Health
Vol.5 No.8E(2013), Article ID:36061,6 pages DOI:10.4236/health.2013.58A5001
Studies of non-metallic organic disinfectants on inactivation of avian influenza viruses
![]()
Wiley Lab/Avian Virology, Animal Diagnostic Laboratory, Department of Veterinary and Biomedical Sciences, Pennsylvania State University, University Park, USA; hxl15@psu.edu
Copyright © 2013 Huaguang Lu. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 26 May 2013; revised 27 June 2013; accepted 15 July 2013
Keywords: AVian Influenza Virus; Non-Metallic Disinfectants; Neutral Electrolyzed Water; M22 Organic Disinfectant; Superoxy Food Wash; Hydrogen Peroxide; Clorox Germicidal Bleach; Clidox-S
ABSTRACT
Six different kinds of non-metallic or organic disinfectants were obtained in this research study including “Neutral Electrolyzed Water”, “M22” organic disinfectant solution, Superoxy Food Wash disinfectant, Hydrogen Peroxide, Clorox Germicidal Bleach and Clidox-S. The effectiveness of these disinfectants was studied against various subtypes of avian influenza virus (AIV). The virus-disinfectant mixtures were prepared in serial dilutions of each disinfectant with a constant virus titer and incubated at ambient temperature in different time intervals for virus inactivation. The virus inactivation results were determined by virus recovery in embryonating chicken eggs. Among the six different kinds of nonmetallic disinfectants obtained for this research project, Neutral Electrolyzed Water, “M22” solution, Clorox Germicidal Bleach and Clidox-S were effectively inactivated AIV with appropriate working dilutions and reaction times. Superoxy Food Wash disinfectant and Hydrogen Peroxide were found having limited effect on virus inactivation with extended exposure times of more than 2 hours. These research findings provide scientific data to poultry industry with guidelines to select and use non-metallic organic disinfectants for poultry flock sanitation and disinfection to effectively prevent and control of avian influenza outbreaks.
1. INTRODUCTION
Avian influenza and other avian viral diseases have always been a major concern to the poultry industry. Because certain subtypes such as H5N1 and H7N9 of avian influenza virus (AIV) or other avian viruses could become highly pathogenic and pose lethal threat to domestic poultry and public health, it is critical to prevent outbreaks and destroy the highly pathogenic viruses quickly when an outbreak occurred. In practice, various “strong” disinfectants are commonly used in poultry premises and processing plants to prevent and control of microbial contamination [1,2]. However, “strong” disinfectants contain harsh chemicals or drugs that have negative public perception issues and also they are not health for water and feed additives and processing plant sanitization [3]. Alcohols (ethanol, isopropanol), chlorine compounds, formalin, glutaraldehydes and phenols are common gradients for most chemical disinfectants [1].
Ethanol or isopropanol in concentrations of 70% - 75% are good general-use disinfectants. They are most effective against lipophilic viruses, less effective against nonlipid viruses, and ineffective against bacterial spores. Because of their quick evaporation rate, it may be difficult to achieve sufficient contact time [4,5].
Chlorine-containing solutions have universal disinfectant activity [6]. With proper concentration and sufficient contact times, hypochlorite solutions can be considered chemical sterilants since they will inactivate bacterial spores. The downside is that chlorine compounds are quickly inactivated by excess organic materials and are corrosive to metals and tissues. Consequently their use in labs has some limitations. Since the free chlorine is inactivated by light and air, disinfectant chlorine solutions are best made fresh before use.
Formalin is a 37% solution of formaldehyde gas in water. Diluted to 5% formaldehyde it is an effective disinfectant; at 0.2% - 0.4% it can inactivate bacteria and viruses. Unlike chlorine, formalin does not corrode stainless steel. It has a pungent, irritating odor; exposures must be limited due to its toxicity and carcinogenicity [7].
Glutaraldehydes are closely related to formaldehyde but seem to be more biologically active. Glutaraldehydes are effective against all types of bacteria, fungi, and viruses.
With sufficient contact time they kill bacterial spores. While glutaraldehyde vapors are less irritating than formaldehyde (formalin), they remain irritating to the eyes, mucous membranes, and upper respiratory tract. Exposures should be minimized by confining use to a properly functioning chemical fume hood [8].
