Health
Vol.5 No.7A5(2013), Article ID:34895,6 pages DOI:10.4236/health.2013.57A5001
Predicting vasospasm after aneurismal subarachnoid hemorrhage with C reactive protein levels*
![]()
1Neurosurgeon at Hospital das Clínicas de Botucatu-UNESP, Botucatu, Brazil; #Corresponding Author: frromero@ig.com.br, romeroncr@gmail.com
2Neurosurgery at São Paulo State University Medical School, Botucatu, Brazil
3Thoracic Surgery at São Paulo State University Medical School, Botucatu, Brazil
Copyright © 2013 Flávio R. Romero et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 21 April 2013; revised 26 May 2013; accepted 15 June 2013
Keywords: Intracranial Vasospasm; Subarachnoid Hemorrhage; C Protein; Intracranial Aneurism
ABSTRACT
Aim: The interest of inflammatory marker increased in the last years, even in preventing clinical outcome after subarachnoid hemorrhage (SAH). Our objective was to study the relationships between C-reactive protein levels and clinical outcome and the development of cerebral vasospasm after aneurismal SAH. Methods: One hundred adult patients with aneurismal SAH were prospectively evaluated. Glasgow Coma Scale (GCS) score, Hunt and Hess grade, Fisher grade, CT scans, digital subtraction angiography studies, transcranial doppler (TCD) and daily neurological examinations were recorded. Serial serum CRP measurements were obtained on daily between admission and 10th days. Glasgow Outcome Scale (GOS) and the modified Rankin Scale (mRS) were used to predict outcome. Results: A progressive increase in the CRP levels from the admission to the 3rd postictal day was observed, followed by a slow decrease until the 9th day. Hemodynamic changes in TCD were associated with higher serum CRP levels. Patients with lower GCS scores presented with increased CRP levels. Patients with higher Hunt and Hess grades on admission developed significantly higher CRP serum levels. Patients with higher admission Fisher grades showed increased levels of CRP. A statistically significant inverse correlation was established in our series between CRP serum levels and GOS and mRS scores on discharge and CRP levels. Conclusion: Increased CRP levels were strongly associated with poor clinical outcome. CRP levels can predict cerebral vasospasm and delayed ischemic deficits with higher statistic significance. There are relationships between hemodynamic chances in TCD and higher CRP levels.
1. INTRODUCTION
Cerebral vasospasm is the major cause of delayed morbidity following aneurysmal subarachnoid hemorrhage (SAH). Despite recent advances in the management of patients with ruptured cerebral aneurysms, case fatality rates remain high (35% to 50%) [1-3]. More than 40% of survivors experience long-term cognitive and functional limitations [1-5].
A multiple inflammatory mechanisms are directly involved in the pathogenesis of cerebral vasospasm, with increased levels of various soluble adhesion molecules (such as E-selectin, intercellular adhesion molecule-1, and vascular adhesion molecule-1) and cytokines (such as IL-6 and IL-1) have been detected in the plasma and cerebrospinal fluid (CSF) of patients with SAH [2,3,6-9].
Identifying risk factors may improve clinical prediction and allow for more effective treatment of vasospasm. The measurement of sensitive inflammatory markers such as CRP significantly increases our ability to predict with accuracy and prevent or appropriately treat coronary thrombotic events [10-13]. Previous clinical studies have shown that elevated levels of high-sensitivity CRP could predict the development of coronary vasospasm [8,9].
Interest in inflammatory markers for cerebral vasospasm has increased, particularly when related with neurologic outcome.
In our study, we measured the CRP serum levels in patients suffering SAH, and analyzed the relationships between systemic CRP levels and the severity of SAH.
2. METHODS
Between January 2009 and February 2013, 100 adult patients were selected for a prospective cohort study at Hospital Ipiranga and Hospital das Clínicas in Botucatu, Brazil. The inclusion criteria were as follows: 1) diagnosis of aSAH and cerebral aneurysms established by a CT scan and four-vessel DS angiography study; 2) patient age > 18 years; 3) patient admission to our institutions within the first 24 hours postictus. The exclusion criteria were concomitant or recent acute myocardial infarction, recent surgery (≤30 days) prior to the event, and/or clinical or laboratory evidence of chronic systemic infection. In addition, patients who died before completing 10 days of treatment were not included.
