Journal of Water Resource and Protection
Vol.2 No.4(2010), Article ID:1670,7 pages DOI:10.4236/jwarp.2010.24033
Radionuclide Contents and Physicochemical Water Quality Indicators in Stream, Well and Borehole Water Sources in High Radiation Area of Abeokuta, Southwestern Nigeria
1Radiation and Health Physics Research Laboratory, Department of Physics, University of Ibadan, Ibadan, Nigeria
2Ionizing Radiation Division, National Institute of Standards and Technology, Gaithersburg, USA
3Department of Chemistry, University of Ibadan, Ibadan, Nigeria
E-mail: jibirinn@yahoo.com, nnamdi.jibiri@mail.ui.edu.ng, nnamdi.jibiri@nist.gov
Received December 28, 2009; revised January 25, 2010; accepted February 19, 2010
Keywords: Radionuclides, Gamma Ray Spectroscopy, Physicochemical Quality, Drinking Water Quality, High Background Radiation, Radiation Ingestion Effective Dose
ABSTRACT
Water samples from streams, hand-dug wells and boreholes in high background radiation areas in Abeokuta, Nigeria have been collected in order to determine the activity concentrations of 40K, 226Ra and 232Th in the samples as well as their physicochemical characteristics. These parameters were evaluated in order to determine the quality of these water sources to the local population, who use these water resources for drinking and domestic activities. Measurements of radioactivity in the water samples were carried out using γ-ray spectroscopy, while standard chemistry methods were used for the physicochemical determinations of these quality parameters. A total of fourteen representative water samples from streams (7), boreholes (4), and hand dug wells (3) were collected for study. The determined activity concentrations of the radionuclides in these samples were used to calculate the effective dose to the population from due to ingestion of and drinking the locally available water. The total annual ingestion effective doses were found to vary between 115.00 ± 1.15µSv and 1362.30 ± 438.02 µSv. The physicochemical parameters where found to be lower than the prescribed standard safe limits in the water sources except for the nitrate and phosphate levels which were particularly high in the water samples from boreholes and hand-dug wells. The radiation effective ingestion dose due to ingestion of water from dug wells and streams was found to be higher than the dose due to ingestion of water from borehole sources in the studied areas. The results obtained in this study, have been taken as a baselines for physicochemical parameters and activity concentrations of natural radionuclides in water samples within Odeda and Obafemi-owode parts of Abeokuta, Nigeria.
1. Introduction
Water is a universal solvent on Earth whose main sources include rivers, springs, wells, boreholes and other fresh water bodies. These sources provide water for drinking and domestic uses including cooking, and will generally constitute an exposure pathway for contaminants to reach the population [1]. Statutorily, potable water supply in Nigeria had been supplied by the government-owned public water utilities (GPWU) in the past. The GPWUs provide this supply from conventional water treatment plants which use water from artificial reservoirs, flowing perennial streams, lakes, and deep boreholes. As the country’s population grows and industrial activity increases, the supply of water by the GPWUs becomes inadequate in both quality and quantity. Only about 48% of the total population was estimated to be served by improved water sources [2], leaving about 52% of the population without safe domestic water. Between 1990 and 2004, the fraction of the population with access to safe water supplies dropped by one percentage point. Ground water can become contaminated by domestic sewage, feedlots and surface runoff, and other pollution sources, such as quarrying activities. Where the subterranean aquifers may also become contaminated in areas where the sub-surface geology permits rapid downward movement of water sources from the surface or where ground/well water sources are tapped near the surface.
