Materials Sciences and Applications
Vol.5 No.3(2014), Article ID:43626,7 pages DOI:10.4236/msa.2014.53015
Preparation and Gas Barrier Characteristics of Polysilazane-Derived Silica Thin Films Using Ultraviolet Irradiation
T. Ohishi*, S. Sone, K. Yanagida
Department of Applied Chemistry, Faculty of Engineering, Shibaura Institute of Technology, Tokyo, Japan
Email: *tooishi@sic.shibaura-it.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 23 December 2013; revised 26 January 2014; accepted 11 February 2014
ABSTRACT
The gas barrier film formation technique using simultaneous photo-irradiation and heat-treatment has been researched on alicyclic polyimide film coated with a polysilazane solution. A fine SiO2 thin film on polyimide film was formed at low temperatures, which greatly improved the substrate’s gas barrier characteristics by this technique. The values of gas barrier characteristics depended on the substrate temperature at the time of photo-irradiation. For photo-irradiated thin film heat-treated to 150˚C, the water vapor transmission rate and oxygen transmission rate fell below the equipment measurement limit of 0.02 g/m2/day and 0.02 cm3/m2/day, respectively. This polyimide film with a gas-barrier film coating has good transmittance in the region of visible light, heat resistance, and flexibility.
Keywords:Polysilazane; Photo-Irradiation; Gas Barrier Characteristics; Alicyclic Polyimide Film

1. Introduction
The development of information appliances such as mobile phones and televisions and electronics-related products such as solar batteries has been quite active in recent years as the demand for products with even higher functionality and performance grows. As part of this trend, attention is focusing on flexible electronics for nextgeneration electronic products with flexible functions, such as flexible displays, e-paper, and flexible solar batteries having thin, light, and bendable features. The component deemed indispensable for these flexible products is the flexible substrate, which has generally been fabricated using organic resin films having flexible characteristics. Ordinary organic films, however, have poor gas barrier characteristics with respect to water vapor and oxygen, which can lead to degraded performance caused by the oxidation of device elements formed on the flexible substrate. The development of flexible substrates having good gas barrier characteristics is therefore anticipated as a solution to this serious problem. In addition, flexible devices like displays require good transmittance in the region of visible light and heat-resistance characteristics in addition to good gas barrier characteristics. One approach to improving the gas barrier characteristics of standard organic films is to form an inorganic thin film on top of the organic film. A simple method for doing so is to coat the organic film with a solution that includes an inorganic precursor. However, forming a fine inorganic thin film requires heat treatment at high temperatures [1] [2] , so it has not yet been possible to obtain sufficient performance due to problems associated with the heat-resistant temperature of organic films. On the other hand, high-performance, multi-layer films can be formed using elaborate high-vacuum film formation equipment as in the sputtering method, vacuum deposition method and CVD method [3] , but the high costs associated with such equipment present another problem. The above situation calls for the development of simple and low-cost low-temperature film formation technology for preparing such substrates.
In this study, we report on low-cost film formation technology for forming a gas barrier film on alicyclic polyimide (PI) film using a polysilazane-based coating method and photo-irradiation. Alicyclic PI film has good transmittance characteristics (visible light region transmittance: 90% T) and heat-resistance characteristics (Tg: 300˚C) but poor gas barrier characteristics. If gas barrier characteristics can be improved here, it should be possible to enhance the utility of substrates for flexible devices. Polysilazane, the base material for forming a gas barrier film, is inorganic polymer precursor having silicon-nitrogen bond (Si-N) that changes to SiO2 when subjected to heat treatment under oxygen or water vapor [4] -[8] . It has also been reported that polysilazane can be transformed to SiO2 by irradiating it with excimer light [9] and that gas barrier characteristics can be improved in this way [10] [11] , but there have been no reports on the effect of applying such photo-irradiation while heating.
For the study reported here, we used a low-pressure mercury lamp as an optical energy source and investigated how applying both photo-irradiation and heat affected the molecular structure of polysilazane film and its gas barrier characteristics.