Patient demographics, clinical status on admission Glasgow Coma Scale (GCS) (GCS scores and Hunt and Hess grades), head CT scans, severity of the SAH blood clot load (Fisher grades), location of a ruptured aneurysm (standard four-vessel DS angiography) and neurological examinations on admission and daily thereafter were recorded. Surgical clipping was performed in 57 (57%) of the 100 patients for 72 (57.14%) of the 126 aneurysms, whereas endovascular treatment was used in 43 patients (43%) for 54 (42.86%) of the 126 aneurysms. The selection of surgical versus endovascular treatment was based on criteria, such as the anatomical location of the lesion, the size and morphological features of the aneurysm, the presence of multiple aneurysms, the presence of a mass effect caused by the aneurysm and/or an associated hematoma, and the patient’s neurological and general medical condition and preference.
The serum CRP levels were measured daily between admission and the tenth day, and the measurements obtained were recorded. Transcranial Doppler (TD) was performed daily. The patients’ clinical outcome was evaluated using the Glasgow Outcome Scale (GOS) and modified Rankin Scale (mRS) at discharge from our institutions. This study was approved by the local Ethics Committee. Statistical analyses were performed using SAS version 9.1.3.
3. RESULTS
The admission GCS scores ranged from 3 to 15 (mean = 14). Hunt and Hess scores on admission ranged from I to V (mean = 2.5), and Fisher grades from 1 to 4 (mean = 1.5). The GOS scores on discharge ranged from 2 to 5 (mean = 4.2), and the range of mRS scores was from 0 to 5 (mean = 0.8).
A progressive increase in CRP levels from admission to the third day post ictus was observed, followed by a slow decrease until the ninth day (Figure 1). Patients with lower GCS scores presented increased CRP measurements (correlation coefficient methodology; z = −8.912, p < 0.0001, r = −0.89). Patients with higher Hunt and Hess grades on admission developed significantly higher serum CRP levels (correlation coefficient methodology; z = 6.941, p < 0.0001, r = 0.81) (Figure 2). Similarly, patients with higher Fisher grades on admission showed increased levels of CRP (correlation coefficient methodology; z = 7.821, p < 0.0001, r = 0.86) (Figure 3). Occurrences of clinical vasospasm were significantly correlated with higher CRP levels (correlation coefficient methodology; z = 7.921, p < 0.0001, r = 0.75) and patients with hemodynamic changes in transcranial Doppler showed higher CRP levels than patients without vasospasm (correlation coefficient methodology; z = 8.421, p < 0.0001, r = 0.88) (Figure 4).
