Applied Mathematics
Vol.3 No.10A(2012), Article ID:24086,5 pages DOI:10.4236/am.2012.330177
Control Approach to Nuclear Safety
Department of Mathematics, University of Ilorin, Ilorin, Nigeria
Email: krauf@unilorin.edu, omolehin_joseph@yahoo, *jubril_aminu@yahoo.co.uk
Received May 20, 2012; revised September 4, 2012; accepted September 11, 2012
Keywords: Control; Nuclear; Safety; Reactors and Containment
ABSTRACT
Despite the advantages of nuclear technology to mankind, it attendance accident is always catastrophic. In this work, the energy balance equations associated with nuclear technology are structured in parametric form to form the basis of our Mathematical model solvable by the Conjugate Gradient Method (CGM).
1. Introduction
As the nation of the world seek to meet the increasing demands for energy which lead to increases in environmental pollution from the burning of coal, oil and natural gas. The use of fossil fuels for transportation, generating electricity, heat and industrial production account for 85 percent [1] of the world’s energy consumption. The environmental consequences of this heavy reliance on fossil fuel are only being fully realized. Sulfur dioxide emissions are said to cause acid rain. Nitrous oxide are said to cause smog. Particles in the air, from the burning of coal and oil, cause all manners of human lung ailments. The scientific community now believes that emissions of carbon dioxide, resulting from the burning of fossil fuels, is the leading contributor to global warming. Accordingly, the leaders of almost every nation in world met in Kyoto, Japan in December 1997 to establish aggressive limits to future use of fossil fuels. In spite of these concerns, fossil fuels are necessary to meet the world’s energy demand. How long can the planet's environment continue to withstand the pollutant load caused by the emissions from these fossil sources? The answer is not certain, but the leaders of the world concluded in Kyoto that dramatic action is necessary even without having all the scientific data because the consequences of being wrong are too severe. Improvements in energy efficiency, conservation and decrease in the use of fossil fuels will all be required if there is any hope of achieving the aggressive targets established by the political leaders. It is also necessary to develop non-carbon alternatives for energy production. Solar, wind and hydro-power are often mentioned for electric generation as they have been for many years. One of the sources of energy that has obvious answer to the global climate change problem is the nuclear energy. Nuclear energy is a non-fossil, non-carbon and non-air-polluting source of energy that has provided many nations of the world with vast amount of electric power. The United States depends on nuclear energy for twenty percent of its electricity. France, Japan, Germany, Korea, Taiwan, Sweden, Iran to name but a few, also have high reliance on nuclear energy [2]. The expanded role for nuclear power of many nations in their energy portfolios is driven by concerns about global warming, growth in energy demand and relative costs of alternative energy sources. In 2008, 435 nuclear reactors in 30 countries provided sixteen percent of the world’s electricity. In January 2009, 43 reactors were under construction in 11 countries, with several hundred more projected to come on line globally by 2030 [3].
2. Nuclear Reactor Process
Nuclear reactors produce energy through a controlled fission chain reaction. While most reactors generate electric power, some can also produce plutonium for weapons and reactor fuel. Power reactors use the heat from fission to produce steam, which turns turbines to generate electricity. In this respect they are similar to plants fueled by coal and natural gas. The components common to all nuclear reactors include a fuel assembly, control rods, a coolant, a pressure vessel, a containment structure and an external cooling facility.
Thermal reactors operate on the principle that uranium-235 undergoes fission more readily with slow neutrons than with fast ones. Light water (H2O), heavy water (D2O), and carbon in the form of graphite are the most common moderators. Since slow neutron reactors are highly efficient in producing fission in uranium-235, they use fuel assemblies containing either natural uranium (0.7percent U-235) or slightly enriched uranium (0.9 to 2percent U-235) fuel. Rods composed of neutronabsorbing materials such as cadmium or boron are inserted into the fuel assembly. The position of these control rods in the reactor core determines the rate of the fission chain reaction. The coolant is a liquid or gas that removes the heat from the core and produces steam to drive the turbines. In reactors using either light water or heavy water, the coolant also serves as the moderator. Reactors employing gaseous coolants (CO2 or He) use graphite as the moderator. The pressure, made of heavyduty steel, holds the reactor core containing the fuel assembly, control rods, moderator and coolant. The containment structure, compose of thick concrete and steel, inhibits the release of radiation in case of an accident and also secure components of the reactor from potential intruders. Finally, the most obvious components of many nuclear power plants are the cooling towers, the external components, which provide cool water for condensing the steam to water for recycling into the containment structure. Cooling towers are also employed with coal and natural gas plants [4].
3. Type of Thermal Reactors
Most of the nuclear power plants in the world are water-moderated thermal reactors. They are categorized as either light water or heavy water reactors. Light water reactors use purified natural water (H2O) as the coolant/ moderator, while heavy water reactors employ heavy water deuterium oxide (D2O). In light water reactors, the water is either pressured to keep it in superheated form (in a pressurized water reactor, PWR) or allowed to vaporize, forming a mixture of water and steam (in boiling water reactor, BWR). In PWR, superheated water flowing through tubes in the reactor core transfers the heat generated by fission to a heat exchanger, which produces steam in a secondary loop to generate electricity. None of the water flowing through the reactor core leaves the containment structure.
Other thermal reactors are Boiling Water Reactor (BWR), Heavy Water Reactor (HWR), Gas Cooled Reactor, Pebble Bed Reactor and Fast Neutron Reactor.
4. Different Types of Non-Nuclear Electric Power Plants
We have different types of non-nuclear electric power generation. Some are Wind-mills, Hydro-electric power (falling water) plants, Fossil-fuels plant and Solar cell plants.
5. Wind-Mill Plant
This plant is device for tapping the energy of the wind by means of sails mounted on a rotating shafts. It is generally less reliable than the water power, but where water is deficient wind power is an attractive substitute. Wind-mill-plant can be use in areas that suffer from draught or from a shortage of surface water and also in a low-lying area where rivers offer little energy. They were mostly used, converting the energy of the wind into mechanical energy for grinding grain, pumping water and drainage. They produced less energy for electricity [5].