2. Experimental
2.1. Preparation of Silica Thin Films Using Photo-Irradiation
A dibutyl ether solution of polysilazane (NL-110, 20 wt%: AZ Electronic Materials Co.) was used as a precursor for forming thin films. Specifically, 5 wt% dibutyl ether solution of this polysilazane solution was spin-coated (1000 rpm, 60 sec) on top of an alicyclic PI film (thickness 100 μm: Mitsubishi Gas Chemical Co.) subjected to hydrophilic treatment. The film was then photo-irradiated in ambient air using a low-pressure mercury lamp at an intensity of 6.5 mW/cm2 for 20 min. Substrate temperature was varied among 80˚C, 100˚C, 120˚C, and 150˚C for different samples. The thin film was prepared on both sides of the PI film. For comparison purposes, samples subjected only to heat treatment (150˚C, 200˚C, 250˚C, and 300˚C) were also prepared.
2.2. Evaluation
Changes in the molecular structure of these thin-film samples were examined by infrared spectra and the crosssectional structures of the samples were observed by transmission electron microscopy (TEM). Samples for the TEM observations were prepared by focused ion beam (FIB) processing. Elemental analysis in the film was examined by X-ray photoelectron spectroscopy (XPS). UV-VIS absorption spectra were measured in order to examine transparency of the films. Gas barrier characteristics were evaluated by measuring water vapor transmission rate (40˚C, 90% RH: MOCON PERMATRAN-W) and oxygen transmission rate (23˚C, 100% O2: MOCON OX-TRAN). Water vapor transmission greater than 10 g/m2/day was measured by the dish method.
3. Results
3.1. Infrared Spectroscopy
Infrared spectra of thin films prepared under various conditions are shown in Figure 1. Spectra for photo-
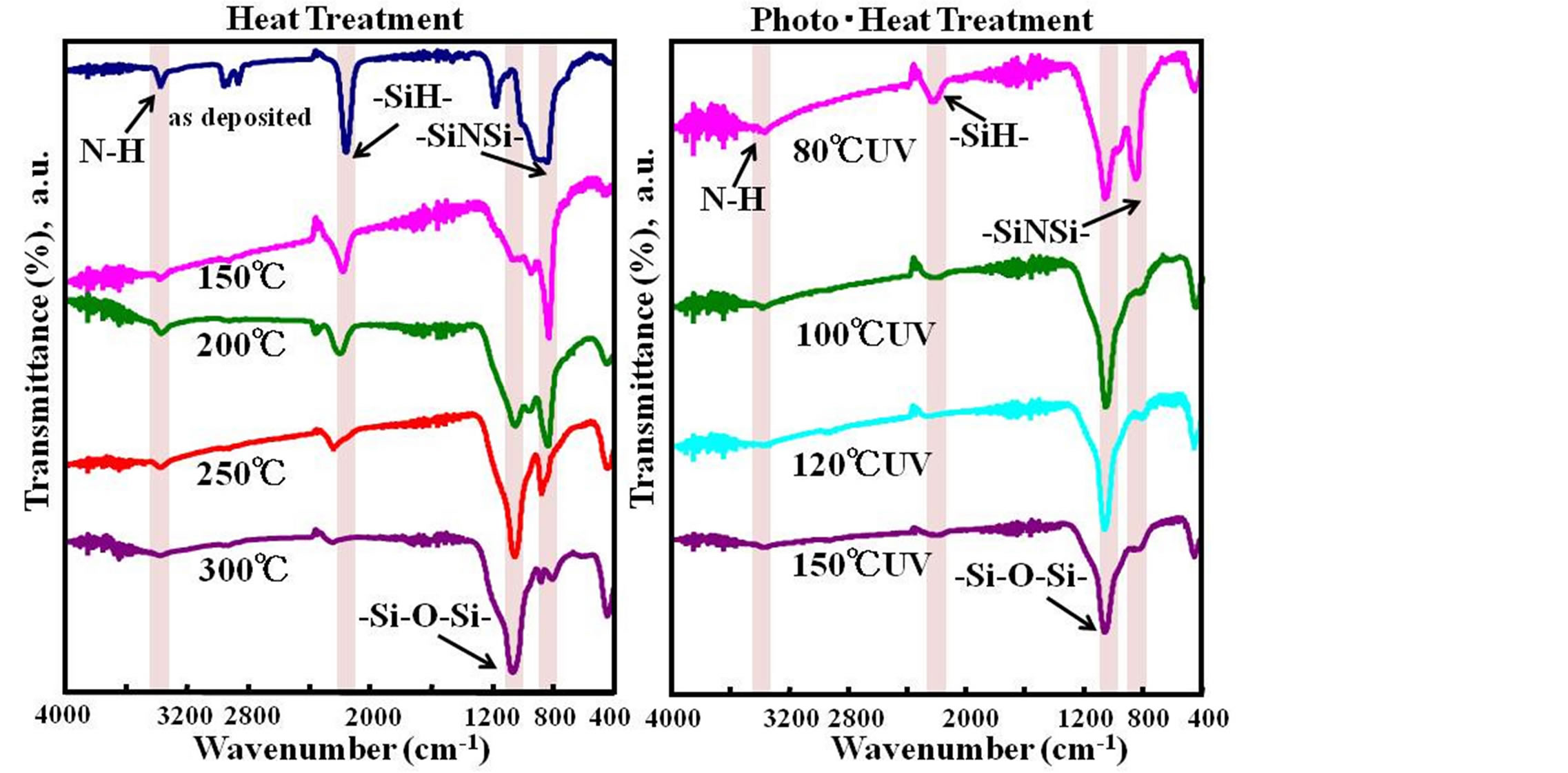
Figure 1. Infrared spectra of the heat treated films and the photo-heat treated films.