There was no statistically significant difference in serum CRP levels between the group of patients undergoing surgical clipping and those undergoing endovascular coil occlusion. With regard to their GOS scores, patients with higher serum CRP levels (correlation coefficient methodology; z = −6.181, p < 0.0001, r = −0.81) (Figure
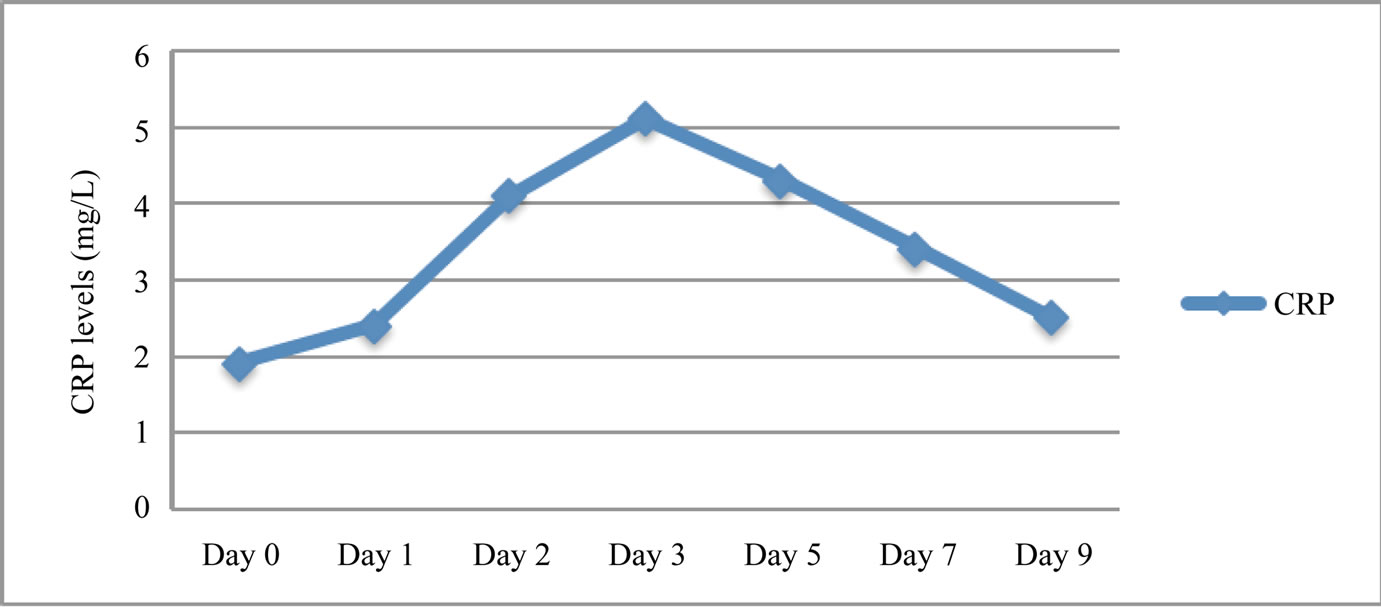
Figure 1. Schematic representation of summated measured CRP serum levels of our patients.

Figure 2. Schematic representation of serum CRP levels with regard to the admission Hunt Hess score (HH).
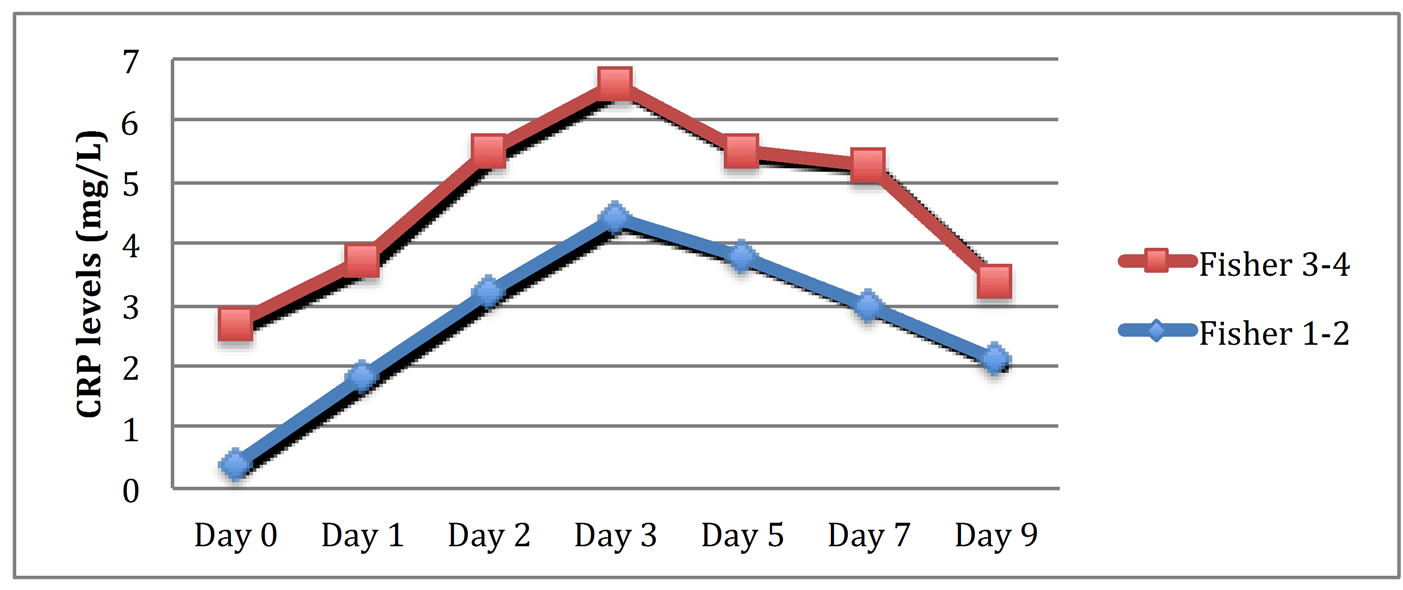
Figure 3. Schematic representation of serum CRP levels in patients with admission fisher score 1 - 2 versus patients with fisher score 3 - 4.