Phenol solutions have been used for many years as a disinfectant. Their usefulness in laboratories is limited, however, because they leave a sticky residue on surfaces following treatment. Concentrated phenol is a highly toxic, corrosive substance that is easily absorbed through the skin. Use of appropriate personal protective equipment is essential [9].
The proper selection and use of disinfectants are important for safety and quality measures of poultry health. Disinfectants have various characteristics that must be considered before one is selected for a particular use. Non-metallic organic or “soft” disinfectants are feasible to use to improve animal health and prevent disease outbreaks. In this research study, six different kinds of nonmetallic disinfectants were obtained for virus inactivation studies on most common avian respiratory and enteric viruses, so as to provide scientific data of their effectiveness on inactivation of the important avian viral pathogens, and provide guidelines and recommendations to poultry industry to apply them correctively and effectively in management practices in poultry farms and hatcheries to prevent poultry from infections and outbreaks of viral diseases, and in poultry meat processing plants for effective sanitization.
2. MATERIALS AND METHODS
“Soft” or Non-Metallic Organic Disinfectants. Six different kinds of “soft” or non-metallic organic disinfectants were obtained for this research study as the following, 1) “Neutral Electrolyzed Water” disinfectant solution (NatureTech Solutions©, Inc. 2440 Butter Rd, Lancaster, PA); 2) “M22” organic disinfectant (Cape Kennedy Plastics, LLC, 5055 State Road 46, Mims, FL); 3) Superoxy Food Wash disinfectant (TRC, Tulsa, OK); 4) Hydrogen Peroxide (CVS Pharmacy, Inc., Woonsocket, RI); 5) Clorox Germicidal Bleach (Wal-Mart local store, State College, PA); and 6) Clidox-S (Pharmacal Research Laboratories Naugatuck, CT).
Avian Influenza Virus (AIV). Various H1 through H9 subtypes of AIV maintained at the Avian Virology laboratory at Wiley lab of Pennsylvania State University were newly propagated and tittered in specific-pathogen-free embryonating chicken eggs (ECE) for this research study.
Virus Inactivation. A virus-disinfectant mixture was prepared in a 15 ml centrifuge tube as a constant virus titer with serial dilutions of a disinfectant. Final virus concentration in the virus-disinfectant mixture were measured between105.0 and 107.0 ELD50 dose/ml (ELD50 = embryo lethal dose 50%). Each virus-disinfectant mixture was incubated at ambient temperature upon it was prepared. A sample of the virus-disinfectant mixture was taken for inoculation of ECE subsequently at certain times of incubation. The virus inactivation results were measured by virus recovery in ECE after 3 - 4 days of post inoculation.
3. RESULTS
Six non-metallic or organic disinfectants obtained from commercial and scientific sources were studied and evaluated their effectiveness on inactivation of various subtypes of AIV. Research findings were summarized as the following.
Effects of Neutral Electrolyzed Water. The Neutral Electrolyzed Water solution effectively inactivated or killed all AIV subtypes tested including H1N1, N2N2, H3N2, H4N8, H5N2, H5N3, H5N9, H6N8, H7N2, N9N1 and H9N2. Effective dilutions of the Neutral Electrolyzed Water were found between 1:2 and 1:5 with sterile tap water. The test viruses were 100% inactivated within 10 min, 15 min, 30 min, 60 min and 120 min of reaction times tested in the effective dilutions (Table 1).
“M22” Organic Disinfectant. The M22 product was effective against AIV H1 through H9 subtypes tested.
Table 1. Neutral Electrolyzed Water on inactivation of avian influenza virus (AIV).
Note: 100% = virus was completely inactivated; 0% = virus was not inactivated.
The M22 working dilutions and reaction times to effectively inactivate these AIV subtypes were measured from 1:100 in 10 - 15 min, 1:500 in 30, 60 and 120 min in laboratory conditions (Tables 2 and 3). Dilutions up to 1:800 or 1:1000 of M22 disinfectant were not able to inactivate H5N2 and H7N2 AIV in 60 min, but were able to inactivate them when extended incubation time to 2 hr (Table 4).
