Health
Vol.5 No.5A(2013), Article ID:31983,7 pages DOI:10.4236/health.2013.55A001
1-Chloromethyl-6,7-dimethoxy-3,4-dihydro-1H-isoquinoline-2-sulfonic acid amide, a derivative of tetrahydroisoquinoline, induces granulocytic differentiation of the human leukemic HL-60 cells via G0/G1 phase arrest
![]()
1Department of Pathology, College of Korean Medicine, Wonkwang University, Iksan, South Korea; *Corresponding Author: omdjbh@wku.ac.kr
2Department of Microbiology and Immunology, Wonkwang University School of Medicine, Iksan, South Korea
3Department of Biological Science, College of Natural Sciences, Wonkwang University, Iksan, South Korea
4Department of Bionanochemistry, School of Natural Sciences, Wonkwang University, Iksan, South Korea
5College of Korean Medicine, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, South Korea
Copyright © 2013 Sung-Min Ju et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 14 March 2013; revised 15 April 2013; accepted 1 May 2013
Keywords: Differentiation; G0/G1 Phase Arrest; HL-60 Cells; Tetrahydroisoquinolines; P27Kip1
ABSTRACT
Tetrahydroisoquinolines are known to have various biological effects, including antitumor activity. This study investigated the effect of 1- chloromethyl-6, 7-dimethoxy-3, 4-dihydro-1H-isoquinoline-2-sulfonic acid amide (CDST), a newly synthesized anticancer agent, on cellular differentiation and proliferation in HL-60 cells. Differentiation and proliferation of HL-60 cells were determined through expression of CD11b and CD14 surface antigens using flow cytometry and nitroblue tetrazolium (NBT) assay, and through analysis of cell cycle using propidium iodide staining, western blot analysis and immunoprecipitation, respectively. CDST induced the differentiation of HL-60, as shown by increased expression of differentiation surface antigen CD11b (but no significant change in CD14 expression) and increased NBT-reducing functional activity. DNA flow cytometry analysis indicated that CDST markedly induced a G0/G1 phase arrest of HL-60 cells. Subsequently, we examined the expression of G0/G1 phase cell cycle-related proteins, including cyclin-dependent kinases (CDKs), cyclins and cyclin dependent kinase inhibitors (CKIs), during the differentiation of HL-60. The levels of CDK2, CDK6, cyclin E and cyclin A were decreased, whereas steady-state levels of CDK4 and cyclin D1 were unaffected. The expression of the p27Kip1 was markedly increased by CDST, but not p21WAF1/Cip1. Moreover, CDST markedly enhanced the binding of p27Kip1 with CDK2 and CDK6, resulting in the reduced activity of both kinases. Taken together, these results demonstrate that CDST is capable of inducing cellular differentiation and growth inhibition through p27Kip1 protein-related G0/G1 phase arrest in HL- 60 cells.
1. INTRODUCTION
Treatment of the promyelocytic leukemia cell lines, including HL-60 cells, with anti-leukemic agents induces differentiation into two cell types of the myeloid lineage: monocyte/macrophage-like cells [1,2] and granulocytelike cells [3]. 12-O-Tetradecanoyl-phorbol-13-acetate and all-trans-retinoic acid (ATRA) are well-known inducers that can stimulate differentiation of HL-60 cells into monocyte/macrophage-like and granulocyte-like phenoltypes, respectively [1,2,4]. After exposure to these agents, HL-60 cells tend to stop cellular proliferating and express the phenotypical and physiological functions specific to mature cells.
Terminal differentiation is usually associated with the exit of the cells from the cell cycle [5-7]. Cell cycle progression in mammalian cells is regulated by a family of enzymes known as cyclin-dependent kinases (CDKs) whose activity is dependent on the binding to specific regulatory subunits called cyclins [5,8]. The activity of the cyclin/CDK complexes is negatively regulated by specific CDK inhibitors, such as p21WAF1/Cip1 and p27Kip1 [8]. The mechanisms for regulation of cell cycle have a fundamental role in the control of cell proliferation and may also be involved in differentiation Tetrahydroisoquinoline alkaloids, based isoquinoline skeleton, have various biological activities according to the structure properties. Tetrahydroisoquinoline alkaloids gain much interest because of their biological activities, including antitumor [9], renal vasoconstriction [10], antihypertensive [11], Parkinson’s disease inhibitor [12]. The derivative of tetrahydroisoquinoline, or 1-Chloromethyl-6,7-dimethoxy-3,4-dihydro-1H-isoquinoline-2-sulfonic acid amide (CDST), has been developed as a new anticancer agent, but the underlying mechanisms remain to be established. In order to understand possible mechanisms for its anticancer/anti-leukemic activities, this study investigated the effects of CDST on proliferation and differentiation of the human promye locytic leukemic HL-60 cells.