6. Hydro-Electric Power
It is the electricity produced from generators driven by water turbines that convert the potential energy in falling or fast-flowing water to mechanical energy. They are usually located in dams that impound rivers; especially in areas with heavy rain fall and hilly or mountainous region. In generation, water is collected or stored at a higher elevation and led downward through large pipes or tunnels to a lower elevation. At the end of its passage down the pipes, the falling water causes turbines to rotate which in turn drive generators which convert the turbines mechanical energy into electricity [6]. Although hydroelectric power plants are not hazardous as the fossil fuels, but it may not meet the electricity demand of a country as fossil fuels would. Also if there are not enough water impounded in a reservoirs during the dry season, the falling water would not be enough to cause the turbine to turn to generate electricity.
7. Fossil-Fuels
The production of steam by fossil-fuels, produce the greatest amount of electrical energy compared to hydroelectric and wind-mills. Fossil-fuels are coal, petroleum, natural gas, oil shales, bitumens, tar sands and heavy oil. All contain carbon and were formed as a result of geologic processes acting on the remains of organic matter produced by photosynthesis. All fossil-fuels can be burned in air or with oxygen to provide heat. The heat may be utilized to produce steam to drive generators that can supply electricity [7]. The use of fossil-fuels is very hazardous to human health as a result of environmental pollutant from burning of the fuels.
8. Solar Cell Plants
This is the best plant that can only be conveniently use on satellites and space where the flow of energy out of the sun (the solar wind) can be harnessed without interference from the atmosphere or the rotation of the earth [8]. Although this type of non-nuclear power plant can also be use on earth, but cannot produce the desire electricity because of the changes in atmospheric conditions and rotation of the earth [9].
9. Advantages of Nuclear Plant over Non-Nuclear Plant
All the non-nuclear power generation discussed above produced low electricity compared to the nuclear reactors. The nuclear reactor released a large amount of energy during the process of fission. Most of this energy released takes form of heat, which is used to produce steam. The steam drives a turbine, the