Clorox Germicidal Bleach. The Clorox Germicidal Bleach at 1:300 dilution was effectively inactivated H1N1, H2N2, H4N2, H5N2 and H7N2 subtypes of AIV at a minimum exposure time of 5 min. Dilutions up to 1:500 inactivated these viruses within a minimum time of 10 - 20 min. Beyond 1:500 dilutions rarely had effect on virus inactivation (Table 5).
Table 2. M22 Solution inactivation on avian influenza viruses (AIV).
Note: 100% = virus was completely inactivated; 0% = virus was not inactivated.
Table 3. M22 Solution inactivation on avian influenza viruses (AIV).
Note: 100% = virus was completely inactivated; 0% = virus was not inactivated.
Table 4. Serial dilutions of M22 disinfectant on inactivation of avian influenza virus (AIV) H5N2 and H7N2 subtypes.
Note: 100% = virus was completely inactivated; 0% = virus was not inactivated. H5N2 = 106.4 ELD50/ml; H7N2 = 106.7 ELD50/ml.
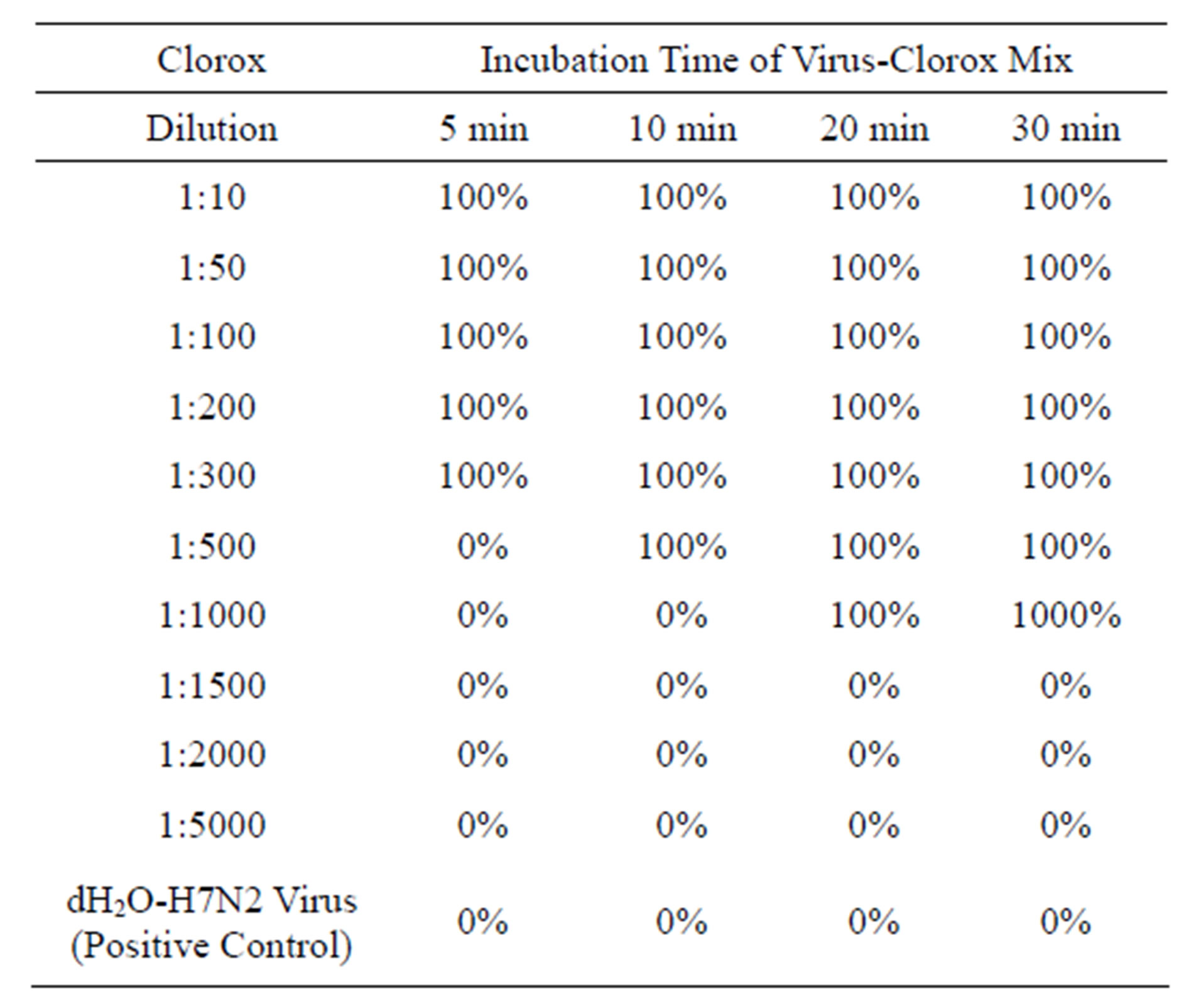
Table 5. Clorox germicidal bleach on inactivation of avian influenza virus H1N1, H2N2, H3N2, H5N2 and H7N2 subtypes.