2. MATERIALS AND METHODS
2.1. Reagents and Antibodies
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), nitrotetrazolium blue chloride (NBT), phorbol 12-myristate 13-acetate (TPA), propidium iodide (PI), ribonuclease A (RNase A), and protease inhibitor cocktail were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). RPE-con-jugated anti-CD11b and FITC-conjugated anti-CD14 antibodies were purchased from DAKO (Glostrup, Denmark). RPMI 1640, fetal bovine serum (FBS) and antibiotic-antimycotic solution were purchased from GIBCO (Grand Island, NY, USA). Protein A/G PLUS-Agarose, antip21WAF1/Cip1, anti-p27Kip1, anti-CDK2, anti-CDK4, antiCDK-6, anti-cyclin D1, anti-cyclin A, and anti-cyclin E antibodies were purchased from Santa Cruz Biotechnology, INC. (Santa Cruz, CA, USA). Anti-p27Kip1 monoclonal antibody was purchased from BD biosciences (San Jose, CA, USA). HRP-conjugated goat anti-rabbit IgG and rabbit anti-mouse IgG were purchased from Invitrogen (Burlington, ON, Canada). 1-Chloromethyl-6, 7-dimethoxy-3,4-dihydro-1H-isoquinoline-2-sulfonic acid amide (CDST) was prepared by the use of iminium ions as intermediates through α-sulfamidoalkylation (Figure 1). CDST was dissolved in DMSO at a concentration 10 mM and stored at −20˚C and diluted in cell culture medium before use.
2.2. Cell Culture
HL-60 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and routinely cultured in RPMI 1640 medium supplemented with 10% FBS in 1:100 dilution of an antibiotic-antimycotic solution at 37˚C in a 5% CO2 incubator.
2.3. Cell Viability
The cells were treated with various concentrations of CDST for 24 h or 72 h. After the indicated time periods, MTT solution was added to each wells and incubated for 4 h. Water-insoluble MTT-formazan crystals were solubilized by adding equal volume of solubilization solution (10% SDS/0.01 N HCl) and incubating the overnight in humidified atmosphere of 5% CO2 at 37˚C. The amount of formazan was determined by Spectra-MAX 250 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) at 570 nm.
2.4. Differentiation Assay
1) NBT reduction assay: The cells were cultured with CDST or ATRA in RPMI-1640 medium containing 10% FBS for 72 h, and then the cell’s NBT reducing activity was determined by the method of Sakashita et al. [13] with a slight modification. The amount of formazan formed was assayed spectrophotometrically at 560 nm in a spectrophotometer. 2) Flow cytometry analysis: The cells were suspended in 100 μl of PBS (pH 7.4) containing 0.25% BSA. After the addition of 10 μl of RPE-labeled anti-CD 11b or FITC-labeled anti-CD 14 antibodies, the cells were incubated in the dark at 4˚C for 30 min, and then fixed in 500 μl of PBS (pH 7.4) containing 1% formaldehyde, and then the level of antibody binding to the cells were quantified using FACS flow cytometry (BD Biosciences, CA, USA).
2.5. DNA Content Analysis
Cell cycle progression was monitored by quantitating cellular DNA content after staining with PI. The cells were fixed with ice-cold 100% ethanol for 1 h, washed with PBS, and then treated with PI/RNase A solution at 37˚C for 1 h in the dark, and analyzed on a FACS flow
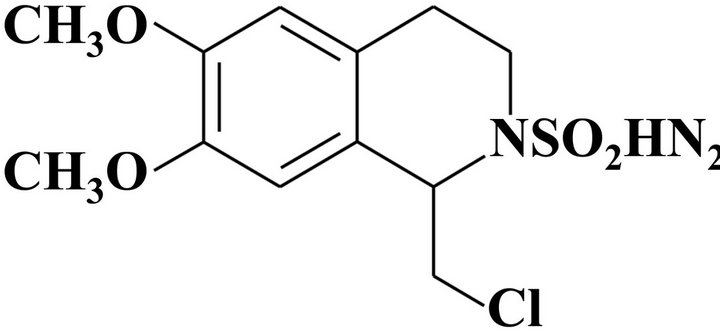
Figure 1. Structure of 1-Chloromethyl-6,7-dimethoxy-3,4-dihydro-1H-isoquinoline-2-sulfonic acid amide (CDST).
cytometry. Cell cycle analysis was determined DNA content from fixed cells stained with PI.