irradiated samples at different substrate temperatures and spectra for samples subjected to only heat treatment are shown.
The only heat-treated samples showed NH (near 3300 cm−1), SiH (near 2200 cm−1), and SiNSi (near 800 cm−1) absorption peaks directly after thin-film formation, suggesting a film structure having Si-N bonds. These absorption peaks decreased as heat-treatment temperature increased in the manner of 150˚C → 200˚C → 250˚C → 300˚C, but at the same time, an absorption peak based on a siloxane bond (-O-Si-O-) near 1100 cm−1 appeared and increased. In fact, only absorption based on this siloxane bond could be observed for heat treatment at 300˚C, indicating a transformation to a SiO2 film. The spectrum for heat treatment at 250˚C showed absorption peaks based on polysilazane, indicating that polysilazane still existed and that the transformation to SiO2 was still incomplete. Looking next at the spectra for the samples that were simultaneously photo-irradiated and heattreated, NH, SiH, and SiNSi absorption peaks based on polysilazane could be observed at 80˚C but so could be a strong absorption peak based on a siloxane bond (-O-Si-O-). This result indicates that the transition to SiO2 was already underway at this temperature. In addition, no polysilazane-based absorption peaks could be observed for films treated at 100˚C or greater, indicating a transition to SiO2 at those temperatures. These results show that the transformation to SiO2 film occurs at an extremely low temperature through simultaneous photo-irradiation and heat treatment.
3.2. X-Ray Photoelectron Spectroscopy (XPS)
Figure 2 shows XPS depth profile of elemental analysis (Si2p, O1s, N1s and C1s) for the film heat treated at 300˚C and the film photo-irradiated at 150˚C. For the 300˚C heat-treated film, Si, O and N were observed in the film, in which N content was about 2 atomic %, indicating that the plysilazane-to-silica conversion was insufficient. On the other hand, for the photo-heat treated film Si and O were observed but N was not observed in the film, indicating that the plysilazane-to-silica conversion was sufficient. Also, C and N due to impurities caused by organic pollution were observed in the outermost surface. The rapid increases in C content around 30 min. sputtering time indicates that sputtering reached the PI film. These results suggest that the polysilazane-to-silica conversion by the photo-heat treatment proceeds easily at low temperature than that by the heat treatment.
