Advances in Bioscience and Biotechnology
Vol.2 No.5(2011), Article ID:7827,5 pages DOI:10.4236/abb.2011.25046
Effect of antigens on colony forming efficiency of stromal clonogenic cells and expression of cytokine genes in primary cultures of bone marrow and spleen of mice
![]()
Gamaleya Institute for Epidemiology and Microbiology, Moscow, Russia.
E-mail: Gorskaya@nearmedic.ru
Received 29 April 2011; revised 30 May 2011; accepted 18 September 2011.
Keywords: Stromal Cells-Immunity
ABSTRACT
Presence of microbial cells preparation (HCL—extract of group A streptococcus) in primary cell cultures of bone marrow (BMCC) and spleen (SCC) of CBA mice induced the expression mRNA of pro-inflammatory cytokines genes: IL-2, IL-6 and IL-8 in BMCC; in SCC cultures mRNA IL-1 β and IL-6 appeared and mRNA IL-4 disappeared. Injection of S. typhimurium antigen complex to mice CBA increased 5 times colony forming efficiency (CFE) and, respectively, content of stromal precursor cells (CFU-F) in femur bone marrow and 9 times in spleen of these animals with maximum at the first day. In both primary BMCC and SCC from immunized animals expression of pro-inflammatory cytokines genes IL-1 β, IL-6, IL-8 and TNF-α was revealed—in BMCC on 1 - 3 days after immunization, and in SCC—up to 15 days. In CBA mice injected with polyvinylpyrrolidone (PVP) CFE and number of CFU-F in BMCC and SCC increased 1, 9 and 1, 8 times only and expression of genes IL-6, IL-8 and TNF-α was observed only on the first day. In СВА/N xid mice there was neither increase of CFU-F in the corresponding organs, nor expression of pro-inflammatory cytokines genes in primary cultures. The data suggest the possibility of positive participation of stromal cells in the development of immune response in organism. Degree of activation of stromal tissue in immunized mice, apparently, correlates with the degree of immune response, supposing a close relationship between stromal tissue and immune system.
1. INTRODUCTION
Stromal cells perform very important functions in the organism—they are responsible for microenvironment of hemopoietic and lymphoid cells and participate in reparation of tissues. It is shown that stromal cells suppress immune response of lymphocytes to transplantation antigens and mitogens in vitro and in vivo [1]. It is also known that mesenchymal stem cells (MSC) express Tolllike receptors (TLR) 1, 2, 3, 4, 5, 6 and 9, respond to TLR ligands and may act as antigen-presenting cells [2]. It is shown in vitro that genes of cytokines and growth factors, including IL-6, IL-8, IL-11, IL-12, IL-14, IL-15, LIF, G-CSF, GM-CSF, M-CSF, FL, SCF are expressed in human MSC [3]. So the participation of these cells in the development of immune response in organism may be suggested. The aim of our study was the investigation of properties of stromal precursor cells in the presence of antigens in vitro and after immunization of animals. We have studied changes of CFE and profile of mRNA cytokines in primary cultures of bone marrow and spleen in CBA and CBA/N mice immunized with S. typhimurium antigen complex or PVP.
2. MATERIALS AND METHODS
Mice CBA (from Kryukovo Central Nursery of Laboratory Animals) and СВА/N (received from Mechnikov Institute for Vaccines and Sera) 18 - 20 g weight were used. All animals were handled in accord with Animal Welfare Act. Complex of S. typhimurium antigens (Gamaleya Institute for Epidemiology and Microbiology) (400 µg per mouse) or PVP (Sigma) (3 µg per mouse) were injected once intraperitoneally in 0.4 ml of physiological solution. It was shown earlier that these doses were optimal to ensure immune response to these antigens. Cellular suspensions of bone marrow and spleen of intact and immunized mice were prepared as described earlier [4]. To determine CFE 1 - 3 × 106 bone marrow cells and 5 × 106 - 1 × 107 spleen cells of intact mice and mice on 1, 3, 6 and 15 days after immunization were explanted into 25 cm2 plastic flasks (Nunc). Medium α-МЕМ (Sigma) with 15% fetal calf serum (Paneco) and antibiotics (penicillin and streptomycin in concentration 100 µg/ml) was used. Cultivation was performed at 37˚C in a humidified mixture of 5% CO2 with air. On 10 - 12 day these cultures consisted of discrete colonies of stromal fibroblasts with small number of macrophages. On day 10 to 12, the cultures were washed with Hanks balanced salt solution (Invitrogen), fixed with ethanol, and stained with azure-eozin. CFU-F colonies containing 50 or more cells were counted, and CFE (number of colonies per 1 × 105 nucleated marrow cells) was calculated.