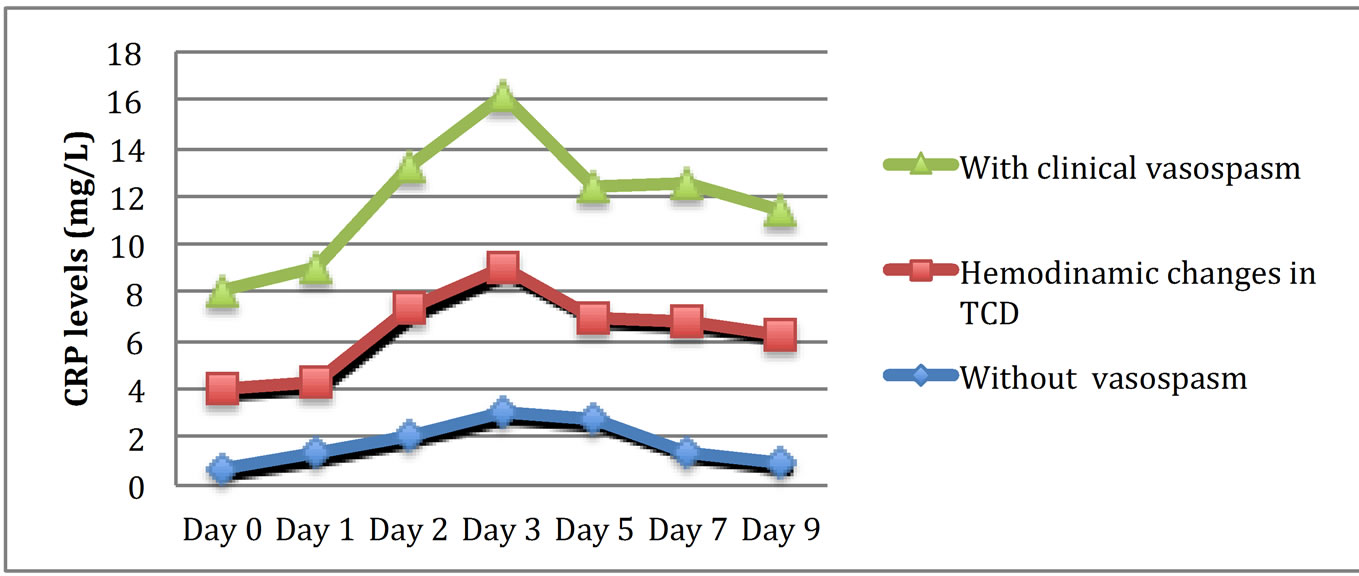
Figure 4. Schematic representation of serum CRP levels in patients with and without vasospasm and with hemodynamic changes in TCD.
5) presented less favorable outcomes. A statistically significant inverse correlation was established in our series between serum CRP levels and GOS scores. A similar statistically strong relationship was found between mRS scores on discharge and CRP measurements (correlation coefficient methodology; z = 6.762, p < 0.0001, r = 0.82) (Figure 6).