3.3. UV-VIS Absorption Spectroscopy
Figure 3 shows the UV-VIS absorption spectra of the films formed on silica substrate and photo-irradiated at various temperatures in comparison with that of the heat-treated films. The as-deposited polysilazane film showed absorption around 300 nm and 800 nm, and also large absorption below 260 nm. These absorption decreased with photo-irradiation, leading to that there were no absorptions in the region of 200 nm - 800 nm from
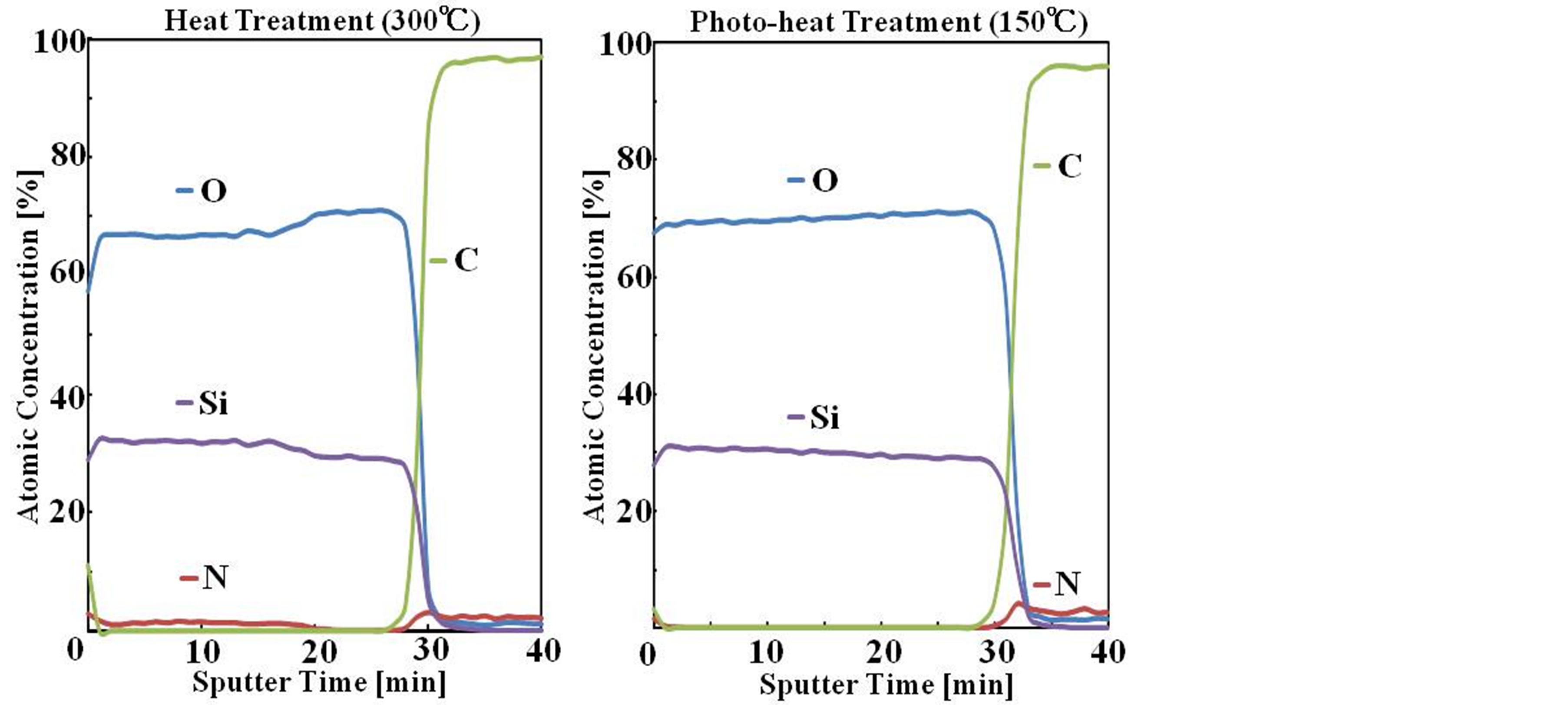
Figure 2. XPS depth profiles of composition of the film photo irradiated at 150˚C and the film heat-treated at 300˚C.
