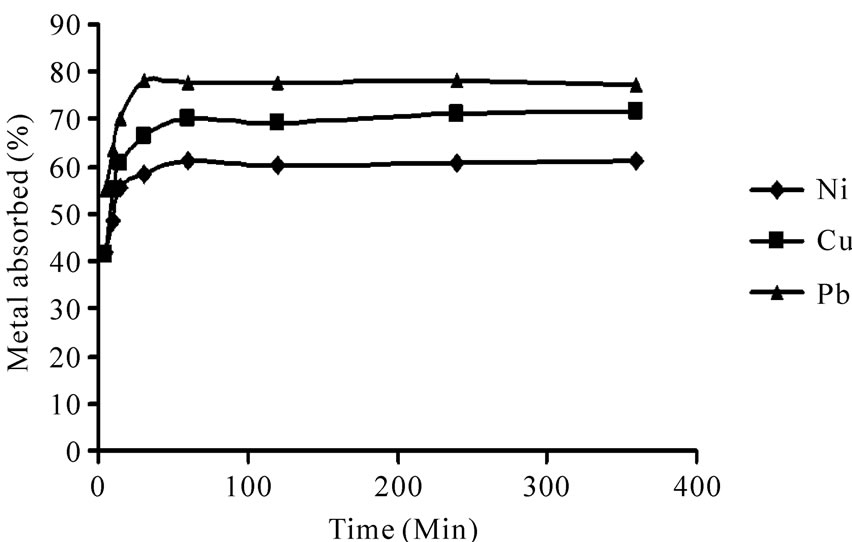
Figure 5. The time-course relationship of the biosorption of Ni(II), Cu(II) and Pb(II) from 10 mg/L metal ion solution, pH 4, 10 g/L P. granatum in shake flask at 100 rpm at room temperature.
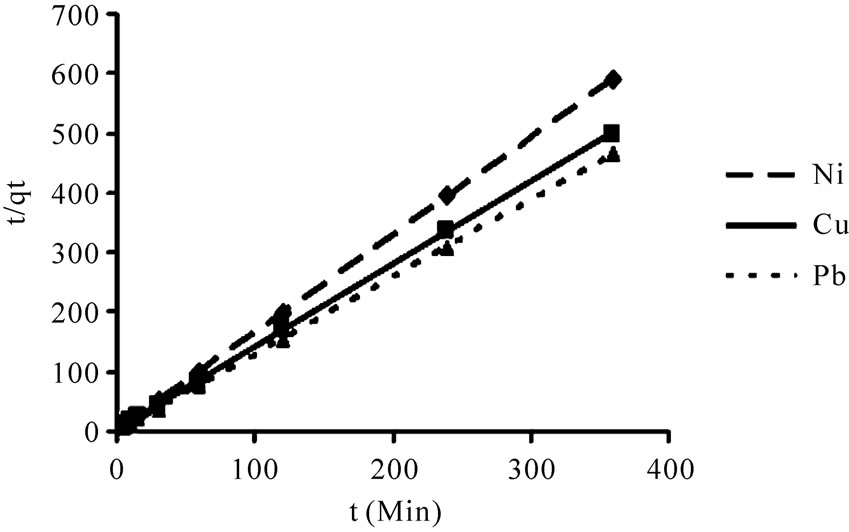
Figure 6. Biosorption of Ni(II), Cu(II) and Pb(II), by P. granatum on the second-order reaction kinetics model, as related to time (t) and the quantity of metal adsorbed at t (qt).
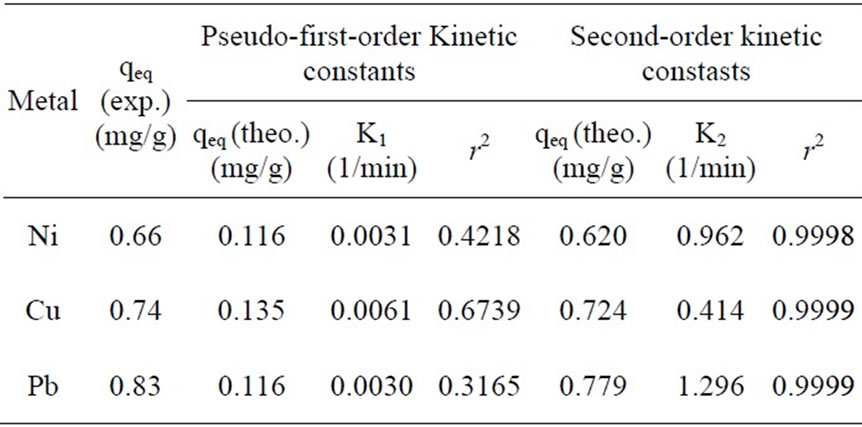
Table 2. Pseudo-first-order and second-order kinetic constants for the sorption of each metal with P. granatum biomass.
among the tested metals which proves its notable application for remediation of environment from lead which is a threatening danger for environment and people’s health. The study of metal’s biosorption by P. granatum leaves indicated that the process conform Langmuir isotherm model, reasonably. The overall adsorption rate of the Ni(II), Cu(II) and Pb(II) can be best described by the second order model which the results of experiments completely confirm this claim.
5. Acknowledgements
We are grateful to Shahid Beheshti University Research Council for financial support of this work.
REFERENCES
- J. O. Nriagu and J. M. Pacyna, “Quantitative Assessment of World Wide Contamination of Air, Water and Soils by Trace Metals,” Nature, Vol. 333, No. 6169, May 1988, pp. 134-139.