2.6. Western Blot Analysis
Cells were lysed with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.5% NaVo4, 0.1% SDS, 1% protease inhibitor cocktail) for 30 min on ice. Cell lysates were centrifuged at 14,000 rpm for 10 min at 4˚C, and the protein concentration was determined using a Bradford assay. Samples containing 40 μg of total protein were resolved by SDS-PAGE gel, and transferred onto a nitrocellulose membrane. Blots were probed with primary antibodies. Immunoreactivity was detected using anti-rabbit or anti-mouse HRP-conjugated secondary immunoglobulin G anti-bodies. Immuno-reactive bands were visualized using the SuperSignal West Pico Chemiluminescent Substrate Kit (Thermo Fisher Scientific, Waltham, MA, USA) and then developed using the Molecular Imager ChemiDoc XRS System (BioRad, Hercules, CA, USA).
2.7. Immunoprecipitation
Cells were lysed with immunoprecipitation lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% Sodium deoxycholate, 1% protease inhibitor cocktail) for 15 min on ice. Cell lysates were centrifuged at 11,000 rpm for 15 min at 4˚C, and then precleared using Protein A/G PLUS-Agarose. Preclered lysates were quantitated using a Bradford assay. 200 μg of the total protein were incubated with anti-CDK2, anti-CDK4 and antiCDK6 polyclonal antibodies for 6 h at 4˚C, followed by incubation with the Protein A/G PLUS-Agarose for 2 h. The immunoprecipitates were boiled in laemmlie sample buffer for 5 min followed by centrifugation.
2.8. Statistical Analysis
Statistical analysis was performed using Microsoft Office Excel 2010 (Microsoft, Redmond, WA). The data were expressed as means ± standard deviation (SD). The statistically significant differences between two groups were calculated by Student’s t-test.
3. RESULTS
3.1. Effect of CDST on Cell Viability of HL-60 Cells
The cytotoxicity of CDST in HL-60 cells was evaluated using the MTT assay. As shown in Figure 2, exposure of HL-60 cells to CDST for 24 h dramatically decreased the viability of HL-60 cells in a concentrationdependent manner. At the concentrations of CDST used in this study (i.e., 10 μM and 20 μM), cell viability was no significant difference in comparison with untreated control.
3.2. Effect of CDST on Differentiation of HL-60 Cells
We examined whether CDST could induce differentiation of HL-60 cells. For this end, HL-60 cells were treated with CDST or ATRA, and the numbers of differentiated cells were determined by measuring NBT-reducing activity. After treatment of HL-60 cells with CDST for 72 h, its differentiation-inducing activity was assayed and compared with ATRA. As shown in Table 1, when HL-60 cells were treated with CDST at concentrations of 10 μM and 20 μM, NBT-reducing activity was increased up to approximately 3.7-fold and 5.1-fold, respectively. Also, differentiation-inducing activety of CDST was comparable to that of ATRA (served as a positive control of differentiation). In CDST-treated cells for 72 h, cell viability reduced about 57.3% and 54, 9% at concentrations of 10 μM and 20 μM CDST, respectively. The induction of HL-60 cell differentiation was also assessed by examining the expression of cell surface
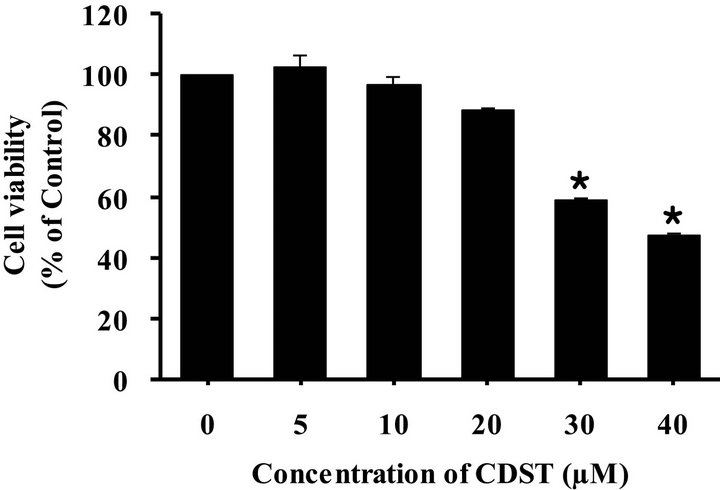
Figure 2. Effect of CDST on cell viability of HL-60 cells. The cells were incubated with various concentrations of CDST for 24 h, and cell viability was measured by MTT assay. Value are means ± SD (N = 3). The statistically significant differences compared with CDST-untreated group were calculated by Student’s t-test. *p < 0.01 with respect to CDSTuntreated control group.