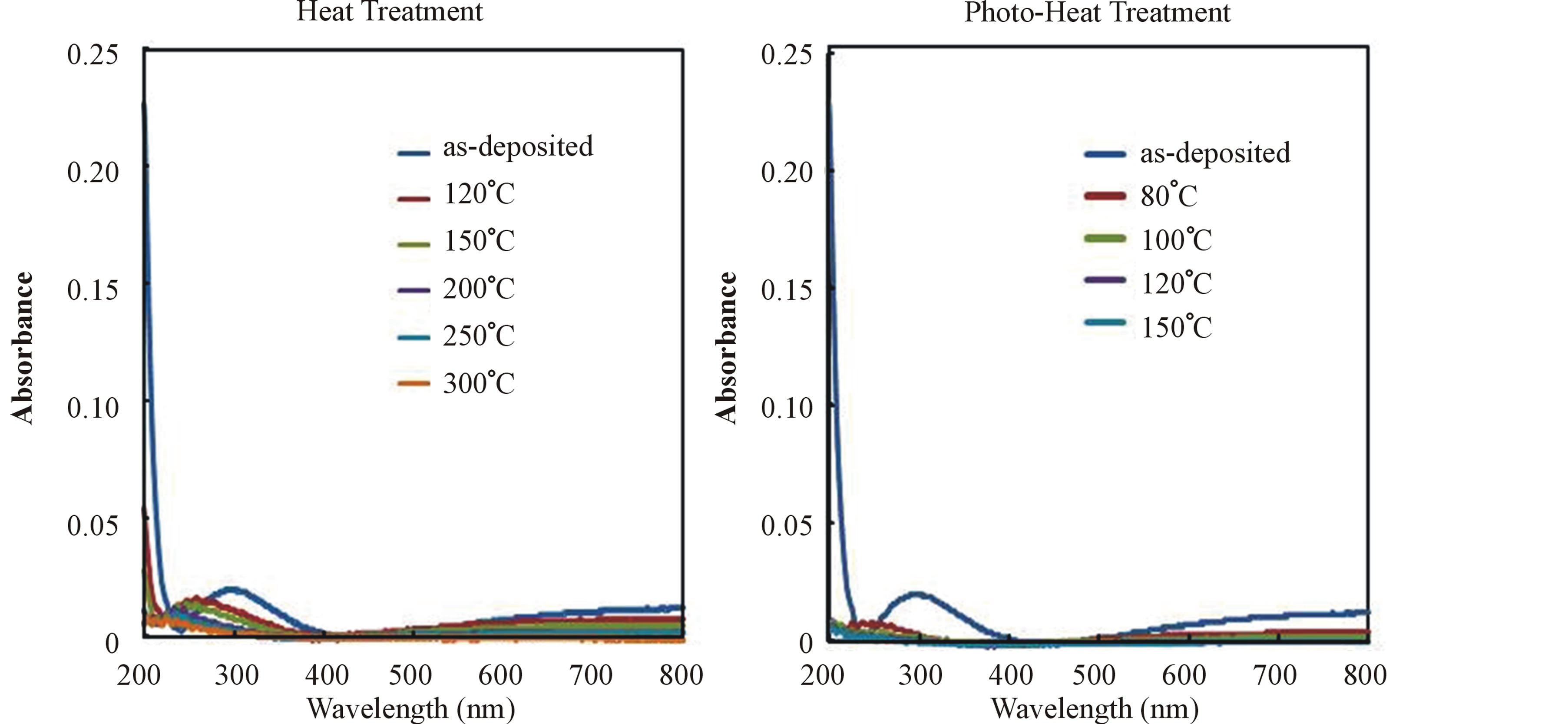
Figure 3. UV-VIS absorption spectra of the heat-treated and photo-heat treated films.
ultraviolet light region to visible light region in the films photo-irradiated at above 100˚C. Finally, the film photo-irradiated at 150˚C became high transparent, indicating that the polysilazane-to-silica conversion was completed.
For the heat-treated films, the absorption peak around 300 nm shifted to shorter wavelength and decreased with rise of heat treatment temperature, and the large absorption below 260 nm and the absorption around 800 nm also decreased with rise of heat treatment temperature. At the temperature of 300˚C there were no absorption peaks in the region of 200 nm - 800 nm.
3.4. Transmission Electron Microscopy (TEM)
Cross-sectional TEM photographs of thin films prepared by photo-irradiation at 150˚C are shown on Figure 4. Thin-film formation on PI film is shown for both a single coat and a double coat of thin film. The thin film was formed uniformly on the base material and the adhesive interface with the PI film was also uniform. No non-uniform sections within the thin film such as SiO2 particles were observed. The thin film was 160 nm thick for the single coat and 333 nm thick for the double coat. The double-coated thin film was uniform with no lamination interface observed.