4. DISCUSSION
Cerebral vasospasm after SAH is common, potentially devastating, and incompletely understood. Inflammation accompanying SAH may be a critical pathway underlying the development of cerebral vasospasm. Inflammation is a complex and multifaceted response aimed to ultimately defend against foreign antigens [8,9,14-18]. In the instance of SAH, a complex series of cellular and molecular events is elicited by the presence of blood clot in the subarachnoid space, culminating in a robust inflammatory response. Although the possible role of inflammation in the genesis of cerebral vasospasm has

Figure 5. Schematic representation of serum CRP measurements in patients with GOS score 4 - 5 versus patients with GOS score 1 - 3 at discharge.
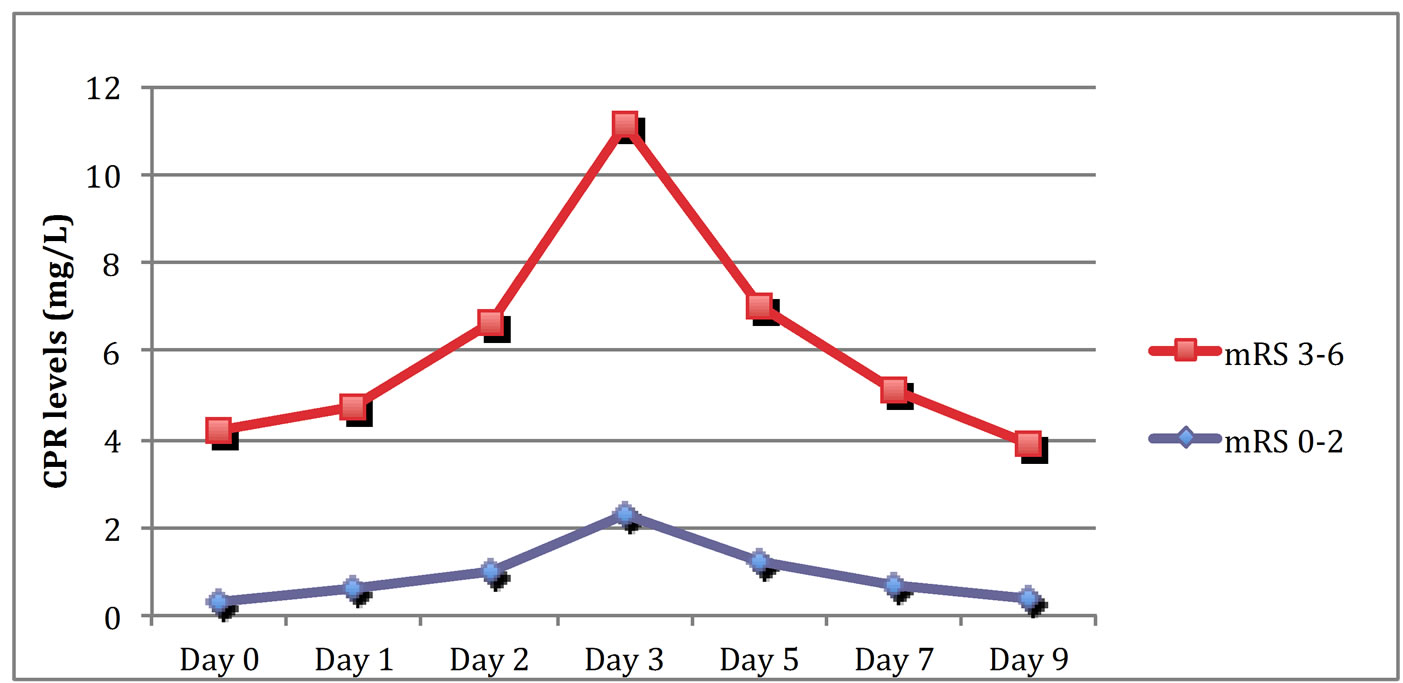
Figure 6. Schematic representation of serum CRP measurements in patients with mRS score 3 - 6 versus patients with mRS score 0 - 2 at discharge.
been recognized for some time, its cellular and molecular basis and putative importance have not been more clearly defined until recently [2,3,19,20].