The local economies in the Odeda and Obafemi-owode areas in Ogun State, Nigeria are characterized by agricultural and stone quarrying activities while, hand-dug wells, streams, and boreholes are the major sources of water for drinking and other domestic activities. Metal pollution has been reported in shallow well water samples in the neighboring town, Ago-Iwoye has been reported [1], and radioactivity has been detected in borehole water in a neighboring state [3] and in part of Ogun state [4]. Furthermore, baseline studies of terrestrial outdoor gamma dose rate levels in Nigeria have shown that the areas under investigation exhibited high concentrations of terrestrial radionuclides [5,6]. Additionally, an outdoor annual effective dose rate of 1.64 mSv due to environmental radiation has recently been reported for Abeokuta [7]. It is therefore anticipated that radionuclide concentrations may be elevated in the different sources of water in these areas. Ground water is particularly important as it accounts for about 88% of safe drinking water in rural areas, where the population is widely dispersed and the infrastructure needed for treatment and transportation of surface water does not exist [8]. Hand dug wells however, are still the most common source of water in rural communities, and a large percentage of the rural population depends on these wells for their water supply [9]. Therefore, the present work is considered imperative and has the following objectives: 1) to determine radionuclide content in different sources of water for drinking and domestic use by the local populations, 2) to evaluate the water quality using physicochemical indicators, 3) to evaluate the internal radiological hazard in different sources of water, and 4) to generate radiometric data that may be useful for future radiological evaluation and epidemiological studies in these areas.
2. Materials and Methods
2.1. Sampling
The study area Abeokuta is situated in the sub-humid tropical region of Southwestern Nigeria. The city enjoys a tropical climate with distinct wet and dry season periods of about 130 days. The mean annual rainfall and temperature are about 1,270 mm and 28℃ respectively while the estimated mean annual potential evaporation is 1,100 mm. Geologically, the city is underlain by crystalline pre-Cambrian basement complex of igneous and metamorphic origin noted for their rather poor groundwater bearing properties and high content of radionuclides [7]. The city is drained mainly by River Ogun which passes through it and the drainage pattern is generally dendritic. The study area which covers a geographical area of 1,256 km2 comprises of Abeokuta South, Abeokuta North, parts of which Odeda and Obafemiowode study areas are located. The geological map of Ogun State Nigeria showing the geological setting of the sampling locations in Abeokuta is shown in Figure 1. A total of fourteen water samples were collected from the two areas. Seven (7) samples were from streams, three (3) were from well water, and four (4) were from borehole water samples. An effort was made to ensure that all

Figure 1. The map of Ogun State showing the geological formations of the sampling locations in Abeokuta.
sources of the local population’s water supply were sampled. Each water sample was collected with a one-liter plastic container which had been previously washed and rinsed with dilute acid (0.1 M HCl). The collected samples were acidified with 1 M of concentrated hydrochloric acid to obtain a pH < 2. This acidification was performed in order to avoid adsorption of radionuclides onto the walls of the container.
2.2. Measurements
2.2.1. Radioactivity Measurement
Gamma-ray spectroscopy was employed for the radioactivity measurements. The spectrometer used was a 7.6 cm × 7.6 cm NaI (Tl) detector, which was well-calibrated, shielded and highly efficient detector (Bircon model, serial number ff-669), coupled to a Canberra series 10 plus Multichannel Analyzer (MCA). The detector resolution measured by Full Width half Maximum (FWHM) is ~8% at the 0.662 MeV of 137Cs peak. The detector is maintained in a vertical position in a Canberra lead cylindrical shield of 10 cm thickness. For the purpose of identifying the various radionuclides that may be present in the samples through the gamma energies they emit, energy calibration of the detector was performed using a set of International Atomic Energy Agency (IAEA) standard sources of known radionuclides with well-defined energies within the range of interest (0.511-2.615 MeV). The water samples were transferred to a 1-L Marinelli sample container, which fits into the detector. The samples were placed on top of the detector and the counting period for each sample was 10 h. The net area count under the photopeak of each of the radionuclides was computed using the firm-ware algorithm of the MCA. The 226Ra and 232Th activities were determined indirectly through the activities of their decay products. The choice of the reference nuclides for the activity determination was made based on the fact that the NaI (Tl) detector used in this study has a poor resolution; hence the peaks of interest to be considered would be sufficiently discriminated and intense. Therefore, the 238U content of the samples was determined from the intensity of the 1.760 MeV gamma-ray peaks of 214Bi, the 232Th content from the intensity of the 2.615 MeV gamma-ray peak from 208Tl, and the 40K content from 1.460 MeV gamma-ray peaks following the decay of 40K. The net area count after background corrections in each photopeak was used to compute of the activity concentration of each of the radionuclides in the samples via the Equation (1) [3,4]:
 (1)
(1)
where Ac = activity of sample, A = full peak area of samples,  = activity concentration of the radionuclide in the standard sample (Bq∙
= activity concentration of the radionuclide in the standard sample (Bq∙ ),
),  = Volume of standard sample (L),
= Volume of standard sample (L), 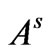 = full peak area of the standard sample and
= full peak area of the standard sample and 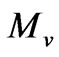 = is the volume of the sample (L).
