Materials Sciences and Applications
Vol.06 No.11(2015), Article ID:60923,10 pages
10.4236/msa.2015.611093
Characterization of Isotactic Polypropylene/Talc Composites Prepared by Extrusion Cum Compression Molding Technique
Rahima Nasrin1,
M. A.Gafur2*,
A. H. Bhuiyan3
1Department of Physics, University of Barisal, Barisal, Bangladesh
2Pilot Plant & Process Development Centre, Bangladesh Council of Scientific & Industrial Research (BCSIR), Dhaka, Bangladesh
3Department of Physics, Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 28 August 2015; accepted 3 November 2015; published 6 November 2015

ABSTRACT
Extrusion-Compression molded isotactic polypropylene (iPP) composites containing 10 wt%, 20wt%, 30 wt%, 40 wt% and 50 wt% of talc filler were studied by scanning electron microscopy (SEM), simultaneous thermal analysis (STA) and physical testing. The scanning electron microscope (SEM) micrographs of neat iPP and composites with 10 wt%, 20 wt%, 30 wt%, 40 wt% and 50 wt% talc content showthat neat PP, 10 wt%, 20 wt%, and 30wt% talc composites surface is smooth in comparison to 40 wt% and 50 wt% talc composites. It is also observed that talc is dispersed uniformly in the matrix and this uniform dispersion is not decreased even with talc content as high as 30wt% talc. The composites of 40 wt% and 50 wt% talc contain more crack, agglomerates or larger particles. Bulk density of the composites decreases with the increase of talc content. With the increase of percentage of talc and period of immersion, the water absorption (WA) increases. Thermal analyses indicate a considerable increase of thermal stability of the composites with filler addition.
Keywords:
Isotactic Polypropylene, Water absorption, Thermal Behavior, talcs

1. Introduction
Fillers-reinforced polymer composites have attracted much attention to the researchers due to the fact that inclusion of fillers in polymers remarkably alters structural, physical, mechanical and thermal behavior of final products [1] .
Isotactic polypropylene (iPP) has found a wide range of applications in the food packaging, electrical, and automotive industries. Inorganic fillers, such as talc, mica and silica, ceramics, etc. are widely used in iPP to improve their mechanical and thermal properties [2] . The commonly used fillers in iPP are white clay, calcium carbonate, and talc, whose effects in different properties of the composites were described in the literature [2] -[5] . Properties of injection molded iPP/talc composites were investigated by some researchers [2] [4] . The aim of the work is to investigate various properties of iPP/talc composites prepared by extrusion cum compression molding technique. With these aims, this research has been done and details of structural, physical and thermal behavior of these composites are represented in this paper.
2. Experimental Works
2.1. Raw Materials
Composites used in this work were prepared from iPP and talc. Commercial grade iPP was purchased from BASF, Germany. The density of PP is 0.91 gm/cc and its melting temperature is 438K. Talc is collected from local market and is in the form of powder. Different chemical component of talc are presented Table 1. The density of talc is 0.56gm/cc and melting temperature 1073 K.
2.2. Sample Preparation
Five different composites were prepared by iPP and talc powder according to the mass ratio (10-X) PP: X talc, Where X =1, 2, 3, 4, 5. Besides these one pure iPP sample was also prepared. The mixtures were kept in separate pot and then mixed uniformly as much as possible. The different mixtures were melted by extrusion machine. Three heaters of extrusion machine were switched “ON” for about one hour. The barrel was heated for about one hour at 513K. After heating for one hour the mixture was put into the feed hopper. The motor was then switched on to feed the batch from the feed hopper into the barrel. The molten composite material was then collected through the die in the form of rod. For easier handling these were cooled in a water bath during collection. The rods were then cut with a hacksaw. For converting the rod shape samples into disc shape sample 450kN Press (machine Paul-Otto Weber GmbH. Germany) were used. The rod shape samples were molded by this machine. The heating temperature and initial pressure were set at 180˚C and 50kN, respectively. After reaching the set temperature, the pressure was increased up to 100kN, continued heating at that temperature for 15 min and stopped the heating system.