| Incubation Time of Virus-Clorox Mix | ||||
| Dilution | 5 min | 10 min | 20 min | 30 min |
| 1:10 | 100% | 100% | 100% | 100% |
| 1:50 | 100% | 100% | 100% | 100% |
| 1:100 | 100% | 100% | 100% | 100% |
| 1:200 | 100% | 100% | 100% | 100% |
| 1:300 | 100% | 100% | 100% | 100% |
| 1:500 | 0% | 100% | 100% | 100% |
| 1:1000 | 0% | 0% | 100% | 1000% |
| 1:1500 | 0% | 0% | 0% | 0% |
| 1:2000 | 0% | 0% | 0% | 0% |
| 1:5000 | 0% | 0% | 0% | 0% |
| dH2O-H7N2 Virus (Positive Control) | 0% | 0% | 0% | 0% |
Note: 100% = virus was completely inactivated; 0% = virus was not inactivated. H1N1 = 106.2 ELD50/ml; H2N2 = 106.5 ELD50/ml: H3N2 = 106.4 ELD50/ml; H5N2 = 106.4 ELD50/ml; H7N2 = 106.7 ELD50/ml.
Clidox-S Disinfectant. The Clidox-S disinfectant consists of two separated components of Base and Activator. A working solution of Clidox-S 1:3:1 (Base:H2O:Activator) disinfectant, which was prepared by manufacturer’s instruction, inactivated H1N1, H3N2, H5N2 and H7N2 subtypes of AIV in 5 min (Table 6).
Hydrogen Peroxide. The Hydrogen Peroxide at 1:10 and 1:50 dilutions inactivated H1N1, H2N2, H3N2, H5N2 and H7N2 subtypes of AIV at exposure times of 120 min. These dilutions had no effect on virus inactivation at exposure times of less than 60 min (Table 7).
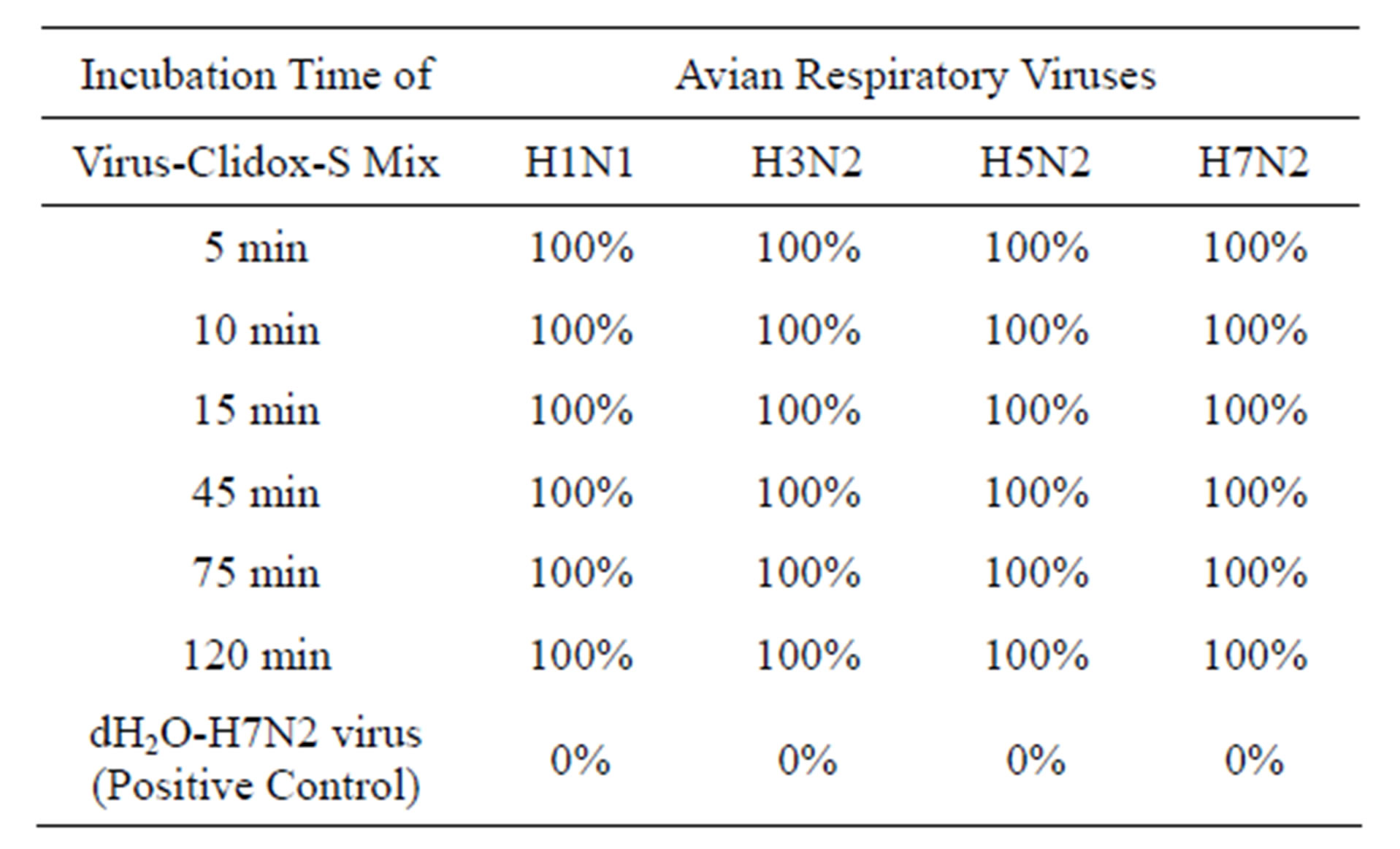