= is the volume of the sample (L).
2.2.2. Water Quality Measurement
At the collection site, the temperature of each of the water samples were taken using a calibrated thermometer, while the pH levels were determined using the glass electrode method with a standard calibrated pH meter. The electrical conductivity of each sample was determined in the laboratory using a conductivity meter. The total suspended solid (TSS), chemical oxygen demand (COD), biological oxygen demand (BOD), alkalinity and dissolved oxygen (DO) parameters were determined according to a standard procedure [10]. The nitrate contents were determined by the Phenoldisulphonic acid method [11]. Phosphate was determined colorimetrically as molybdophosphoric acid [12], while sulphate was analyzed according to the procedure in standard methods of examination of water and effluents [10].
2.2.3. Radiological Evaluation
To estimate the equivalent dose to members of the public from the ingestion of radionuclides in drinking water, the parameters required are the concentration of the radionuclides in water (measured in Bq∙L-1), the daily consumption rate of water (L∙d-1), and the dose conversion factor for each particular radionuclide. These parameters allow for the annual dose from each individual radionuclide consumed in water to be calculated using the following equation:
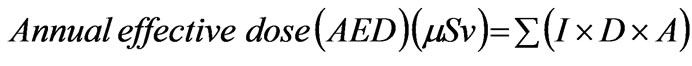 (2)
(2)
where I is the dose per unit intake (mSv∙Bq-1), D is the annual water consumption (L∙y-1) and A is the radionuclide concentration in (Bq∙L-1). Dose per unit intake by ingestion for adult members of the public was obtained from International Commission of Radiological Protection (ICRP) [13] while yearly consumption of water for an individual is assumed to be 500 L∙y-1 [14].
3. Results and Discussions
3.1. Activity Concentrations of the Radionuclides
The activity concentrations of 40K, 238U, and 232Th have been determined in each of the water samples from the Odeda and Obafemi-owode areas. Cesium-137 was not detected in any of the samples, which implies that the radioactivity in the collected samples was only due to natural radioactive elements. The results from the determination of the activity concentrations of the radionuclides are presented in Table 1. As can be observed from the table, the activity concentration in the stream water

Table 1. The activity concentration and annual effective dose (AED) in stream, dug well and borehole water sources in the study areas.
samples ranged from below detection (BD) to 87.71 ± 19.41 Bq∙L-1 for 40K, BD to 9.71 ± 2.62 Bq∙L-1 for 226Ra and 0.10 ± 0.01 ~ 8.02 ± 2.71 Bq∙L-1 for 232Th. For the wellwater samples, the activity concentrations ranged from 197.78 ± 23.85 ~ 270.78 ± 23.85, 2.58 ± 0.09 ~ 3.65 ± 0.04 and BD ~ 4.45 ± 2.05 Bq∙L-1 for 40K, 226Ra and 232Th respectively. For the borehole water samples, the activity concentrations ranged from BD ~ 185.72 ± 16.61 Bq∙L-1, 0.51 ± 0.02 ~ 6.77 ± 0.23 Bq∙L-1 and 0.87 ± 0.05 ~ 2.59 ± 0.13 Bq∙L-1 for 40K, 226Ra and 232Th respectively. The activity concentrations of all of the radionuclides except for 40K were higher in stream samples than in dug well and borehole water sources. The concentrations of 226Ra and 232Th obtained in this study compared relatively well with the results obtained by Ajayi and Achuka [4] in drilled and dug well drinking water in neighboring towns also in Abeokuta. However, the results obtained for 40K concentrations in this study were ten times higher than the results by Ajayi and Achuka. This discrepancy may be attributed to the stone quarrying activities in the areas where the geological formations are largely comprised of granites. It may also be suggested that since the streams may not be used for drinking purposes other than cooking and washing of cloths, and that water from the dug wells and boreholes should further be treated to purify them including treatment that would remove radium (226Ra). This is particularly important because water -mineral interactions in an aquifer may produce significant natural radionuclide levels especially radium in groundwater that can constitute a long term health hazard. Two short-lived polonium daughters, notably 218Po and 214Po, produced from radon-222 decay can cause severe damage to the lung and possibly lung cancer by alpha radiation when natural radium (Ra) or radon (Rn) is ingested. Most regions in the world with