In the acute stages of inflammation, the local homeostatic milieu is altered, resulting in expression of adhesion molecules that evoke leukocyte adherence to the endothelium, with subsequent migration, activation, and release of effector substances [8,21]. Leukocytes are also critical constituents of the inflammatory response. Leukocytes may act by direct effects on the vasculature or indirectly through the elaboration and propagation of the inflammatory response [22]. For example, neutrophils may produce and release reactive metabolites of oxygen that evoke endothelial dysfunction and calcium influx [2,23]. Furthermore, leukocytes may release a variety of other substances (including leukotrienes and other lipid mediators) that have powerful vascular effects. In addition, leukocytes produce a host of factors (such as cytokines) that further activate and propagate immune responses [24,25].
Experimental and clinical evidence suggests that Intercellular Adhesion Molecule 1 (ICAM-1) mediated leukocyte migration may play a crucial role in the pathogenesis of cerebral vasospasm [9-13]. SAH increases endothelial ICAM-1 expression [1,3-5,26,27] and resultant perivascular leukocyte migration [1,3-5,9,14,15]. Furthermore, serum ICAM-1 level correlates with the onset of cerebral vasospasm [1,3,6-9]. Perivascular chemokine-activated inflammatory cells synthesize and release endothelin-1, a potent vasoconstrictor, as well as superoxide free radicals, leading to inactivation of nitric oxide (NO) and vasoconstriction [1,3,6-9,26,27]. AntiICAM-1 antibodies decrease leukocyte migration and attenuate cerebral vasospasm after SAH [1,3,6-9].
CRP is a sensitive inflammatory marker, with synthesis in hepatocytes is strongly stimulated by interleukin-6 [1-3,6,7,20,21]. Additionally, IL-1, which has been implicated in the pathogenesis of cerebral vasospasm, also represents a strong stimulus for CRP synthesis [1-5]. So, elevated concentrations of CRP may well be associated with an increased possibility of developing cerebral vasospasm and subsequently a DIND [1,3,22-25,28].
There was a strong inverse correlation between admiting GCS scores and CRP levels in serum (r = −0.89 and r = −0.82, respectively). Hunt and Hess and Fisher grades were also correlated in a statistically significant fashion with the CRP measurements in our cohort. These data clearly indicates that CRP levels significantly relate with the severity of aSAH and occurrence of vasospasm. Furthermore, the elevated CRP levels were associated with worse clinical outcome, as expressed in GOS and mRS scores. Additionally, we found no statistically significant differences in the occurrence of angiographic vasospasm between patients undergoing surgical treatment and those undergoing endovascular coil occlusion.
Our strict inclusion criteria minimized the influence of other confounding factors such as systemic infection or concomitant systemic conditions, and statistical analysis is compelling to define the influence of CRP levels on vasospasm occurrence and neurological final outcomes. Unfortunately, the clinical significance of elevated serum CRP measurements in patients sustaining aSAH is confounded by the fact that most of these patients may have other concomitant systemic infections or pathological conditions that could potentially result in increased CRP serum concentrations. Additionally, the surgical manipulation in these patients could influence the systemic CRP levels.
It is well known that clinical outcome in patients with aSAH is multifactorial. The association between CRP levels systemically with the clinical outcome may well be influenced by other parameters in a complex and frequently unpredictable way. In addition, CRP represents a sensitive but nonspecific inflammatory marker. A largescale, multicenter, prospective clinical study is necessary to validate our results and to determine the role of serum CRP in the identification of patients at high risk for developing cerebral vasospasm.
5. CONCLUSION
Clinical and radiologic severity of SAH correlated significantly with serum CRP levels, since patients with low GCS scores and high Hunt and Hess and Fisher grades presented elevated serum CRP levels. Additionally, CRP levels were able to predict occurrences of vasospasm and poor clinical outcomes in a statistically significant fashion. Routine use of CRP levels may identify patients who are at a high risk of developing cerebral vasospasm and may have a positive impact on therapeutic strategies and the future management of patients with aSAH.