- B. W. Atkinson, F. Bux and H. C. Kasan, “Considerations for Application of Biosorption Technology to Remediate Metal—Contaminate Industrial Effluents,” Water of South Africa, Vol. 24, No. 2, April 1998, pp. 129-135.
- F. A. Abu Al-Rub, M. H. El-Naas, F. Benyahia and I. Ashour, “Biosorption of Nickel on Blank Alginate Beads, Free and Immobilized Algal Cells,” Process Biochemistry, Vol. 39, No. 11, July 2004, pp. 1767-1773.
- B. Volesky, “Detoxification of Metal Bearing Effluents: Biosorption for the Next Century,” Hydrometallurgy, Vol. 59, No. 2, February 2001, pp. 203-216.
- D. Kratochvil and B. Volesky, “Advaces in the Biosorption of Heavy Metals,” Trends in Biotechnology, Vol. 16, No. 7, July 1998, pp. 291-300.
- S. P. K. Sternberg and R. W. Dorn, “Cadmium Removal Using Cladophora in Batch, Semi-Batch and Flow Reactors,” Bioresource Technology, Vol. 81, No. 3, February 2002, pp. 249-255,
- N. Akhtar, M. Iqbal, S. I. Zafar and J. Iqbal, “Biosorption Characteristics of Unicellular Green Alga Chlorella Sorokiniana Immobilized in Loofa Sponge for Removal of Cr(III),” Journal of Environmental Sciences, Vol. 20, No. 2, February 2008, pp. 231-239.
- S. S. Ahluwalia and D. Goyal, “Microbial and Plant DeRived Biomass for Removal of Heavy Metals from Waste-Water,” Bioresource Technology, Vol. 98, No. 12, September 2007, pp. 2243-2257.
- S. R. Popuri, A. Jammala, K. V. N. S. Reddy and K. Abburi, “Biosorption of Hexavalent Chromium Using Tamarind (Tamarindus Indica) Fruit Shell-A Comparative Study,” Electronic Journal of Biotechnology, Vol. 10, No. 3, July 2007, pp. 358-367.
- R. Gupta, P. Ahuja, S. Khan, R. K. Saxena and M. Mohapatra, “Microbial Biosorbents: Meetings Challenges of Heavy Metals Pollution in Aqueous Solution,” Current Science, Vol. 78, No. 8, April 2000, pp. 967-973.
- I. Langmuir, “The Adsorption of Gases in Plane Surfaces of Glass, Mica and Platinum,” Journal of American Chemical Society, Vol. 40, No. 9, 1918, pp. 1361-1403.
- N. Ünlü and M. Ersoz, “Removal of Heavy Metal Ions by Using Dithiocarbamated-Sporopollenin,” Separation and Purification Technology, Vol. 52, No. 3, January 2007, pp. 461-469.
- W. J. Weber, “Adsorption Theory, Concepts and Models. In: Adsorption Technology: A Step by Step Approach to Process Evaluation and Application,” Marcel Dekker, New York, 1985.
- S. Lagergren, “Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens,” Handlingar, Vol. 24, No. 4, 1898, pp. 1-39.
- Y. S. Ho and G. McKay, “The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat,” Water Research, Vol. 34, No. 3, February 2000, pp. 735-742.
- R. Gong, Y. Ding, H. Liu, Q. Chen and Z. Liu, “Lead Biosorption and Desorption by Intact and Pretreated Spirulina Maxima Biomass,” Chemosphere, Vol. 58, No. 1, January 2005, pp. 125-130.
- P. King, N. Rakesh, S. B. Lahari, Y. P. Kumar and V. S. R. K. Prasad, “Biosorption of Zing onto Syzygium Cumini L.: Equilibrium and Kinetic Studies,” Chemical Engineering Journal, Vol. 144, No. 2, October 2008, pp. 181-187.
- A. Saeed, M. W. Akhter and M. Iqbal, “Removal and Recovery of Heavy Metals from Aqueous Solutionusing Papaya Wood as a New Biosorbent,” Separation and Purification Technology, Vol. 45, No. 1, September 2005, pp. 25-31.
- P. Salehi, B. Asghari and F. Mohammadi, “Removal of Heavy Metals from Aqueous Solutions by Cercis Siliquastrum L.,” Journal of Iranian Chemical Society, Vol. 5, No. 1, October 2008, pp. S80-S86.
- E. Romera, F. González, A. Ballester, M. L. Blázquez, and J. A. Muñoz, “Comparative Study of Biosorption of Heavy Metals Using Diffrent Types of Algae,” Bioresource Technology, Vol. 98, No. 17, December 2007, pp. 3344-3353.
- J. M. Tobin, D. G. Cooper and R. J. Neufeld, “Uptake of Metal Ions by Rhizopus Arrhizus Biomass,” Applied and Environmental Microbiology, Vol. 47, No. 4, October 1984, pp. 821-824.
- K. Vijayaraghavan and Y. S. Yun, “Bacterial Biosorbents and Biosorption,” Biotechnology Advances, Vol. 26, No. 3, May-June 2008, pp. 266-291.
- S. Al-Asheh, F. Banat, R. Al-Omari and Z. Duvunjak, “Predictions of Binary Sorption Isotherms for the Sorption of Heavy Metals by Pine Bark Using Single Isotherm Data,” Chemosphere, Vol. 41, No. 5, September 2000, pp. 659-665.