elevated radioactivity in the ground water are underlain by granitic or crystalline bedrock, due to rainout and washout of radon daughter [5,6]. Another possible source of radionuclides in the water sources is application of Nitrogen-Phosphorus-Potassium (N-P-K) fertilizers to the soil by the farmers, to improve crop yield. This is considered necessary as soil fertility in the investigated areas is rather poor for agricultural purposes due to the decades of continuous use of same farms for agricultural activities. It has been reported that fertilizers may be rich in natural radioactive elements and consequently, that their application may enrich these radioactive elements in the soil and plants [15,16]. An inorganic fertilizer used in agricultural activities, derives its basic raw materials from rock phosphate and this concentrates naturally occurring radionuclides [16]. The continuous application of fertilizer in farms could enhance the radioactivity level in that field. This in turn increases the radionuclide concentration in water sources through runoff and leaching and by water ingestion pathway will result to radiation dose burden to the different tissues and organs of the body. Also the acidity of water may be increased by the presence of elevated levels of nitrates associated with agricultural land use and this is believed to increase the amount of radium (226Ra) that dissolves into ground and surface water from contact with sands and soils. The interstitial movement of radionuclides is controlled by the flow rate of water and the retardation of the isotopes and daughter products by the adsorption processes which occur between the soluble isotopes such as 226Ra and the aquifer solids, as well as by dissolution-precipitation reactions. The extent to which this interstitial movement results in differential radionuclide distributions in the water sources and fertilizer application affected the water characteristics in areas was not investigated; this may be a subject for future work.
3.2. Physicochemical Water Quality Indicators
The values of the physicochemical water quality parameters for the three different types of water supplies in the areas are presented in Table 2. Also presented in Table 2 are the standard safe limits of the determined physicochemical parameter as recommended by an international body, the World Health Organization (WHO) [14] and the national regulatory agency, the Federal Environmental Protection Agency (FEPA) [17]. As can be observed from the table, the pH of the samples from all the three water sources was within the range 6.7 to 8.2 which falls in the internationally accepted standard range (6.5-8.5) for drinking water. The concentrations of total suspended solids (TSS) in the borehole and well waters were 0.40 ± 0.28 mg∙L-1 and 0.58 ± 0.05 mg∙L-1, respectively. The TSS levels were much lower than the specified safe limit of 10 mg∙L-1 by WHO and FEPA and this low level is an indication that there is little or no significant impact on turbidity. The conductivity level of a drinking water is related to the total dissolved solids (TDS) concentrations. The low conductivity levels of 0.40 ± 0.28 μS∙cm-1, 0.58 ± 0.05 μS∙cm-1 and 0.46 μS∙cm-1 suggest low TDS levels in these water samples. The phosphate and nitrate concentrations were higher than the prescribed values which is an indication that these waters can serve as medium for algae growth which, is undesirable and unhealthy in a portable water supply.
These elevated levels may further suggest the need for more treatment of the water sources in these areas. The dissolved oxygen (DO) levels at Odeda and Obafemiowode streams were 7.0 mg∙L-1 and 13 mg∙L-1 respectively, while their corresponding levels of chemical oxygen demand (COD) were respectively 20.0 mg∙L-1 and 40 mg∙L-1. These DO and COD levels suggest that the streams contain a low organic load which may possibly support aquatic life. The nitrate level of the water samples ranged from 27.8-250 mg∙L-1. This is oders of magnitude greater than prescribed limit of about 20 mg∙L-1 by WHO and FEPA [14,17]. The observed high level of nitrate in the water sources may originate primarily from fertilizers, septic systems, because it is the end product of the aerobic decomposition of organic nitrogenous matter. Signifi-

Table 2. The physicochemical parameters of the different sources of water supplies in the study areas.