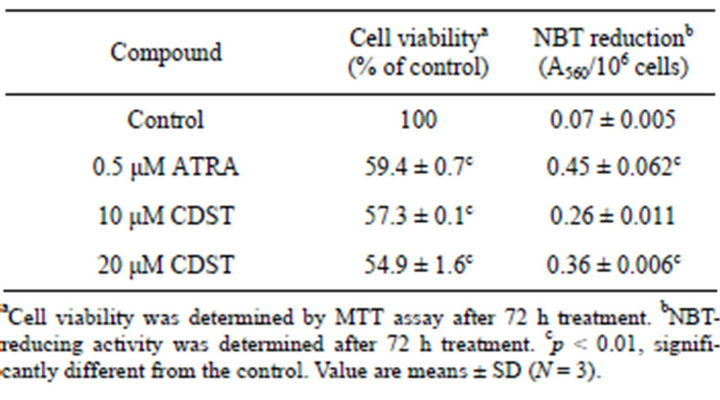
Table 1. Effects of CDST on viability and differentiation of HL-60 cells.
antigens (e.g., CD11b and CD14). CD11b (FITC-labeled) expression was used as a marker of granulocytic differentiation, while CD14 (RPE-labeled) expression was only found in monocytic differentiation. After HL-60 cells were incubated with or without 20 μM CDST for 72 h, cell surface markers were immunolabeled and measured by a flow cytometry. As shown in Figure 3, in comparison with the untreated cells, the amount of CD11bpositive cells in CDST-treated HL-60 cells were increased significantly (66.87%). However, the expression of monocytic CD14 antigen was not significantly increased (11.03%). CD11b and CD14 in CDST-HL-60 cells were normally expressed by approximately 7.51% and 3.93%, respectively. These results suggest that CDST has ability to induce granulocytic differentiation of HL-60 cells.
3.3. Effect of CDST on the Cell Cycle Distribution and Expression of Cell Cycle-Regulatory Proteins in HL-60 Cells
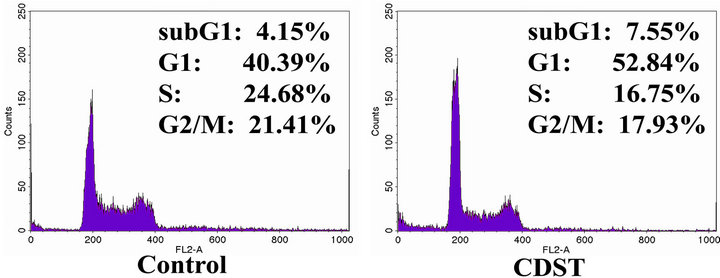
The effect CDST on the cell cycle progression in HL- 60 cells was determined by FACS. ATRA, a differentiating agent, inhibits cell proliferation and arrests leukemic cells in G0/G1 phase [14]. In a previous study, it was suggested that the maintenance of G0/G1 phase might be necessary for HL-60 granulocytic differentiation [15]. HL-60 cells were incubated with or without 20 μM CDST for 72 h and analyzed by DNA flow cytometry. As shown in Figure 4(a), the cell population in G1 phase of CDST-treated HL-60 cells was increased from 40.39% to 52.84%, whereas cell population in G2/M and S phases was decreased from 21.41% to 17.93% and from 24.68% to 16.75%, respectively. Thus, our data obtained from DNA flow cytometric analysis indicate that CDST is capable of inducing a G0/G1 phase arrest of cell cycle in HL-60 cells.
We next examined the levels of G1 cell cycle regulatory proteins in CDST-treated HL-60. The CDK4/6-cyclin D and CDK2-cyclin E complexes play important
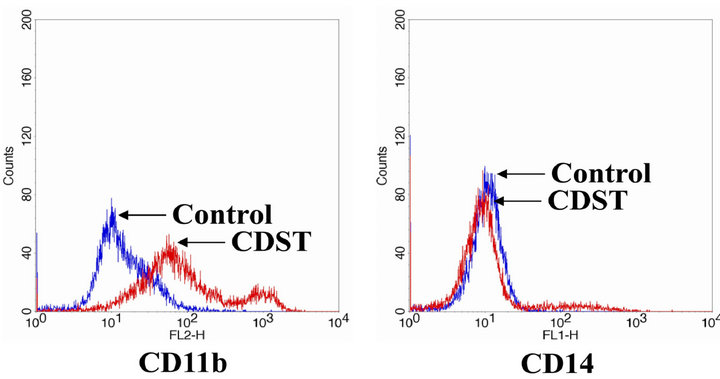
Figure 3. The differentiation-inducing effect of CDST on HL- 60 cells. Cells were treated with 20 μM CDST for 72 h, and cells were assessed by FACS analysis using RPE-conjugated anti-CD11b or FITC-conjugated anti-CD14 mono-clonal antibodies.
regulatory roles in G1 phase cell cycle progression [15]. The CDK4/6-cyclin D complexes are involved in early G1 phase, while CDK2-cyclin E complex is necessary for the G1/S phase transition [15]. HL-60 cells were treated with 20 μM CDST for 24, 48, and 72 h and then harvested for Western blotting. The protein levels of CDK2, CDK6, cyclin E and cyclin A were significantly decreased in response to CDST, whereas steady-state levels of CDK4 and cyclin D1 were unaffected (Figure 4(b)). These results imply that decreased expression of CDK2, CDK6, cyclin E and cyclin A proteins may be responsible for the CDST induced G0/G1 phase arrest.