2.3. Characterization
2.3.1. Surface Morphology
The morphology of the iPP sample and composites with 10 wt%, 20 wt%, 30 wt%, 40 wt%, 50 wt% talc was studied by a scanning electron microscope (SEM) (Philips XL 30, Netherlands) with a maximum operating voltage of 10kV of the apparatus. The sample surface was coated with a thin gold layer by a sputtering prior to SEM measurement.
2.3.2. Bulk Density (BD)
Bulk density was calculated using following formula
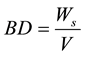 (1)
(1)
Table 1.Chemical composition of Talc.
where,
BD = Bulk Density of the specimen in Kg/m3,
Ws = Weight of the specimen in Kg, and
V = Volume of the specimen in m3.
In this way the bulk density of each sample is measured[6] .
2.3.3. Water absorption(WA)
Water intake specimen was prepared according to ASTM Designation [7] . The test specimen was 76mm length and width above 25mm. The total water absorption was calculated following the rules given below:
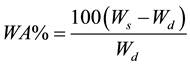 (2)
(2)
where
Wd = Dry weight of the specimen,
Ws = Saturated weight at the specimen after submersion in distill water.
In all cases a protective gel coat (araldite) was applied on the cut sides to prevent penetration of water from cut sides.
2.3.4. Thermalmeasurements
Melting and degradation temperatures of the neat iPP sample and the composites were monitored by a thermo gravimetric-differential thermal analyzer (TG-DTA) [Seiko-Ex-STAR-6300, Japan]. The measurements using TG-DTA were carried out from room temperature to 600˚C at a heating rate of 20˚C∙min−1 using nitrogen gas flow. While the DTA traces give the melting and degradation temperatures as determined from the DT signal versus temperature curves, the TGA runs exhibit the weight retained of the sample with temperature.
3. Results and Discussions
3.1. Surface Morphology (SEM)
The SEM micrographs of iPP and composites with 10 wt%, 20 wt%, 30 wt%, 40 wt% and 50 wt% talc content are shown in figure 1. It is observed that the surface structure changes due to the percentage of talc in PP. It can also be seen from micrographs that iPP, 10 wt%, 20 wt% and 30wt% talc compotes surface is smooth in comparison to 40 wt% and 50 wt% talc composites. It is also observed that talc is dispersed uniformly in the matrix and this uniform dispersion is not decreased even with talc content as high as 30 wt% talc. On the surface of the composites of 40 wt% and 50 wt% talc composites contain more crack, void, agglomerates or larger particles. These observed agglomerates were created during fracture by extraction of some talc crystals from their places on fractured surface.
3.2. Physical properties
3.2.1. Bulk Density
Figure 2 shows the effect of talc addition on bulk density of composite materials. It is observed that with the increase of talc addition the density of the composites decreases.
3.2.2. Water absorption
In figure 3 it is observed that water absorption (WA) increases with increasing period of immersion and increase of percentage of talc. Since talchas more affinity for water than iPP, so with the addition of talc, the water absorption of the composite fairly rises. Similar effect was found by M. Maniruzzaman et al. [9] the pulque fibre reinforced LDPE composites. With increasing period of immersion the water absorption increases in the pulque fibre reinforced LDPE composites.
Figure 1. The SEM micrographs of (a) Ipp; (b) 10 wt%; (c) 20 wt%; (d) 30 wt%; (e) 40 wt%; and (f) 50 wt% iPP-talc composites.
Figure 2. Effect of talc addition on bulk density of PP-talc composites.
Figure 3. Immersion time versus water absorption (%) curves of PP and PP-talc composites.
3.3. Thermal Analyses
Figure 4(a) shows the TGA, DTA and DTG curves of iPP. The TGA curve shows that major degradation occurs in one stage. The onset temperature is 389.6˚C. The 50% degradation occurs at about 415.9˚C and the maximum slope is at 413.5˚C. The ash content is 2.4%. DTA curve shows two endothermic peaks; one represents melting peak temperature at 166.5˚C and the other represents peak degradation temperature at 431.4˚C. DTG curve depicts that the maximum degradation occurs at the temperature of 432.8˚C with the rate of 1.17 mg/min.
Figure 4(b) shows the TGA, DTA and DTG curves of composites with 20wt% talc. The TGA curve shows that major degradation occurs in one stage. It is found that initial weight loss starts at about 398.2˚C. The 50% degradation occurs at about 427.5˚C and the maximum slope is at 435.5˚C. The ash content is 26.3%. DTA curve shows two endothermic peaks; one is at 166.9˚C and the other is at 434.7˚C. DTG curve depicts that the maximum degradation occurs at the temperature of 452.1˚C with the rate of 1.97 mg/min.