3.5. Gas Barrier Characteristics
The results of measuring water vapor transmission rate (WVTR) for a single coat of thin film are listed in Table 1. For heat-treated films, it can be seen that WVTR tends to decrease as the treatment temperature increases dropping to a value of 1.36 g/m2/day for the thin film treated at 300˚C. Since WVTR is 143 g/m2/day for PI with no thin-film formation, it can be seen that a gas-barrier effect is produced by the formation of a thin film. On the other hand, WVTR in photo-irradiated films drops significantly with increase in substrate temperature. Specifically, while a WVTR of 130 g/m2/day for irradiated PI at room temperature (with no substrate heating) is not much lower than that for PI with no irradiation and no thin-film formation, WVTR decreases with rise in substrate temperature in the manner of 80˚C (15.3 g/m2/day) → 100˚C (3.42 g/m2/day) → 120˚C (1.84 g/m2/day) → 150˚C (0.17 g/m2/day). In short, WVTR becomes as small as 0.17 g/m2/day for a photo-irradiated film at 150˚C.
The results of measuring water vapor transmission and oxygen transmission for both single-coated and double-coated thin films photo-irradiated at 150˚C are shown in Table 2. The WVTR in the case of a doublecoated thin film was smaller than the equipment’s measurement limit of 0.02 g/m2/day. The oxygen transmission
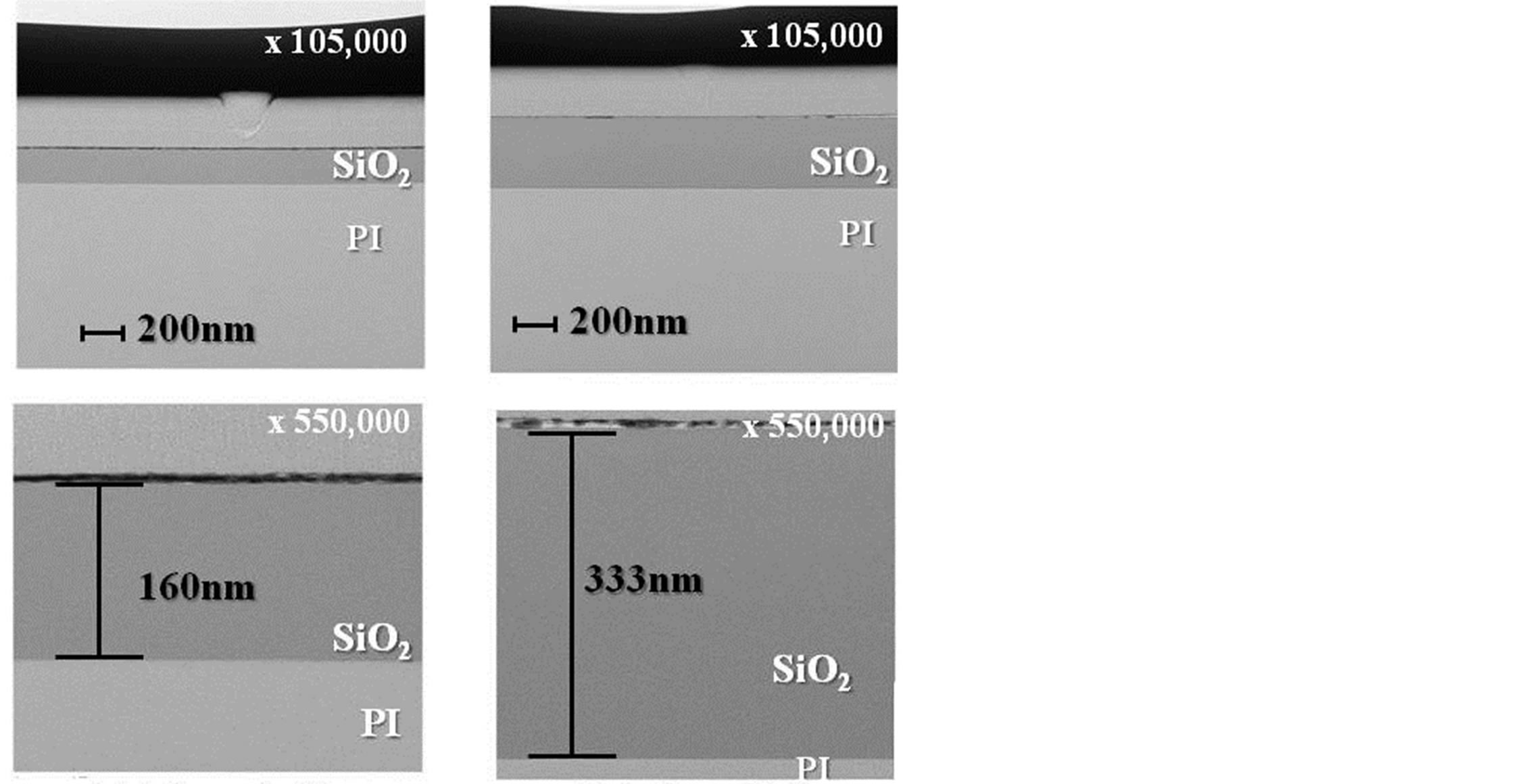 (a) (b)
(a) (b)
Figure 4. Cross-sectional TEM photographs of gas barrier films. (a) 1 coated film; (b) 2 coated film.