mechanical energy of which is converted to electricity by a generator. The whole process takes place in a safety environment that is not hazardous to the human. The nuclear reactor eliminates most of the disadvantages of the non-nuclear energy by generating enough energy for consumption; does not involved emission of carbon which is very dangerous to the environment; does not involved burning of coal and oils that are the causes of all manners of lung ailments and also does not dependent on fossil-fuels.
10. Main Result
There were three major nuclear reactor accidents they are the three mole island in Pennsylvania, USA in 1979 [3], the Chernobyl RBMK in Soviet Union in 1986 [10] and Fukushima nuclear reactor accident in Tokyo, Japan in 2011 [11].
With the above analysis on nuclear accidents, nuclear technology is necessary for power generation if the safety concerns can be adequately addressed and that is why this work is purely devoted to the safety aspect and not to the weapons production. The fission from the reactors produce energy in form of heat that releases steam to turn turbine to generate electricity, therefore it is necessary to find equations for rate of heat that can be use for the purpose of our work. See [12-14].
11. The Energy Balance for Reactors
The following results were obtained through energy balance equation and were arranged in terms of rate of heats of the reactors (i.e. batch, continuous stirred tank, semi batch and plug flow). See [15,16].
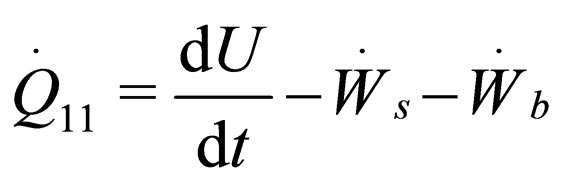 (1)
(1)
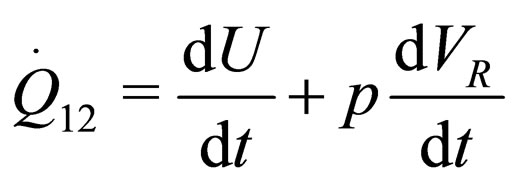 (2)
(2)
 (3)
(3)
 (4)
(4)
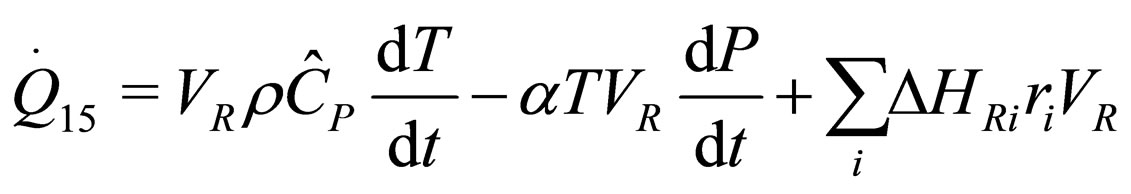 (5)
(5)
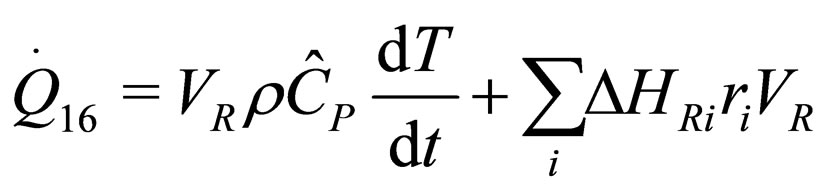 (6)
(6)
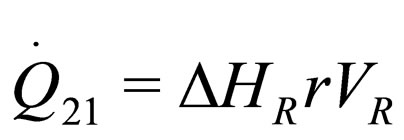 (7)
(7)
 (8)
(8)
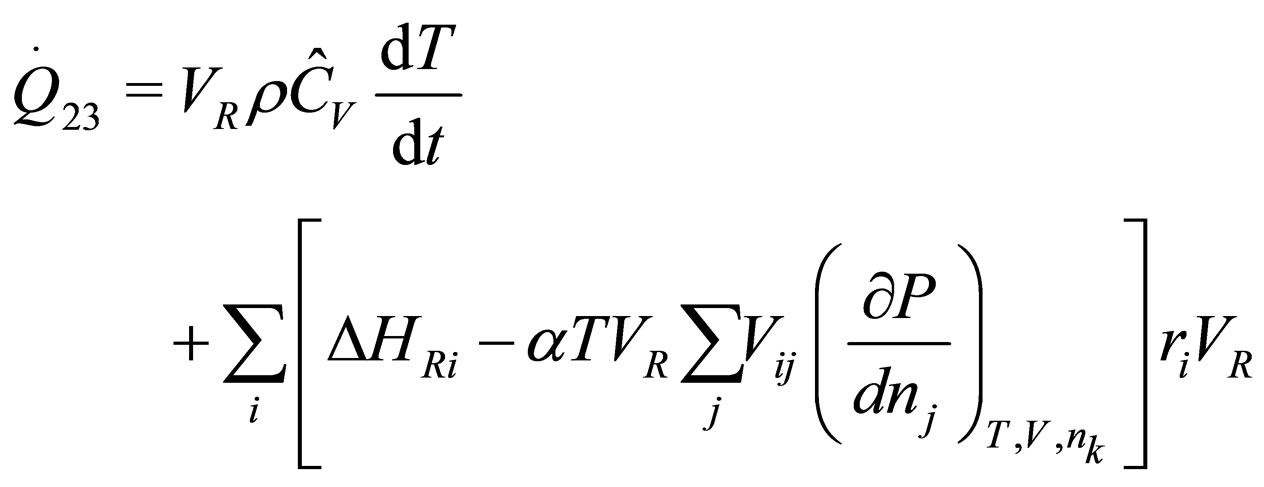 (9)
(9)
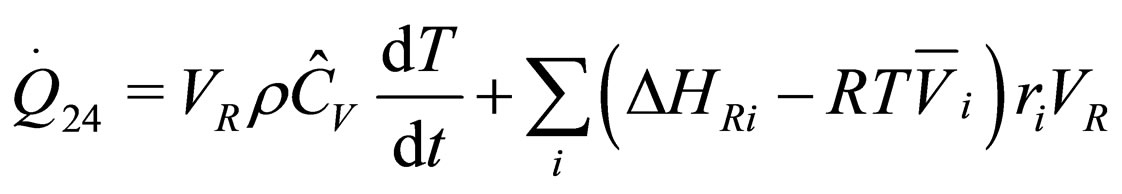 (10)
(10)
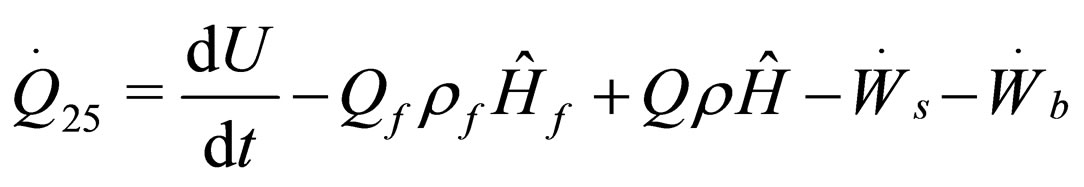 (11)
(11)
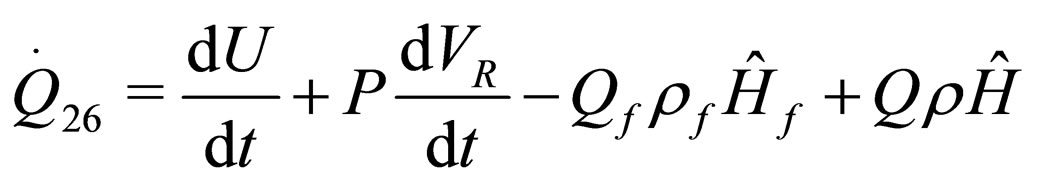 (12)
(12)
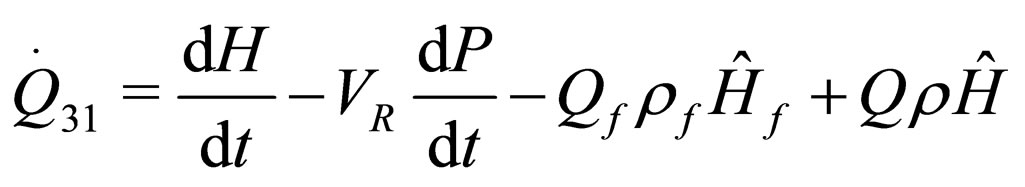 (13)
(13)
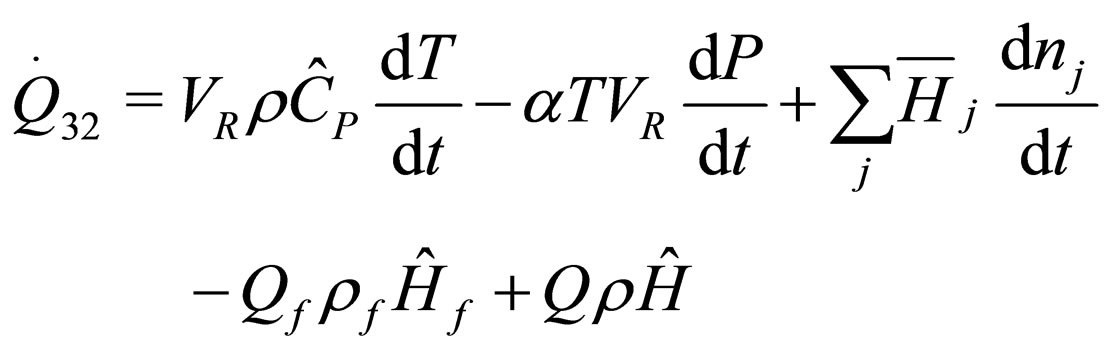 (14)
(14)
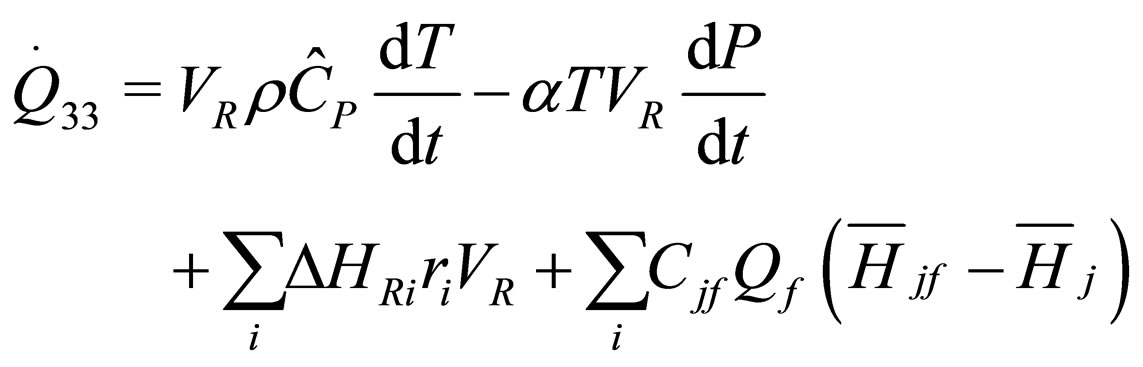 (15)
(15)
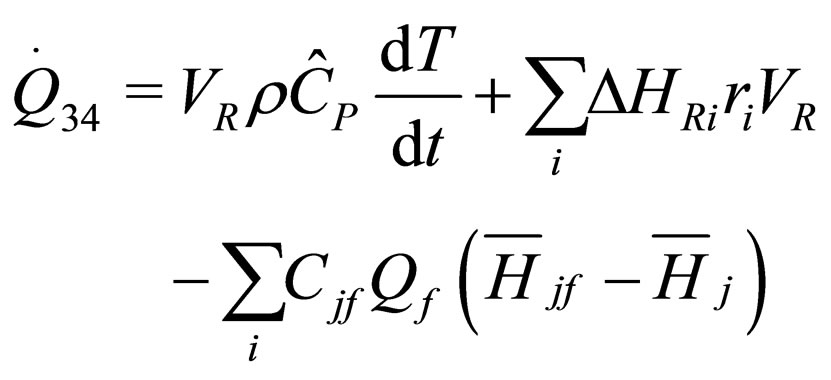 (16)
(16)
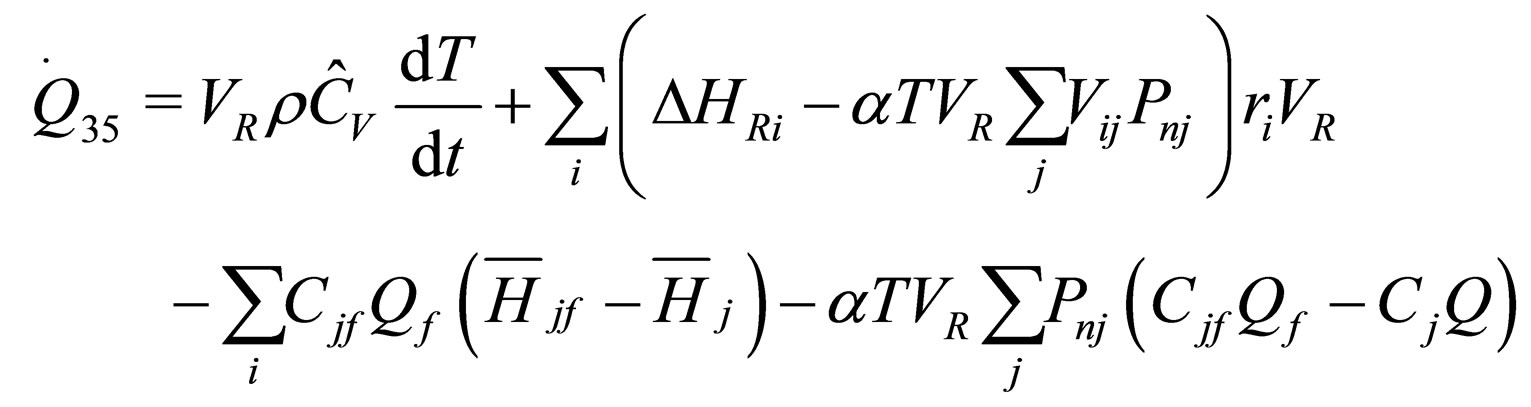 (17)
(17)
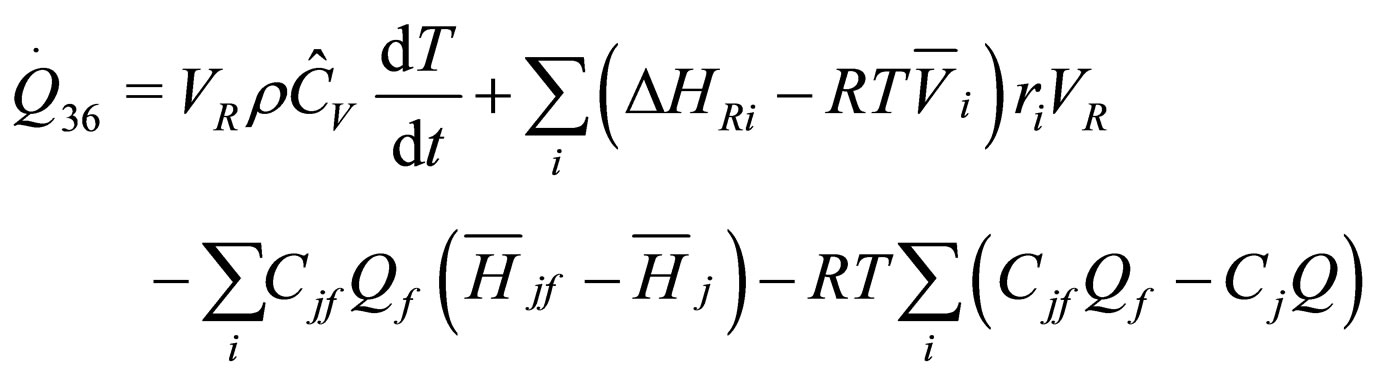 (18)
(18)
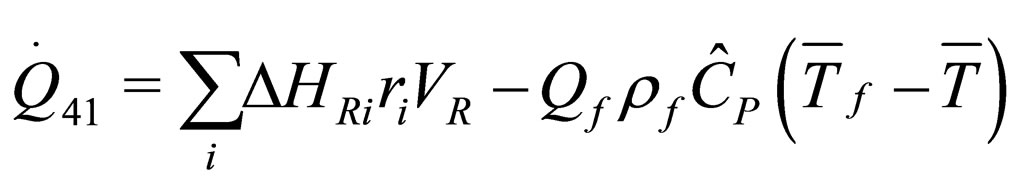 (19)
(19)
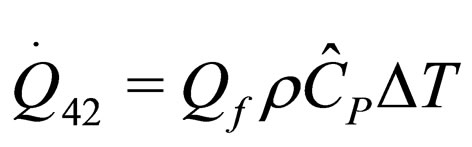 (20)
(20)
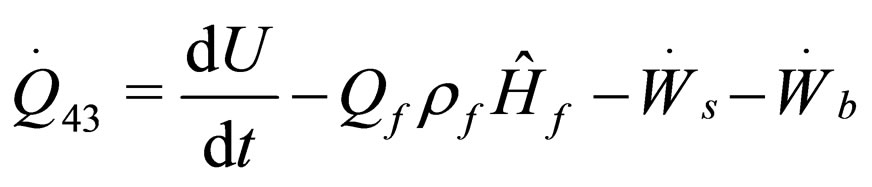 (21)
(21)
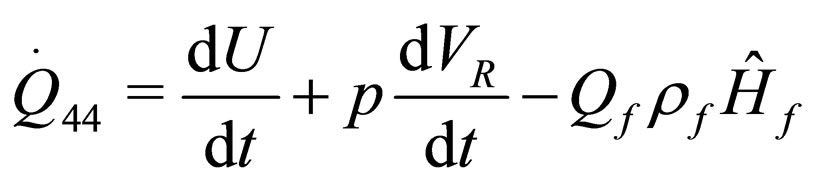 (22)