REFERENCES
- Bengzon, J., Grubb, A., Bune, A., Heillstrom, K., Lindstrom, V. and Brandt, L. (2003) C-reactive protein levels following standard neurosurgical procedures. Acta Neurochirurgica, 145, 667-671. doi:10.1007/s00701-003-0083-5
- Romero, F.R., Bertolini, E.G., Figueiredo, E.G. and Teixeira, M.J. (2012) Serum C-reactive protein levels predict neurological outcome after aneurysmal subarachnoid hemorrhage. Arq Neuropsiquiatr, 70, 202-205.
- Fountas, K.N., Tasiou, A., Kapsalaki, E.Z., Paterakis, K.N., Grigorian, A.A. and Lee, G.P. (2009) Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Neurosurgical Focus, 26, 22-31. doi:10.3171/2009.2.FOCUS08311
- Kramer, A.H., Gurka, M.J., Nathan, B., Dumont, A.S., Kassell, N.F. and Bleck, T.P. (2008) Statin use was not associated with less vasospasm or improved outcome after subarachnoid hemorrhage. Neurosurgery, 62, 422-430. doi:10.1227/01.neu.0000316009.19012.e3
- Suzuki, R., Masaoka, H., Hirata, Y., Marumo, F., Isotani, E. and Hirakawa, K. (1992) The role of endothelin-1 in the origin of cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery, 77, 96-100. doi:10.3171/jns.1992.77.1.0096
- Vajkoczy, P., Meyer, B., Weidauer, S., Raabe, A., Thome, C., Ringel, F., et al. (2005) Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: Results of a randomized, double-blind, placebo-controlled, multicenter Phase IIa study. Neurosurgery, 103, 9-17. doi:10.3171/jns.2005.103.1.0009
- Goddard, A.J.P., Raju, P.P.J. and Gholkar, A. (2004) Does the method of treatment of acutely ruptured intracranial aneurysms influence the incidence and duration of cerebral vasospasm and clinical outcome? Journal of Neurology, Neurosurgery & Psychiatry, 75, 868-872. doi:10.1136/jnnp.2003.033068
- Berk, B.C., Weintraub, W.S. and Alexander, R.W. (1990) Elevation of C-reactive protein in ‘‘active’’ coronary artery disease. American Journal of Cardiology, 65, 168- 172. doi:10.1016/0002-9149(90)90079-G
- Carr, W.P. (1983) The role of the laboratory in rheumatology. Acute-phase proteins. Rheumatic Diseases Clinics of North America, 9, 227-239.
- Dumont, A.S., Dumont, R.J., Chow, M.M., Lin, C., Calisaneller, T., Ley, K.F., et al. (2003) Cerebral vasospasm after subarachnoid hemorrhage: Putative role of inflammation. Neurosurgery, 53, 123-135. doi:10.1227/01.NEU.0000068863.37133.9E
- Hansen-Schwartz, J. (2004) Cerebral vasospasm: A consideration of the various cellular mechanisms involved in the pathophysiology. Neurocritical Care, 1, 235-246. doi:10.1385/NCC:1:2:235
- Hergenroeder, G., Redell, J.B., Moore, A.N., Dubinsky, W.P., Funk, R.T., Crommett, J., et al. (2008) Identification of serum biomarkers in brain-injured adults: Potential for predicting elevated intracranial pressure. Journal of Neurotrauma, 25, 79-93. doi:10.1089/neu.2007.0386
- Hoshi, T., Shimizu, T., Kito, K., Yamasaki, N., Takahashi, K., Takahashi, M., et al. (1984) Immunological study of late cerebral vasospasm in subarachnoid hemorrhage: Detection of immunoglobulins, C3, and fibrinogen in cerebral arterial walls by immunofluorescence method. Neurologia Medico-Chirurgica, 24, 647-654. doi:10.2176/nmc.24.647
- Rothoerl, R.D., Schebesch, K.M., Kubitza, M., Woertgen, C., Brawanski, A. and Pina, A.L. (2006) ICAM-1 and VCAM-1 expression following aneurysmal subarachnoid hemorrhage and their possible role in the pathophysiology of subsequent ischemic deficits. Cerebrovascular Diseases, 22, 143-149. doi:10.1159/000093243
- Rankin, J. (1957) Cerebral vascular accidents in patients over the age of 60. Scottish Medical Journal, 2, 200-215.