Table 1. WVTR of the heat-treated and photo-heat treated films.
Table 2. Gas barrier characteristics of the 1 coated and 2 coated films.
rate (OTR), meanwhile, was smaller than the equipment’s measurement limit of 0.02 cm3/m2/day for both single-coated and double-coated thin films, which was a significant decrease from that of PI with no thin-film formation.
4. Discussion
Gas barrier characteristics greatly improved when photo-irradiating the thin film and those values depended on the substrate temperature at the time of photo-irradiation. Although no significant decrease in WVTR could be observed in photo-irradiated film with no substrate heating compared to film with no photo-irradiation, a major decrease in that value could be observed in photo-irradiated film at a substrate temperature of just 80˚C. This value continued to decrease greatly with rise in substrate temperature to 100˚C, 120˚C, and 150˚C. The reason for this is considered to be that polysilazane-derived molecular structures still remained in 80˚C photo-irradiated film but that the film was transforming to SiO2 and forming a finer and denser SiO2 film for 100˚C-and-higher photo-irradiated film, as shown in IR spectra, XPS data, UV-VIS spectra and TEM observation. The refractive index of the as-deposited film showed 1.56 and that value decreased with photo-heat treatment. The refractive index of the film photo-irradiated at 150˚C was 1.47 that was close to the refractive index of silica glass, 1.46. The decrease in refractive index observed can be ascribed to polysilazane-to-silica conversion, not to increased porosity, by TEM observation. These results suggest that the photo-heat treatment promotes the conversion to silica and the densification of the film. It is presumed that both the chemical-bond-breaking effect of optical energy at the 254 nm and 185 nm wavelengths of the low-pressure mercury lamp and the film-oxidation effect due to O3, O (1D) reactive oxygen generated from oxygen in air contributed to the oxidation reaction in polysilazane film. Optical energy at the 254 nm and 185 nm wavelengths is coincident with 113 kcal/mol and 155 kcal/mol, respectively. Each chemical bond energy of Si-N and N-H in polysilazane is 105 kcal/mol and 92 kcal/mol, respectively [12] . Optical energy at the 254 nm and 185 nm wavelengths of the low-pressure mercury lamp is sufficient to cleave the chemical bond of Si-N and N-H. We point out here that no major decrease in WVTR could be observed in film that was photo-irradiated after 150˚C heat treatment (90.3 g/m2/day) or in film that was heat-treated at 150˚C after photo-irradiation (116 g/m2/day). This observation indicates that substrate heating at the time of photo-irradiation has a major effect on the conversion to and the fineness of SiO2 film. It is presumed here that substrate heating quickens the diffusion of reactive oxygen in the film and promotes an oxidation reaction directly after polysilazane bond breaking by optical energy thereby contributing to the generation of a fine SiO2 film. The details of this mechanism are now under study.
Film thickness also had an effect on gas barrier characteristics. Compared to single-coated film, WVTR of double-coated film decreased significantly dropping to a value below the equipment’s measurement limit of 0.02 g/m2/day. On the other hand, the oxygen transmission rate dropped below the equipment’s measurement limit of 0.02 cm3/m2/day for both single-coated and double-coated film. Cross-sectional TEM observations of doublecoated film show a uniform and fine SiO2 film with no lamination interface. We therefore achieved good gas barrier characteristics in this study for both water vapor and oxygen, and we consider that the WVTR and oxygen gas transmission rate can be further improved by optimizing film thickness in the proposed technique. The adhesion between the thin film and PI film was strong—no film peeling could be observed even by a tape test conforming to a JIS standard (Japan Industrial Standard). Finally, the developed substrate has good flexibility, transmittance in the region of visible light, and heat resistance and should therefore be effective as a substrate for use in the field of flexible electronics.