(22)
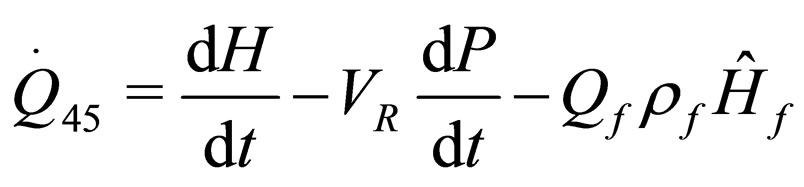 (23)
(23)
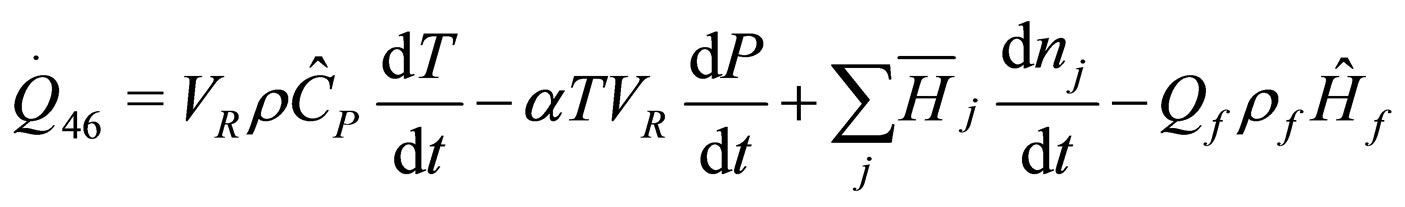 (24)
(24)
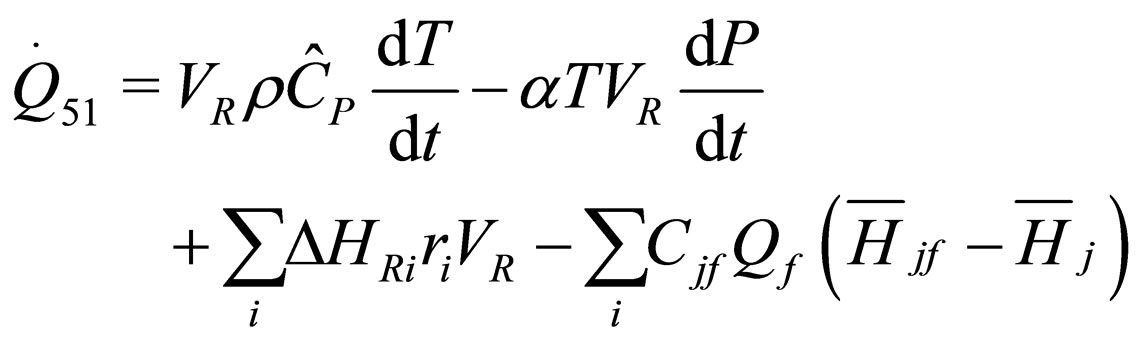 (25)
(25)
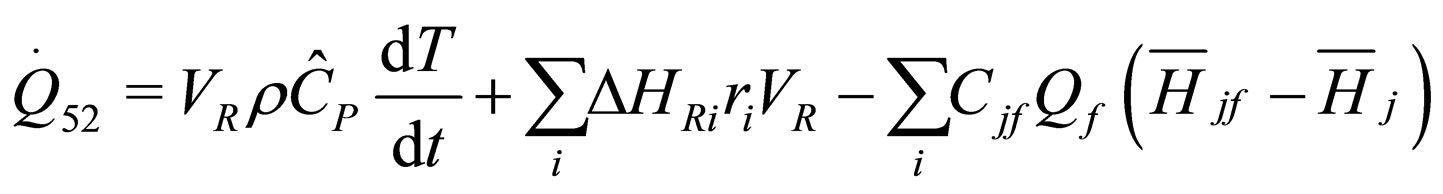 (26)
(26)
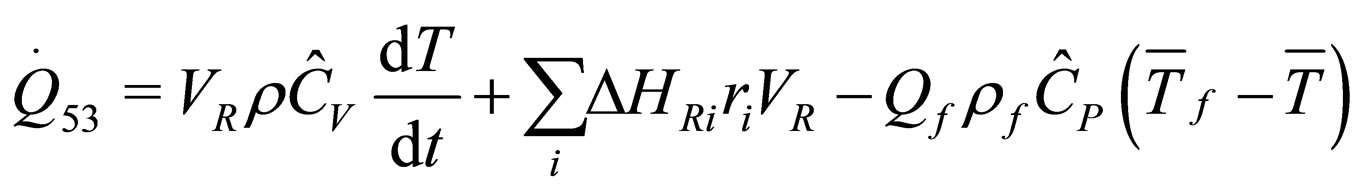 (27)
(27)
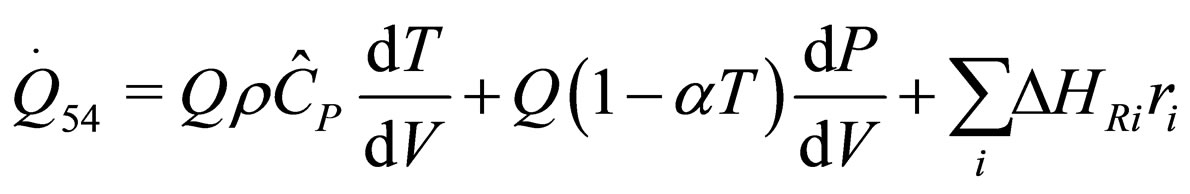 (28)
(28)
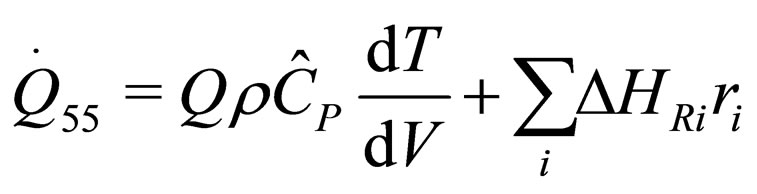 (29)
(29)
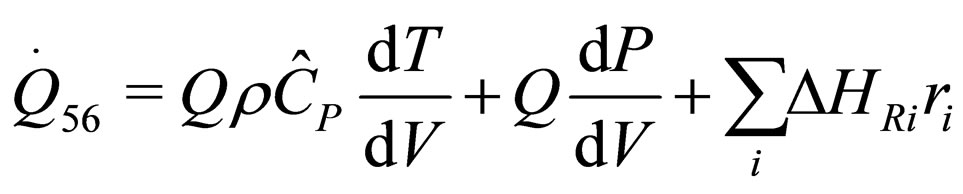 (30)
(30)
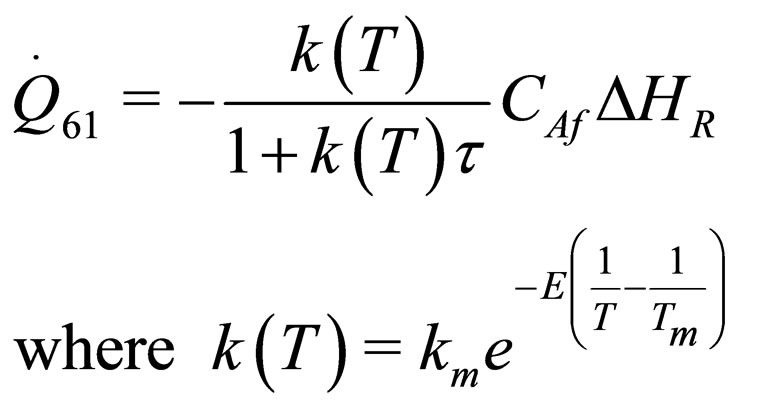 (31)
(31)
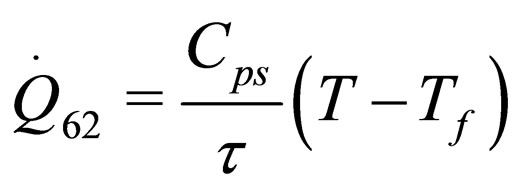 (32)
(32)
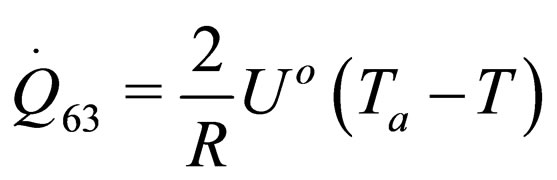 (33)
(33)
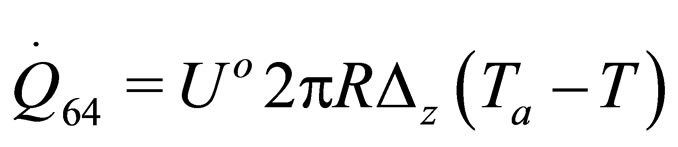 (34)
(34)
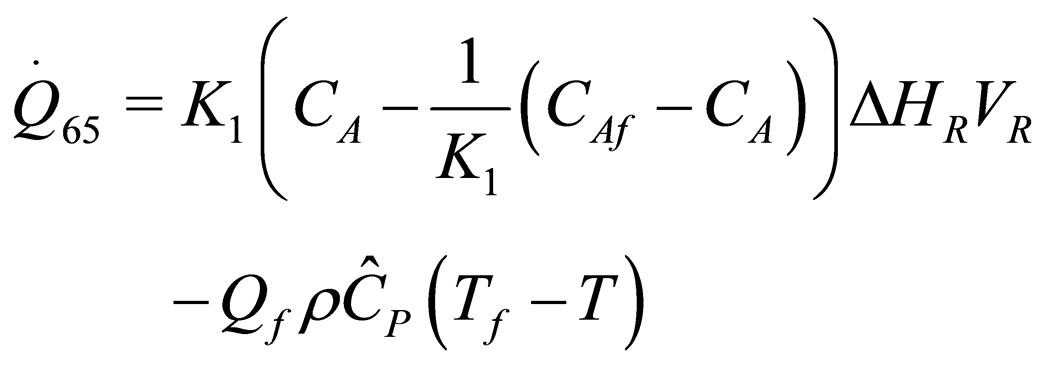 (35)
(35)
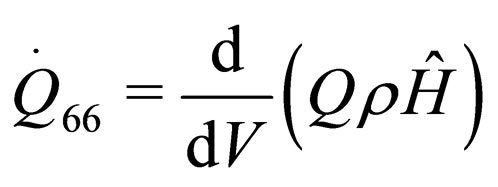 (36)
(36)
If the above thirty-six parametric equations and nuclear tokens can be used for the structure model for the construction of nuclear reactors, then the safety will be maximized and disaster minimized. This will later be structured into mathematical model in form of quadratic functional. The model will be solved using Conjugate Gradient Method algorithm, with MATLAB as a support soft-ware. See [17,18].
In particular, we shall use the following quadratic functional for further work.