Figure 5(a) shows the TGA, DTA and DTG curves of composites with 30wt% talc. The TGA curve shows that major degradation occurs in one stage. It is found that onset temperature is at 423.7˚C. The 50% degradation occurs at about 446.5˚C and maximum slope is at 454.5˚C. The ash content is 23.3%. DTA curve shows two endothermic peaks; one is at 167.8˚C and the other is at 450.6˚C. DTG curve depicts that the maximum degradation occurs at the temperature of 454.8˚C with the rate of 3.36 mg/min.
Figure 5(b) shows the TGA, DTA and DTG curves of composites with 50% talc. The TGA curve shows that major degradation occurs in one stage. It is found that initial weight loss starts at about 403.2˚C. The 50% degradation occurs at about 432.4˚C and the maximum slope is at 447.7˚C. The ash content is 34.6%. DTA curve shows three endothermic peaks at 171.7˚C, 326.6˚C and 438.1˚C. DTG curve depicts that the maximum degradation occurs at the temperature of 447˚C with the rate of 1.73mg/min.
From Table 2 it is evident that thermal stability of iPP-talc composites increases to maximum value for 30% talc addition, but it is decreased with further addition.
Table 3 shows melting shifts towards higher temperature with the addition of talc. It is also evident that the peak degradation temperature also increases to maximum for 30%talc-pp composites, then it decreases. For 40%-50%talc composites addition 3rd peak is obtained.
Table 4 describes that both the maximum degradation temperature and degradation increases with the increase of talc addition up to 30%, then decreases with further addition.
Figure 6 represents the comparison of TGA, DTA and DTG curves of iPP and iPP-talc composites. From TGA curve it is evident that major degradation occurs at one stage for PP and composites. From the TGA curve it is also observed that with the addition of talc, the TGA curve of composites shift towards higher temperature. It shows that composite with 30 wt% talc is the most thermally stable than others and iPP is least thermally stable. This shift to high temperature may be attributed that the composite becomes more thermally stable with the addition of talc. DTA curves show two endothermic peaks for 0, 10, 20, and 30 wt% talc content and three endothermic peaks for 40 and 50 wt% talc content. The DTG curve shows that the peak of PP is below the peak of


Figure 4. TGA, DTA and DTG curves of (a) iPP; and (b) 20 wt% iPP-talc composites.


Figure 5.TGA, DTA and DTG curves of (a) 30 wt%; and(b) 50 wt% iPP-talc composites.
Figure 6. TGA, DTA and DTG curves of the PP sample and PP-talc composites with various contents of talc.
Table 2.TGA results for iPP and iPP-talc composites.
Table 3.DTA for PP and PP-talc composites.
Table 4. DTG of PP and PP-talc composites.
different wt% of iPP-talc composites. From DTG curve it is also noticed that the maximum degradation occurs for 30% talc at the temperature 454.8˚C with the rate of 3.36 mg/min. The reason for increasing degradation temperature of the composites may be based on the influence of impurity. If it is assumed that talc has higher volumetric heat capacity and thermal conductivity than iPP, then the composite materials will preferably absorb more heat as compared to the pure iPP sample. As a result of the colligative thermodynamic effect, the temperature of the composite material will increase and the iPP chains start to degrade at higher temperatures in the composites than the iPP sample. So figure 4analyses show that with addition of talc in different concentration iPP-talc composite become more thermally stable. Such increase of thermal stability in copper filled low density polyethylene, talc filled polypropylene and TiO2 filled PP was observed by Luyt et al.[10] , Zhou et al.[1] and Farhad Mina et al.[11] respectively.
4. Conclusions
The structural, physical and thermal properties of iPP-talc composites are studied. The SEM micrographs of iPP and composites with 10 wt%, 20 wt%, 30 wt%, 40 wt% and 50 wt% talc content show that PP, 10 wt%, 20 wt%, and 30wt% talc surface is smooth comparison to 40 wt% and 50 wt% talc composites. The composites of 40 wt%and 50 wt% talc contain more crack, agglomerates or larger particles. Bulk density of the composites decreases with the increase of talc content. With the increase of percentage of talc and period of immersion, WA increases. From the TGA, DTA, and DTG curves it is seen that composites become more thermally stable than neat iPP and composites with 30 wt% talc is the most thermally stable than others.