3.4. Association of P27Kip1 with Cell Cycle-Regulatory Proteins in CDST-Treated HL-60 Cells
Because CDST induced a G1 arrest in HL-60 cells, we subsequently examined the change in the p21WAF1/Cip1 and p27Kip1 proteins, which are the CKIs related with the G1 phase arrest. In the progression of the cell cycle, the CKIs p21WAF1/Cip1 and p27Kip1 proteins cause cell cycle
 (a)
(a)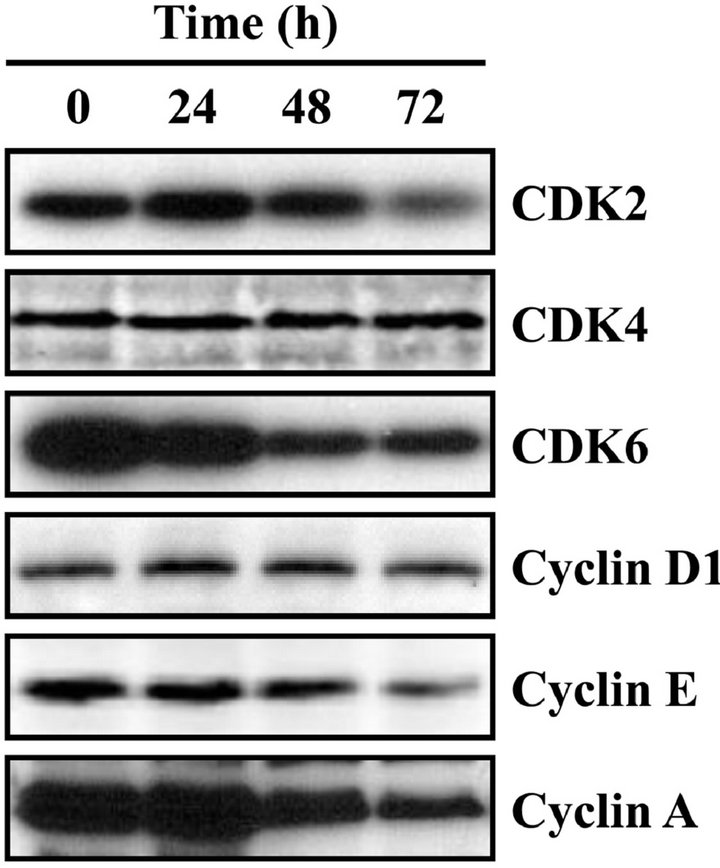 (b)
(b)
Figure 4. Effect of CDST on the cell cycle progression of HL- 60 cells. (a) Cells were exposed to 20 μM CDST for 72 h, washed and then harvested. The cells were fixed and stained with PI, and the DNA content was analyzed by a flow cytometry. (b) The cells were harvested at the indicated times after incubation with CDST. Cells were the lysed, and the supernatants were subjected to Western blot analysis using antibodyies against CDK2, CDK4, CDK6, cyclin D1, cyclin E and cyclin A.
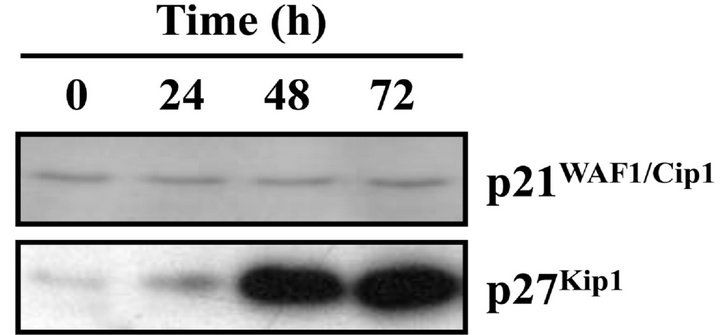
arrest in the Gl phase through suppression of CDK activity [15]. After CDST treatment of HL-60 cells, the p27Kip1 protein level increased in a time-dependent manner, whereas no detectable change was observed in the level of the p21WAF1/Cip1 (Figure 5(a)). The level of p27Kip1 began to increase slightly after 24 h incubation with CDST and had clearly increased at 48 h. These results suggested that induction of p27Kip1 protein was involved in G0/G1 phase arrest of CDST-treated HL-60 cells.