cant sources of nitrate have been reported to be due to chemical fertilizers from cultivated land and drainage from livestock feedlots, as well as domestic and some industrial waters [1]. Nitrate is one of the most common groundwater contaminants in rural areas. It is regulated in drinking water primarily because excess levels can cause methemoglobinemia, or “blue baby” disease. Although nitrate levels that affect infants do not pose a direct threat to older children and adults, they do indicate the possible presence of other more serious residential or agricultural contaminants, such as bacteria or pesticides. Also, fertilizer nitrogen that is not taken up by plants; volatilized, or is carried away by surface runoff leaches to the groundwater in the form of nitrate. This not only makes the nitrogen unavailable to crops, but also can elevate the concentration in water above the levels acceptable for water drinking quality.
3.3. Total Annual Effective Dose Rate from Daily Intakes of 40K, 226Ra and 232Th from Water
Using Equation (2), the total annual effective dose for the different water samples was calculated. These results are presented in Table 1. As can be observed from the table, the annual effective dose due to ingestion of these sources of water to the local population ranged from 292.40 to 1201.75 µSv (0.29 to 1.20 mSv) in Odeda, while in the Obafemi-owode area, the annual effective dose ranged from 115.00 to 1362.30 µSv (0.11 ~ 1.36 mSv). The calculated doses from the two drinkable dug well samples exceeded the 1 mSv∙y-1 exposure limits for the members of the public [18]. From a radiological health standpoint, however, these slightly elevated effective doses due to ingestion of water from sources in the studied areas are not expected to pose any significant health concerns to the local population since there are other optional available water sources. Also it does not represent a large value when compared with extreme local natural radiation value (~100 mSv∙y-1) to which harmful effects is not likely [16,18].
4. Conclusions
The gamma-ray spectrometric methods have been used to determine the natural radionuclide concentrations in streams, hand dug-wells and boreholes; major water sources to the local population in Odeda and Obafemiowode parts of Abeokuta, Nigeria. The physicochemical parameters of these water sources were also measured using the American Public Health Association (APHA) series of Standard Methods of Examination of Water and Effluent. The activity concentration in the stream water samples ranged from below detection (BD) to 87.71 ± 19.41 Bq∙L-1 for 40K, BD ~ 9.71 ± 2.62 Bq∙L-1 for 226Ra and 0.10 ± 0.01 ~ 8.02 ± 2.71 Bq∙L-1 for 232Th. For the wellwater samples, the activity concentrations ranged from 197.78 ± 23.85 ~ 270.78 ± 23.85, 2.58 ± 0.09 ~ 3.65 ± 0.04 and BD ~ 4.45 ± 2.05 Bq∙L-1 for 40K, 226Ra and 232Th respectively. For the borehole water samples, the activity concentrations ranged from BD ~ 185.72 ± 16.61 Bq∙L-1, 0.51 ± 0.02 ~ 6.77 ± 0.23 Bq∙L-1 and 0.87 ± 0.05 ~ 2.59 ± 0.13 Bq∙L-1 for 40K, 226Ra and 232Th respectively. The activity concentrations of all of the radionuclides except for 40K were higher in stream samples than in dug well and borehole water sources. The activity concentrations of the radionuclides were used to calculate the ingestion effective doses due to the water sources. The total annual effective dose due to the radionuclides ranged from 0.29 to 1.20 mSv in the Odeda area and from 0.11 to 1.36 mSv in the Obafemi-owode area. The majority of the physiochemical water quality parameters for the various water samples; BOD, TSS, DO, COD etc. were lower than the accepted safety limits with the exception of nitrate. Self-purification of the stream water supply is presumed to be responsible for the lower values of the physicochemical indicators in these samples compared to the more elevated levels in samples from dug wells and boreholes. The results obtained in this study, have been taken as a baselines for physicochemical parameters and activity concentrations of natural radionuclides in the water samples within the two areas under investigation. However, it is recommended that uranium (226Ra) reduction options be advocated to further reduce the exposures levels to the local population. Additionally, general treatment of the water sources, especially from the dug wells and boreholes be carried out in order to ensure a purequality drinking water supply for the local population.