In conclusion, it is seen that a thermally stable iPP-talc composite can be developed for industrial and scientific application.
Acknowledgements
The authors thank the authority of the Bangladesh University of Engineering and Technology (BUET) to provide financial support for this investigation. The authors are grateful to the department of materials and metallurgical Engineering, BUET and Bangladesh Council of Scientific & Industrial Research (BCSIR) to allow facilities for this research.
Cite this paper
RahimaNasrin,M. A.Gafur,A. H.Bhuiyan, (2015) Characterization of Isotactic Polypropylene/Talc Composites Prepared by Extrusion Cum Compression Molding Technique. Materials Sciences and Applications,06,925-934. doi: 10.4236/msa.2015.611093
References
- 1. Zhou, Y.X., Rangari, V., Mahfuz, H., Jeelani, S. and Mallick, P.K. (2005) Experimental Study on Thermal and Mechanical Behavior of Polypropylene, Talc-Polypropylene and Polypropylene-Clay Nanocomposites. Materials Science and Engineering, A402, 109-117.
- 2. Tjong, S.C. and Li, R.K.Y. (1997) Mechanical Properties and Impact Toughness of Talc Filled β-Crystalline Phase Polypropylene Composites. Journal of Vinyl and Additive Technology, 3, 89-95.
http://dx.doi.org/10.1002/vnl.10171 - 3. Farhad Md, M., Nasima, B. and Jellur Md., R., Gafur, M.A. and Buiyan, Md.A.H. (2009) Structures and Performance of White Clay-Filled Isotactic Polypropylene Composites Prepared by Double Molding Techniques. Polymer-Plastics Technology and Engineering, 48, 1275-1281.
http://dx.doi.org/10.1080/03602550903204139 - 4. Samsudin, M.S.F., Mohd Ishak, Z.A., Jikan, S.S., Ariff, Z.M. and Arifin, A. (2006) Effect of Filler Treatments on Rheological Behavior of Calcium Carbonate and Talc-Filled Polypropylene Hybrid Composites. Journal of Applied Polymer Science, 102, 5421-5426.
http://dx.doi.org/10.1002/app.25054 - 5. Leong, Y.W., Abu Bakar, M.B., Mohd Ishak, Z.A. and Arifin, A. (2005) Effects of Filler Treatments on the Mechanical, Flow, Thermal, and Morphological Properties of Talc and Calcium Carbonate Filled Polypropylene Hybrid Composites. Journal of Applied Polymer Science, 98, 413-426.
http://dx.doi.org/10.1002/app.21507 - 6. ASTM Designation; C 134-76, Standard Test Method for Size and Bulk Density of Refractory Brick and Insulating Firebrick.
- 7. ASTM (1988) Designation; D 570-81; Standard Test Method for Water Absorption of Plastic.
- 8. Kuriger, R.J. and Khirul Alam, M. (2002) Thermal Properties of Carbon Fiber Reinforced Polypropylene. BSME-ASME International Conference on Thermal Engineering, 31 December 2001-2 January 2002.
- 9. Maniruzzaman, M., Haque Md, M. and Gafur, M.A. (2008) Properties of Pulque Fibre Reinforced LDPE Composites. Textile Asia, 6, 37-39.
- 10. Luyt, A.S., Molefi, J.A. and Krump, H. (2006) Thermal, Mechanical and Electrical Properties of Copper Powder Filled Low-Density and Linear Low-Density Polyethylene Composites. Polymer Degradation and Stability, 91, 1629-1636.
http://dx.doi.org/10.1016/j.polymdegradstab.2005.09.014 - 11. Mina, F.Md., Seema, S., Matin, R., Rahman, J.Md., Bijoy, S.R., Gafur, M.A. and Bhuiyan, A.H. (2008) Improved Performance of Isotactic Polypropylene/Titanium Dioxide Composites: Effect of Processing Conditions and Filler Content. Polymer Degradation and Stability, 1-6.
NOTES
*Corresponding author.