Table 6. Clidox-S 1:3:1 disinfectant on inactivation of avian influenza virus H1N1, H3N2, H5N2 and H7N2 subtypes (Clidox-S 1:3:1 = Base:Activator:H2O).
Note: 100% = virus was completely inactivated; 0% = virus was not inactivated. H1N1 = 106.2 ELD50/ml; H3N2 = 106.4 ELD50/ml; H5N2 = 106.4 ELD50/ml; H7N2 = 106.7 ELD50/ml.
Table 7. Hydrogen peroxide (H2O2) on inactivation of avian influenza virus H1N1, H2N2, H3N2, H5N2 and H7N2 subtypes.
Note: 100% = virus was completely inactivated; 0% = virus was not inactivated. H1N1 = 106.2 ELD50/ml; H2N2 = 106.5 ELD50/ml: H3N2 = 106.4 ELD50/ml; H5N2 = 106.4 ELD50/ml; H7N2 = 106.7 ELD50/ml.
Superoxy Food Wash. Superoxy Food Wash at 1:10 dilutions had no effect on H1N1, H2N2, H3N2, H5N2 and H7N2 subtypes of AIV inactivation at exposure times of 15 - 60 min, and inactivated these viruses when exposure time extended to 120 min (Table 8).
4. DISCUSSION
Among the 6 different kinds of non-metallic disinfectants obtained for this research project, “Neutral Electrolyzed Water” disinfectant solution, “M22” organic disinfectant, Clorox germicidal bleach and Clidox-S were effective to disinfect AIV with appropriate working dilutions and reaction time. Superoxy Food Wash disinfectant and Hydrogen peroxide were found having limited effect on AIV inactivation with extended exposure times
Table 8. Superoxy Food Wash disinfectant on inactivation of avian influenza virus H1N1, H2N2, H3N2, H5N2 and H7N2 subtypes.