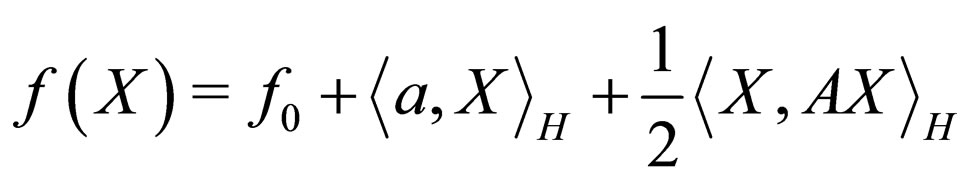
where  i.e. X = (x1x2 x3x4x5x6)T, a = (111111)T, f0 = 1 and
i.e. X = (x1x2 x3x4x5x6)T, a = (111111)T, f0 = 1 and
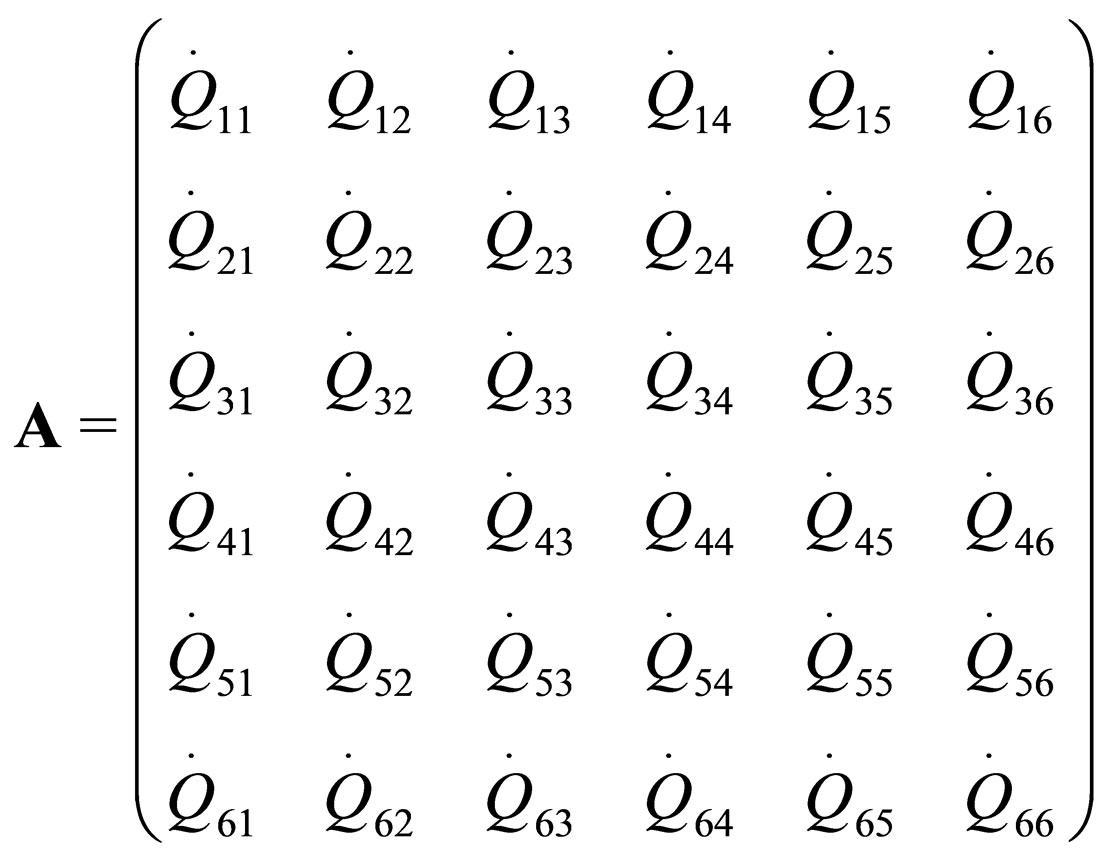
where 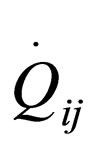 are the rate of heat of the reactors, and i, j = 1, 2, 3, 4, 5, 6. See [19].
are the rate of heat of the reactors, and i, j = 1, 2, 3, 4, 5, 6. See [19].
12. Nomenclature
The following nomenclatures are the nuclear tokens.
A = heat transfer area Cj = concentration of species j
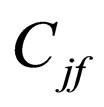 = feed concentration of species j
= feed concentration of species j
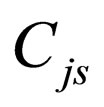 = steady-state concentration of species j
= steady-state concentration of species j
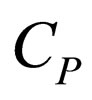 = constant-pressure heat capacity
= constant-pressure heat capacity
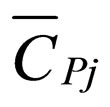 = partial molar heat capacity
= partial molar heat capacity
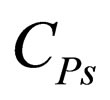 = heat capacity per volume
= heat capacity per volume
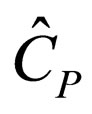 = constant-pressure heat capacity per mass
= constant-pressure heat capacity per mass
 = constant-volume heat capacity per mass
= constant-volume heat capacity per mass
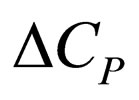 = heat capacity change on reaction,
= heat capacity change on reaction,
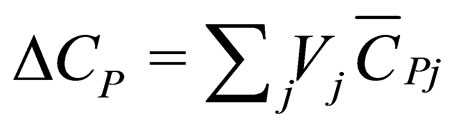
E = total energy
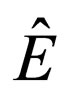 = total energy per mass
= total energy per mass
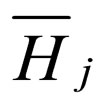 = partial molar enthalpy
= partial molar enthalpy
 = enthalpy per unit mass
= enthalpy per unit mass
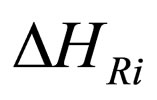 = enthalpy change on reaction,
= enthalpy change on reaction,
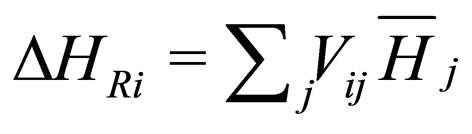
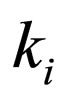 = reaction rate constant for reaction i
= reaction rate constant for reaction i
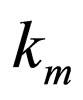 = reaction rate constant evaluated at mean temperature Tm
= reaction rate constant evaluated at mean temperature Tm
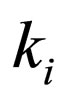 = equilibrium constant for reaction i
= equilibrium constant for reaction i
 = kinetic energy per unit mass
= kinetic energy per unit mass
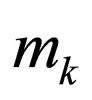 = total mass fow of stream k n = reaction order
= total mass fow of stream k n = reaction order
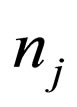 = moles of species j,
= moles of species j, 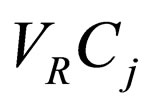
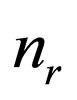 = number of reactions in the reaction network
= number of reactions in the reaction network
ns = number of species in the reaction network
P = pressure
Pj = partial pressure of component j
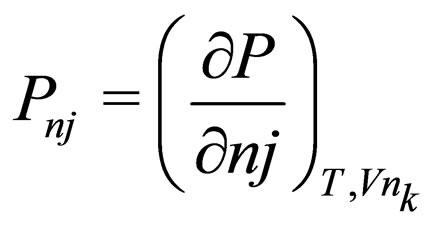
Q = volumetric flow rate Qf = feed volumetric flowrate
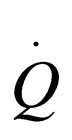 = heat transfer rate to reactor
= heat transfer rate to reactor
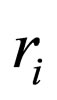 = reaction rate for i th reaction
= reaction rate for i th reaction
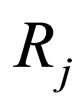 = production rate for j th species
= production rate for j th species
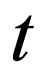 = time
= time
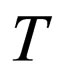 = temperature
= temperature
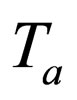 = temperature of heat transfer medium
= temperature of heat transfer medium
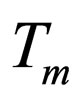 = mean temperature at which k is evaluated
= mean temperature at which k is evaluated
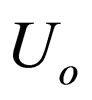 = overall heat transfer coefficient
= overall heat transfer coefficient
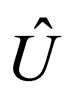 = internal energy per mass
= internal energy per mass
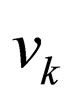 = velocity of stream k
= velocity of stream k
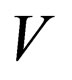 = reactor volume variable
= reactor volume variable
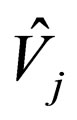 = partial molar volume of species j
= partial molar volume of species j