- Bonita, R. and Beaglehole, R. (1988) Modification of rankin scale: Recovery of motor function after stroke. Stroke, 19, 1497-1500. doi:10.1161/01.STR.19.12.1497
- Van Swieten, J.C., Koudstaal, P.J., Visser, M.C., Schouten, H.J. and van Gijn, J. (1988) Interobserver agreement for the assessment of handicap in stroke patient. Stroke, 19, 604-607. doi:10.1161/01.STR.19.5.604
- Lynch, J.R., Blessing, R., White, W.D., Gracott, H.P., Newman, M.F. and Laskowitz, D.T. (2004) Novel diagnostic test for acute stroke. Stroke, 35, 57-63. doi:10.1161/01.STR.0000105927.62344.4C
- Suzuki, H., Kanamaru, K., Tsunoda, H., Inada, H., Kuroki, M., Sun, H., et al. (1999) Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. Journal of Clinical Investigation, 104, 59-66. doi:10.1172/JCI5357
- Pluta, R.M. (2005) Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacology & Therapeutics, 105, 23-56. doi:10.1016/j.pharmthera.2004.10.002
- Mazlam, M.Z. and Hodgson, H.J. (1994) Interrelations between interleukin-6, interleukin-1 beta, plasma C-reactive protein values, and in vitro C-reactive protein generation in patients with inflammatory bowel disease. Gut, 35, 77-83. doi:10.1136/gut.35.1.77
- Kassell, N.F., Sasaki, T., Colohan, A.R.T. and Nazar, G. (1985) Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke, 16, 562-572. doi:10.1161/01.STR.16.4.562
- Kasuya, H., Weir, B.K., Nakane, M., Pollock, J.S., Johns, L., Marton, L.S., et al. (1995) Nitric oxide synthase and guanylate cyclase levels in canine basilar artery after subarachnoid hemorrhage. Journal of Neurosurgery, 82, 250-255. doi:10.3171/jns.1995.82.2.0250
- Hung, M.J., Cherng, W.J., Yang, N.I., Cheng, C.W. and Li, L.F. (2005) Relation of high-sensitivity C-reactive protein level with coronary vasospastic angina pectoris in patients without hemodynamically significant coronary artery disease. American Journal of Cardiology, 96, 1484- 1490. doi:10.1016/j.amjcard.2005.07.055
- Deshmukh, V.R., Kakarla, U.K., Figueiredo, E.G., Zabramski, J.M. and Spetzler, R.F. (2006) Long-term clinical and angiographic follow-up of unclippable wrapped intracranial aneurysms. Neurosurgery, 58, 434-442.
- Mayberg, M.R., Batjer, H.H., Dacey, R.G. Jr, Diringer, M., Haley, E.C., Heros, R.C., et al. (1994) Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke, 25, 2315-2328. doi:10.1161/01.STR.25.11.2315
- Kasuya, H., Weir, B.K., Nakane, M., Pollock, J.S., Johns, L., Marton, L.S., et al. (1995) Nitric oxide synthase and guanylate cyclase levels in canine basilar artery after subarachnoid hemorrhage. Journal of Neurosurgery, 82, 250-255. doi:10.3171/jns.1995.82.2.0250
- Cecon, A.D., Figueiredo, E.G., Bor-Seng-Shu, E., Scaff, M. and Teixeira, M.J. (2008) Extremely delayed cerebral vasospasm after subarachnoid hemorrhage. Arq Neuropsiquiatr, 66, 554-556. doi:10.1590/S0004-282X2008000400024
NOTES
*Conflicts of interest: none.