5. Acknowledgments
The authors wish to thank the International Foundation for Science (IFS) Stockholm in Sweden, for providing the grant used in carrying out this study under its Food science program.
REFERENCES
- O. Fasunwon, J. Olowofela, O. Akinyemi and O. Akintokun, “Contaminants Evaluation as Water Quality Indicators in Ago-Iwoye, Southwestern Nigeria,” African Physical Review, Vol. 2, 2008, pp. 110-116.
- World Health Organization, “Meeting the MDG Drinking Water and Sanitation Target: The Urban and Rural Challenge of the Decade,” WHO Press, New York, 2006.
- A. O. Awodugba and P. Tchokossa “Assessment of Radionuclide Concentrations in Water Supply from BoreHoles in Ogbomosoland, Western Nigeria,” Indoor and Built Environment, Vol. 17, 2008, pp. 183-186.
- O. S. Ajayi and J. Achuka, “Radioactivity in Drilled and Dug Well Drinking Water of Ogun State, Southwestern Nigeria and Consequent Dose Estimates,” Radiation Protection Dosimetry, Vol. 135, No. 1, 2009, pp. 54-63.
- I. P. Farai and N. N. Jibiri, “Baseline Studies of Terrestrial Outdoor Gamma Dose Rate Levels in Nigeria,” Radiation Protection Dosimetry, Vol. 88, No. 3, 2000, pp. 247-254.
- N. N. Jibiri, “Assessment of Health Risk Associated with Terrestrial Gamma Radiation Dose Rate Levels in Nigeria,” Environment International, Vol. 27, No. 1, 2001, pp. 21-26.
- I. P. Farai and U.E. Vincent, “Out-Door Radiation Level Measurement in Abeokuta Nigeria by Thermoluminiscent Dosimetry,” Nigerian Journal of Physics, Vol. 18, No. 1, 2006, pp. 121-126.
- U. Amakom, “Financing Water Supply and Sanitation at the Local Government Level, Nigeria Pilot Study,” Draft Report Submitted to Water Aid, Abuja, 2007.
- Water Aid Nigeria, “United States Environmental Protection Agency (EPA): Groundwater and Drinking Water, Water Quality Policy,” EPA Report, No. 810/K-92-001, 2006.
- American Public Health Association (Water Works Association/Water Environment Federation), “Standard Methods for the Examination of Water and Waste Water,” 20th Edition, Washington D.C., 1998.
- Z. Marckenzo, “Separation and Spectrophotometric Determination of Elements,” John Wiley and Sons Ltd., London, 1986.
- A. C. Twort, F. M. Raw and F. W. Crawley, “Water Supply,” Edward Arnold Press, London, 1995.
- P. C. Jackson, “Age-dependent Doses to Members of the Public from Intake of Radionuclides: Part 5 Compilation of Ingestion and Inhalation Dose Coefficients,” Physics in Medicine and Biology, Pergamon Press, Oxford, Vol. 41, No. 12, 1996.
- World Health Organization, “Guidelines for Drinking Water Quality: Health Criteria and Other Supporting Information,” 2nd Edition, Geneva, 1997, pp. 367-370.
- N. N. Jibiri, I. P. Farai and S. K. Alausa, “Estimation of Annual Effective Dose Due to Natural Radioactive Elements in Ingestions of Foodstuffs in Tin Mining Area of Jos Plateau, Nigeria,” Journal of Environmental Radioactivity, Vol. 94, No. 1, April 2007, pp. 31-40.
- N. N. Jibiri, I. P. Farai and S. K. Alausa, “Activity Concentrations of 226Ra, 228Th, and 40K in Different Food Crops from a High Background Radiation Area in Bitsichi, Jos Plateau, Nigeria,” Radiation and Environmental Biophysics, Vol. 46, No. 1, 2007, pp. 53-59.
- Federal Environmental Protection Agency, “National Guidelines and Standards for Industrial Effluents, Gaseous Emissions and Hazardous Waste Management in Nigeria,” Federal Environmental Protection Agency Decree, No. 58, 1988.
- United Nations Scientific Committee on the Effects of Atomic Radiation, “Sources, Effects and Risks of Ionizing Radiation,” Report to the General Assembly, New York, 2000.