5. Conclusion
The gas barrier film formation technique using simultaneous photo-irradiation and heat-treatment has been researched on alicyclic polyimide film coated with a polysilazane solution. This technique enables the formation of a fine SiO2 thin film on polyimide film at low temperatures and greatly improves the substrate’s gas barrier characteristics. For photo-irradiated thin film heat-treated to 150˚C, the water vapor transmission rate and oxygen transmission rate fell below the equipment measurement limit of 0.02 g/m2/day and 0.02 cm3/m2/day, respectively. This polyimide film with a gas-barrier film coating has good transmittance in the region of visible light, heat resistance, and flexibility and should therefore prove useful as a film for flexible electronics.
Acknowledgements
This work was partially supported by Grant-in-Aid from Japan Science and Technology Agency.
References
- Brinker, C. J. and Scherer, G.W. (1990) Sol-Gel Science. Academic Press, Boston.
- Seyferth, D. and Wiseman, G.H. (1984) High-Yield Synthesis of Si3N4/SiC Ceramic Materials by Pyrolysis of a Novel Polyorganosilazane. Journal of the American Ceramic Society, 67, C132-C133.
- Taga, Y. and Akashi, K. (2007) Passivation Films on Organic Film Substrates Designed for Organic Electroluminescence Device. The Journal of the Vacuum Society of Japan, 50, 735-738. http://dx.doi.org/10.3131/jvsj.50.735
- Kamiya, K., Tange, T., Hashimoto, T., Nasu, H. and Shimizu, Y. (2001) Formation Process of Silica Glass Thin Films from Perhydropolysilazane. Research Reports of the Faculty of Engineering, Mie University, 26, 23-31.
- Kubo, T., Tadaoka, E. and Kozuka, H. (2004) Formation of Silica Coating Films from Spin-On Polysulazane at Room Temperature and Their Stability in Hot Water. Journal of Materials Research, 19, 635-642. http://dx.doi.org/10.1557/jmr.2004.19.2.635
- Kubo, T., Tadaoka, E. and Kozuka, H. (2004) Preparation of Hot Water-Resistant Silica Thin Films from Polysilazane Solution at Room Temperature. Journal of Sol-Gel Science and Technology, 31, 257-261. http://dx.doi.org/10.1023/B:JSST.0000047999.87439.c2
- Kubo, T. and Kozuka, H. (2006) Conversion of Perhydropolysilazane-to-Silica Thin Films by Exposure to Vapor from Aqueous Ammonia at Room Temperature. Journal of the Ceramic Society of Japan, 114, 517-523. http://dx.doi.org/10.2109/jcersj.114.517
- Ohishi, T. (2003) Gas Barrier Characteristics of a Polysilazane Film Formed on an ITO-Coated PET Substrate. Journal of Non-Crystalline Solids, 330, 248-251. http://dx.doi.org/10.1016/j.jnoncrysol.2003.09.022
- Naganuma, Y., Tanaka, S., Kato, C. and Shindo, T. (2004) Formation of Silica Coating from Perhydropolysilazane Using Vacuum Ultraviolet Excimer Lamp. Journal of the Ceramic Society of Japan, 112, 599-603. http://dx.doi.org/10.2109/jcersj.112.599
- Prager, L., Dierdorf, G., Liebe, H., Naumov, S., Stojanovic’, S., Heller, R., Wennrich, L. and Buchmeiser, M.R. (2007) Conversion of Perhyropolysilazane into SiOx Network Triggered by Vacuum Ultraviolet Irradiation. Chemistry—A European Journal, 13, 8522-8529. http://dx.doi.org/10.1002/chem.200700351
- Kobayashi, Y., Yokota, H., Fuchita, Y., Takahashi, A. and Sugawara, Y. (2013) Characterization of Gas Barrier Silica Coating Prepared from Perhydropolysilazane Films by Vacuum Ultraviolet Irradiation. Journal of the Ceramic Society of Japan, 121, 215-218. http://dx.doi.org/10.2109/jcersj2.121.215
NOTES

*Corresponding author.