The p27Kip1 protein bind to cyclin-CDK complexes to inhibit their catalytic activity and induce cell-cycle arrest [16,17]. To demonstrate whether increased p27Kip1 protein bind to cyclin-CDK complexes, we examined the amount of p27 Kip1 associated with cyclin-CDK complexes. The CDK2, CDK4, and CDK6 complexes were immunoprecipitated from the HL-60 cells, which were treated with or without CDST, and the co-immuno-precipitated p27 Kip1 level in immune complex was determined by Western blot analysis using anti-p27 Kip1 monoclonal antibody. As shown in Figure 5(b), the p27 Kip1 levels in the CDK2 and CDK6 immune complexes of the CDST-treated cells were distinctively higher than in those of the untreated cells. However, the CDK4 immune complex exhibited no significant difference in the level
 (a)
(a)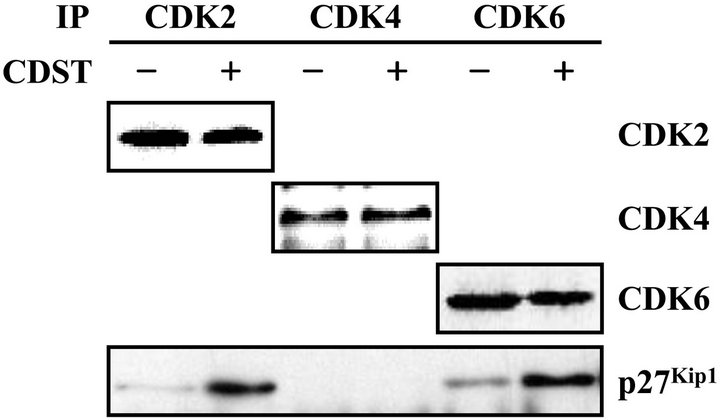 (b)
(b)
Figure 5. Association of p27Kip1 with CDKs in CDST-treated HL-60 cells. (a) The cells were treated with 20 μM CDST for various times. The levels of p21WAF1/Cip1 and p27Kip1 ex-pression were determined by Western blot analysis using anti-p21WAF1/Cip1 and anti-p27Kip1 antibodies. (b) The cells were treated with (+) or without (−) 20 μM CDST for 48 h. The total lysates were immunoprecipitated using anti-CDK2, anti-CDK4, or anti-CDK6 antibodies.
of p27Kip1 protein between CDST-treated and -untreated cells. Taken together, these results suggested that p27Kip1 protein might play a key role in G1 phase arrest through its increased binding to CDK2 and CDK6 in CDST-treated HL-60 cells.
4. DISCUSSION
CDST used in the study included aminosulfonyl group in nitrogen of tetrahydropyridine ring that was synthesized using intramolecular α-sulfamidoalkylation reaction. Synthesized CDST has isoquinoline skeleton and ring type sulfamide carrying biological activity. This study has demonstrated that CDST can induce cellular differentiation and inhibit cell proliferation in the human leukemia HL-60 cells. The differentiation-inducing effect of CDST was confirmed with a functional NBT reduction assay and the expression of cell surface antigens. According to our results, CDST-induced differentiation resulted in a marked increase in NBT reducing activity and expression of the cell surface antigen CD11b, indicating granulocyte differentiation. Thus, CDST is thought to have the ability to induce the differentiation of HL-60 cells into mature cells, those of granulocytic lineage.
Cell differentiation is regulated in a cell cycle-dependent manner. For instance, the differentiation of hematopoietic cells is accompanied with G0/G1 phase cell cycle arrest [18-20]. In this study, the cell cycle analysis revealed that CDST could markedly induce a G0/G1 phase arrest in HL-60 cells, along with a significant reduction in G2/M and S phases. It has been demonstrated that inhibition of the G1/S transition induces growth arrest and granulocyte differentiation of HL-60 cells, which is mediated by a block of cell cycle progression at the G0/G1 phase [21,22]. The p21WAF1/Cip1 and p27Kip1 proteins are a cyclin-dependent kinase inhibitor that is one of the regulators of cell cycle progression in the G1/S transition [23]. Here we have shown that treatment of HL-60 cells with CDST resulted in increased p27Kip1 expression, but no significant change in p21WAF1/Cip1 expression. These results suggest that p27 Kip1 expression by CDST may be important for the G0/G1 phase arrest and granulocytic differentiation of HL-60 cells.