Note: 100% = virus was completely inactivated; 0% = virus was not inactivated. H1N1 = 106.2 ELD50/ml; H2N2 = 106.5 ELD50/ml: H3N2 = 106.4 ELD50/ml; H5N2 = 106.4 ELD50/ml; H7N2 = 106.7 ELD50/ml.
of more than 2 hours. These research findings provide poultry industry with guidelines to select and use nonmetallic organic disinfectants for effectively prevent and control of avian respiratory viral diseases.
Many commercial disinfectants include formaldehyde and other toxic chemical ingredients that release gases called volatile organic compounds. These compounds can cause shortand long-term health problems to animal workers. According to the US Environmental Protection Agency, volatile organic compounds can cause nose, throat, skin and eye irritation, headaches, nausea, loss of coordination, cancer and damage to the kidneys, liver and central nervous system.
To disinfect or kill AIV and other microorganisms without using chemical products, non-metallic organic or “soft” disinfectants provide nontoxic and inexpensive alternatives to harsh chemical products. The non-metallic organic disinfectants do not contain harsh chemicals but they have antioxidant properties which are active against a broad spectrum of microorganisms which attach products and contaminate process plants and equipment [10- 12]. Research findings in this study have demonstrated that the non-metallic disinfectants are effective, safe and feasible to use against AIV in laboratory conditions. The effectiveness of these disinfectants was also studied against other avian respiratory and enteric viruses including Newcastle disease virus, infectious bronchitis virus, pigeon herpesvirus, avian reovirus, and fowl adenovirus. Results on inactivation of these avian viruses, which were not included in this report, were very similar to AIV inactivation results. Further studies shall be conducted in field conditions to sanitize environment and prevent birds from virus infections so as to provide guidelines to use in poultry flocks, poultry houses, hatcheries and processing plants.
Neutral Electrolyzed Water is the most well studied non-metallic disinfectant as a sanitizer in recent years. Research findings have indicated that Neutral Electrolyzed Water is effective on inactivation of common foodborne pathogens including Escherichia coli, Salmonella enteritidis, Listeria monocytogenes, and other microorganisms on the surface of vegetables and fruits [13-16], egg shells and poultry carcasses [17-19], sea food products [20], and food processing equipment [15,21]. Neutral Electrolyzed Water provides a novel disinfection system and a promising disinfection method as it would allow reducing significant amount of free chlorine commonly used for the disinfection of vegetable and meat processing plants by the food industry, and could represent an alternative to sodium hypochlorite which is another most widespread disinfectant used by fresh-cut industries. Neutral Electrolyzed Water and other non-metallic disinfectants would certainly constitute a much safe and practical way to ensure food safety. They have great potential in application in human health, animal health, and particularly the food industry.
5. ACKNOWLEDGMENTS
The Pennsylvania Poultry Industry Egg/Broiler Check-Off Research Program funded for this research project. The author sincerely thanks all technical staff in assisting the conduct of this research at the avian virology lab. The author would like to acknowledge NatureTech Solutions© Inc (Lancaster, PA) and Cape Kennedy Plastics LLC (Mims, FL) for their constantly providing the “Neutral Electrolyzed Water” and “M22” solution for this research, respectively.
REFERENCES
- Chou, J.K. (1996) Classes of disinfectants and their uses. Pet Bird Magazine, Ezine. http://www.birdsnways.com/wisdom/ww6eiv.htm
- Ruano, M., El-Attrache, J. and Villegas, P. (2001) Efficacy comparisons of disinfectants used by the commercial poultry industry. Avian Disease, 45, 972-977. doi:10.2307/1592876
- McDonnell, G. and Russell, A.D. (1999) Antiseptics and disinfectants: Activity, action, and resistance. Clinical Microbiology Reviews, 12, 147-179. http://cmr.asm.org/content/12/1/147.full
- Johannes, K., Knobloch, M. and Matthias, A. (2002) Horstkotte, holger rohde, paul-michael kaulfers and dietrich mack. Alcoholic ingredients in skin disinfectants increase biofilm expression of Staphylococcus epidermidis. Journal of Antimicrobial Chemotherapy, 49, 683-687. doi:10.1093/jac/49.4.683
- Turpin, K. (2013) Ethanol vs. isopropyl alcohol to disinfect. http://www.ehow.com/about_6540795_ethanol-vs_-isopropyl-alcohol-disinfect.htmll
- John, H.W. (1926) Chlorine-containing solutions. US Patent number: 1588288.