 = reactor volume
= reactor volume
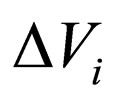 = change in volume upon reaction i,
= change in volume upon reaction i,
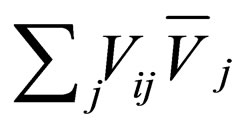
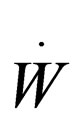 = rate work is done on the system
= rate work is done on the system
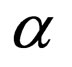 = coefficient of expansion of the mixture,
= coefficient of expansion of the mixture,
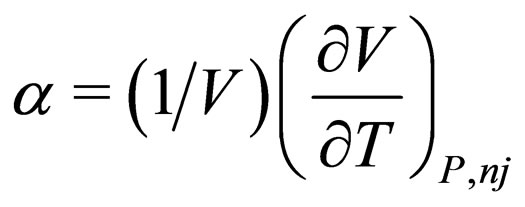
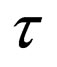 = reactor residence time,
= reactor residence time, 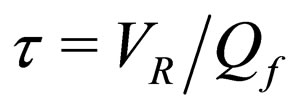
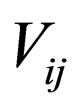 = stoichiometric coefficient for species j in reaction i
= stoichiometric coefficient for species j in reaction i

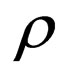 = mass density
= mass density
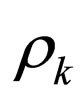 = mass density of stream k
= mass density of stream k
REFERENCES
- A. C. Kadak, et al., “A Response to the Environmental and Economic Challenge of Global Warming,” Massachusetts Institute of Technology, Massachusetts, 1998, pp. 1-6.
- IAEA Austria, “Structure of Nuclear Power Plant Design Characteristics in the IAEA Power reactor Information System(PRIS),” International Atomic Energy Agency (IAEA), Vienna, 2007, pp. 9-17.
- S. Frank, “Nuclear Reactors,” Kennesaw State University, Kennesaw, 2009, pp. 1-13. http//www.chemcases.com/nuclear/nc-10.html
- Wikipedia, “Boiling Water Reactor Safety System,” The Free Encyclopedia, 2011, pp. 2-9. http://en.wikipedia.org/wiki/Boiling-water-reator-safety-systems
- Encyclopaedia Britannica, “Windmill Power,” (Ultimate Refrence Suite) Chicago Encyclopaedia Britannica, Chicago, 2010.
- Encyclopaedia Britannica, “Hydroelectric Power,” (Ultimate Refrence Suite) Chicago Encyclopaedia Britannica, Chicago, 2010.
- C. K. Otto, “Fossil Fuel Power,” University of Tennessee, Ultimate Reference Suite Chicago Encyclopaedia Britannica, Knoxville, 2011.
- S. Ashok, “Solar Energy,” Department of Engineering, Pennsylvania State University: Ultimate Reference Suite Chicago Encyclopaedia Britannica, Chicago, 2011.
- J. S. Walker, “The Three Mile Island: ANuclear Crises in Historical Perspective. Berkeley,” University of California Press, Berkeley, 2004, pp. 216-265.
- “The Chernobyl Facts,” 2011. http://www.chernobyl-interntional.org/documents/chernobylfacts2.pdf
- Wikipedia, “The Free Encyclopedia,” Fukushima Daiichi Nuclear Reactor Disaster, Fukushima, 2011, pp. 5-27. http://en.wikipedia.org/wiki/Fukushima-Daiichi-nclear-disaster
- Wikipedia, “The Free Encyclopedia,” Energy in Japan, 2009, p. 1. http://www.en.wikipedia.org/wiki/Electricity-sector-in-Japan
- “Nuclear Fuel Behaviour in Loss-of-Coolant-Accident (LOCA) Conditions,” Nuclear Energy Agency (NEA), 2009, p. 141. http://wwwoecd-nea.org/nsd/reports/2009/nea6846-LOCA.pdf
- “Mitigation of Hydrogen Hazard in Water Cooled Power Reactors,” International Atomic Energy Agency (IAEA), 2001, pp. 3-10. http://www-pub.iaea.org/MTCD/publicatuion/PDF/te-1196-prn.pdf
- “The Enargy Balance for Chemical Reactors,” Nob Hill Publishing, LLC., Adison Wisconsin, 2011, pp. 1-182. http://jbrwww.che.wisc.edu/home/jbraw/chemreacfun/chb/slides-enbal.pdf
- G. H. Brian, “Energy Equations for Reactors,” Department of Chemical Engineering and Material Science, University of California, Davis: Lecture Note, Berkeley, 2010, pp. 9-13.
- J. O. Omolehin, “On the Control of Reaction Diffusion Equation,” Ph.D. Thesis, University of Ilorin, Ilorin, 1991.
- J. O. Omolehin, K. Rauf and D. J. Evans, “Conjugate Gradient Method Approach to Queue Theory,” International Journal of Computer Mathematics, Vol. 82, No. 7, 2005, pp. 829-835. http://www.tandf.co.uk/journals doi:10.1080/00207160412331296670
- J. O. Omolehin, L. Aminu and K. Rauf, “Computational Results on Quadratic Functional Model for the Tokens of Nuclear Safety,” American Journal of Computational Mathematics, 2012 (submitted for publication). http://www.scirp.org/journals/ajcm
NOTES
*Corresponding author.