Among the CDKs that regulate the cell cycle, CDK2, CDK4 and CDK6 are activated in association with the D-type cyclins or cyclin E during the G1 progression and the G1-S transition [24]. This study revealed that the CDK2, CDK6, cycline E and cyclin A were decreased in the CDST-treated HL-60 cells. In addition, the accumulation of the p27Kip1 protein in association with the G0/ G1 arrest was detected in the complexes with CDK2 and CDK6. The interaction between the CDK2/CDK6 and p27Kip1 was associated with suppressed cyclin/CDK activity. Thus, the decreased protein levels of CDK2, CDK6, cyclin E and cyclin A and the reduced kinase activities of CDK2 and CDK6 might result in G0/G1 phase cell cycle arrest in CDST-treated HL-60 cells. It is likely that ATRA-induced terminal differentiation of leukemia cells involves the sequential regulation of cell cycle regulatory genes, coordinating the process of differentiation with arrest in the G0/G1 phase of the cell cycle [25]; namely, G0/G1 phase arrest of the cell cycle is hallmark of terminally differentiation in leukemia cells.
5. CONCLUSION
CDST induces granulocytic differentiation and growth inhibition through p27Kip1 protein-related G0/G1 phase arrest in HL-60 cells. Thus, these results suggest that CDST is a new potent inducer for differentiating the HL- 60 human leukemia cells into granulocytes. Further studies are necessary to elucidate the exact mechanism(s) for CDST-induced differentiation of HL-60 cells.
6. ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (NO. 2010-0029469).
REFERENCES
- Huberman, E. and Callaham, M.F. (1979) Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proceedings of the National Academy of Sciences of the United States of America, 76, 1293-1297. doi:10.1073/pnas.76.3.1293
- Rovera, G., Santoli, D. and Damsky, C. (1979) Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proceedings of the National Academy of Sciences of the United States of America, 76, 2779-2783. doi:10.1073/pnas.76.6.2779
- Collins, S.J., Ruscetti, F.W., Gallagher, R.E. and Gallo, R.C. (1978) Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proceedings of the National Academy of Sciences of the United States of America, 75, 2458-2462.
- Breitman, T.R., Selonick, S.E. and Collins, S.J. (1980) Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proceedings of the National Academy of Sciences of the United States of America, 77, 2946-2940. doi:10.1073/pnas.77.5.2936
- Figarola, J.L., Weng, Y., Lincoln, C., Horne, D. and Rahbar S. (2012) Novel dichlorophenyl urea compounds inhibit proliferation of human leukemia HL-60 cells by inducing cell cycle arrest, differentiation and apoptosis. Investigational New Drugs, 30, 1413-1425. doi:10.1007/s10637-011-9711-8
- Congleton, J., MacDonald, R. and Yen A. (2012) Src inhibitors, PP2 and dasatinib, increase retinoic acid-induced association of Lyn and c-Raf (S259) and enhance MAPKdependent differentiation of myeloid leukemia cells. Leukemia, 26, 1180-1188. doi:10.1038/leu.2011.390
- Kuo, H.C., Kuo, W.H., Lee, Y.J., Wang, C.J. and Tseng, T.H. (2006) Enhancement of caffeic acid phenethyl ester on all-trans retinoic acid-induced differentiation in human leukemia HL-60 cells. Toxicology and Applied Pharmacology, 216, 80-88. doi:10.1016/j.taap.2006.04.007
- Fabiani, R., Rosignoli, P., De Bartolomeo, A., Fuccelli, R. and Morozzi, G. (2008) Inhibition of cell cycle progression by hydroxytyrosol is associated with upregulation of cyclin-dependent protein kinase inhibitors p21(WAF1/ Cip1) and p27(Kip1) and with induction of differentiation in HL60 cells. Journal of Nutrition, 138, 42-48.
- Powan, P., Saito, N., Suwanborirux, K. and Chanvorachote, P. (2013) Ecteinascidin 770, a tetrahydroisoquinoline alkaloid, sensitizes human lung cancer cells to anoikis. Anticancer Research, 33, 505-512.
- Anan, H., Tanaka, A., Tsuzuki, R., Yokota, M., Yatsu, T. and Fujikura, T. (1996) 4-(3,4-Dihydroxyphenyl)-1,2,3,4- tetrahydroisoquinoline derivatives. II. Their renal vasodilation activity and structure-activity relationship. Chemical and Pharmaceutical Bulletin (Tokyo), 44, 1865-1870.
- Watanuki, S., Matsuura, K., Tomura, Y., Okada, M., Okazaki, T., Ohta, M. and Tsukamoto, S. (2011) Synthesis and pharmacological evaluation of 1-isopropyl-1,2,3,4- tetrahydroisoquinoline derivatives as novel antihypertensive agents. Chemical and Pharmaceutical Bulletin (Tokyo), 59, 1029-1037.