- US Department of Labor, Occupational Safety & Health Administration (2013) Substance technical guidelines for formalin. http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10076&p_table=standards
- US Department of Labor, Occupational Safety and Health Administration (2006) Best practices for the safe use of glutaraldehyde in health care. http://www.osha.gov/Publications/glutaraldehyde.pdf
- CAMEO Chemicals Version 2.4. (2010) Phenol solution. http://cameochemicals.noaa.gov/chemical/173822
- Gulati, B.R., Allwood, P.B., Hedberg, C.W. and Goyal, S.M. (2001) Efficacy of commonly used disinfectants for the inactivation of calicivirus on strawberry, lettuce, and a food-contact surface. J Food Prot, 64, 1430-1434. http://www.ncbi.nlm.nih.gov/pubmed/11563523
- Harris, K., Miller, M.F., Loneragan, G.H. and Brashears, M.M. (2006) Validation of the use of organic acids and acidified sodium chlorite to reduce Escherichia coli O157 and Salmonella typhimurium in beef trim and ground beef in a simulated processing environment. Journal of Food Protection, 69, 1802-1807.
- Jean, J., Vachon, J.F., Moroni, O., Darveau, A., KukavicaIbrulj, I. and Fliss, I. (2003) Effectiveness of commercial disinfectants for inactivating hepatitis A virus on agrifood surfaces. Journal of Food Protection, 66, 115-119.
- Abadias, M., Usall, J., Oliveira, M., Alegre, I. and Viñas, I. (2007) Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally-processed vegetables. International Journal of Food Microbiology, 31, 151-158.
- Deza, M.A. and Araujo MGarrido, M.J. (2003) Inactivation of Escherichia coli O157:H7, Salmonella enteritidis and Listeria monocytogenes on the surface of tomatoes by neutral electrolyzed water. Letters in Applied Microbiology, 37, 482-487. doi:10.1046/j.1472-765X.2003.01433.x
- Hricova, D., Stephan, R. and Zweifel, C. (2008) Electrolyzed Water and Its Application in the Food Industry. Journal of Food Protection, 71, 1934-1947.
- Koseki, S., Isobe, S. and Itoh, K. (2004) Efficacy of acidic electrolyzed water ice for pathogen control on lettuce. Journal of Food Protection, 67, 2544-2549.
- Bialka, K.L., Demirci, A., Knabel, S.J., Patterson, P.H. and Puri, V.M. (2004) Efficacy of electrolyzed oxidizing water for the microbial safety and quality of eggs. Poultry Science, 83, 2071-2078.
- Kim, C., Hung, Y.C. and Russell, S.M. (2005) Efficacy of electrolyzed water in the prevention and removal of fecal material attachment and its microbicidal effectiveness during simulated industrial poultry processing. Poultry Science, 84, 1778-1784.
- Park, C.M., Hung, Y.C., Lin, C.C. and Brackett, R.E. (2005) Efficacy of electrolyzed water in inactivating Salmonella enteritidis and Listeria monocytogenes on shell eggs. Journal of Food Protection, 68, 986-990.
- Liua, C., Duanb, J. and Sub, Y.C. (2006) Effects of electrolyzed oxidizing water on reducing Listeria monocytogenes contamination on seafood processing surfaces. International Journal of Food Microbiology, 106, 248-253. doi:10.1016/j.ijfoodmicro.2005.06.020
- Ayebah, B., Hung, Y.C. and Frank, J.F. (2005) Enhancing the bactericidal effect of electrolyzed water on Listeria monocytogenes biofilms formed on stainless steel. Journal of Food Protection, 68, 1375-1380.
- Abbreviations: AIV = avian influenza virus; ECE = embryonating chicken eggs; ELD50 = embryo lethal dose 50%.