- Okuda, K., Kotake, Y. and Ohta, S. (2006) Parkinsonismpreventing activity of 1-methyl-1,2,3,4-tetrahydroisoquinoline derivatives in C57BL mouse in vivo. Biological and Pharmaceutical Bulletin, 29, 1401-1403. doi:10.1248/bpb.29.1401
- Sakashita, A., Nakamaki, T., Tsuruoka, N., Honma, Y. and Hozumi, M. (1991) Granulocyte colony-stimulating factor, not granulocyte-macrophage colony-stimulating factor, co-operates with retinoic acid on the induction of functional N-formyl-methionyl-phenylalanine receptors in HL-60 cells. Leukemia, 5, 26-31.
- Pae, H.O., Seo, W.G., Kim, N.Y., Oh, G.S., Kim, G.E., Kim, Y.H., Kwak, H.J., Yun, Y.G., Jun, C.D. and Chung, H.T. (2001) Induction of granulocytic differentiation in acute promyelocytic leukemia cells (HL-60) by water-soluble chitosan oligomer. Leukemia Research, 25, 339- 346. doi:10.1016/S0145-2126(00)00138-7
- Seo, B.R., Lee, K.W., Ha, J., Park, H.J., Choi, J.W. and Lee, K.T. (2004) Saucernetin-7 isolated from Saururus chinensis inhibits proliferation of human promyelocytic HL-60 leukemia cells via G0/G1 phase arrest and induction of differentiation. Carcinogenesis, 25, 1387-1394. doi:10.1093/carcin/bgh143
- Chen, W.J., Chang, C.Y. and Lin, J.K. (2003) Induction of G1 phase arrest in MCF human breast cancer cells by pentagalloylglucose through the down-regulation of CDK4 and CDK2 activities and up-regulation of the CDK inhibitors p27(Kip) and p21(Cip). Biochemical Pharmacology, 65, 1777-1785. doi:10.1016/S0006-2952(03)00156-4
- Coqueret, O. (2003) New roles for p21 and p27 cell-cycle inhibitors: A function for each cell compartment? Trends in Cell Biology, 13, 65-70. doi:10.1016/S0962-8924(02)00043-0
- Choi, J.H., Shin, K.M., Kim, N.Y., Hong, J.P, Lee, Y.S., Kim, H.J., Park, H.J. and Lee, K.T. (2002) Taraxinic acid, a hydrolysate of sesquiterpene lactone glycoside from the Taraxacum coreanum NAKAI, induces the differentiation of human acute promyelocytic leukemia HL-60 cells. Biological & Pharmaceutical Bulletin, 25, 1446-1450. doi:10.1248/bpb.25.1446
- Chen-Deutsch, X. and Studzinski, G.P. (2012) Dual role of hematopoietic progenitor kinase 1 (HPK1) as a positive regulator of 1α,25-dihydroxyvitamin D-induced differentiation and cell cycle arrest of AML cells and as a mediator of vitamin D resistance. Cell Cycle, 11, 1364- 1373. doi:10.4161/cc.19765
- Kokorina, N.A., Granier, C.J., Zakharkin, S.O., Davis, S., Rabson, A.B. and Sabaawy, H.E. (2012) PDCD2 knockdown inhibits erythroid but not megakaryocytic lineage differentiation of human hematopoietic stem/progenitor cells. Experimental Hematology, 40, 1028-1042. doi:10.1016/j.exphem.2012.08.004
- Fang, Y., Zhou, X., Lin, M., Jing, H., Zhong, L., Ying, M., Luo, P., Yang, B. and He, Q. (2010) The ubiquitin-proteasome pathway plays essential roles in ATRA-induced leukemia cells G0/G1 phase arrest and transition into granulocytic differentiation. Cancer Biology & Therapy, 10, 1157-1167.
- Cooper, S. (2001) Revisiting the relationship of the mammalian G1 phase to cell differentiation. Journal of Theoretical Biology, 208, 399-402. doi:10.1006/jtbi.2000.2228
- Jeon, Y., Lee, K.Y., Ko, M.J., Lee, Y.S., Kang, S. and Hwang, D.S. (2007) Human TopBP1 participates in cyclin E/CDK2 activation and preinitiation complex assembly during G1/ S transition. The Journal of Biological Chemistry, 282, 14882-14890.
- Matsumoto, A. and Nakayama, K.I. (2013) Role of key regulators of the cell cycle in maintenance of hematopoietic stem cells. Biochimica et Biophysica Acta, 1830, 2335- 2340.
- Dimberg, A. and Oberg, F. (2003) Retinoic acid-induced cell cycle arrest of human myeloid cell lines. Leukemia and Lymphoma, 44, 1641-1650.