To determine expression of cytokine genes 5 × 106 - 1 × 107 bone marrow cells and 1 - 2 × 107 spleen cells of intact and immunized mice were explanted into 25 cm2 plastic flasks. On 8 - 10 days these cultures consisted of a layer of fibroblasts (formed as a result of CFU-F colony confluence), macrophages and a small number of hemopoietic and lymphoid cells. HCL—extract of group A streptococcus (Gamaleya Institute for Epidemiology and Microbiology) was added to culture medium of intact mice cells on 9 - 10 day of cultivation in concentration 25 µg/ml for 24 hours. Then culture medium was removed and expression of mRNA of 11 cytokines: interferons (IFN-α and IFN-γ), interleukines (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-18) and TNF-α was determined in culture cells by method of reverse transcription and polymerase chain reaction. RNA isolation was performed by P. Chomczynsky, N. Sacchi with acid guanidine thiocyanate—phenol—chloroform extraction [5]. Reverse transcription and PCR amplification were carried out according to Gelder [6]. Pairs of primers for the following cytokines were used: IFN-α [6], IL-6, IL-8 [7], IL-1β, IL-2, IL-4, IL-10, TNF-α, IFN-γ [8], IL-18, IL-12 [9]. β—actin was used as positive control [7]. Registration of PCR results was carried out electrophoretically in 2.5% agarose gel stained by ethydium bromide. Promega electrophoresis marker (G 1758) was used to identify nucleotide sequence. Presence (sign “+” in the table) or absence (sign “-” in the table) of mRNA activity was determined by results of not less than three independent experiments.
3. RESULTS
Presence of HCL—extract of group A streptococcus in bone marrow cell cultures of CBA mice induced expression of IL-6 and IL-8 genes. In spleen cell cultures mRNA IL-1β and IL-6 appeared; in cultures of bone marrow and spleen mRNA IL-4 disappeared (Table 1).
Injection of S. typhimurium antigen complex to CBA mice induced significant (5, 5 times) increase of CFE and of the content of CFU-F in femur bone marrow of mice with maximum on 1 - 3 days after immunization (Table 2). In bone marrow cultures from immunized animals in contrast to intact ones, the expression of pro-inflammatory cytokines genes was observed—IL-1β and IL-8 (on the first day after immunization), IL-6 (on 1 - 3 days) and TNF-α (on 1 - 6 days). Expression of anti-inflammatory cytokine gene IL-4 was revealed only on day 6 after immunization (Table 3).
CFE and the content of CFU-F in spleen of immunized CBA mice increased 7 and 9 times respectively on the first day after immunization, decreasing to a normal level by 6 day after immunization (Table 2). In spleen cell cultures of these mice there was stimulation of synthesis of m-RNA of pro-inflammatory cytokines: IL-1β (1 - 15 days), IL-6 (1 - 6 days), TNF-α, IL-18, IFN-α (6 - 15 days). Synthesis of m-RNA IL-4 was suppressed on the 6 day after immunization. Synthesis of m-RNA IL-10 was suppressed (on 1 - 6 days); m-RNA IL-6 disappeared on the 15 day after immunization of animals (Table 3).
Injection of PVP to CBA mice increased CFE and, respectively, the content of CFU-F in femur bone marrow and spleen of mice about 2 times on the first day after immunization (Table 2). In bone marrow cultures from immunized animals in contrast to intact ones, expression of anti-inflammatory cytokines genes IL-6, IL-8 and TNF-α was induced (on 1 day), as well as IL-1β (on 3 day) and expression of gene IL-4 (on 1 - 3 days) and IL-10 (on 3 day) was suppressed. In cultures of spleen cells from immunized animals on the first day expression of genes IL1β and IL-6 was already revealed and expression of gene IL-4 was suppressed (Table 3).
When injecting PVP to СВА/N mice CFE and number of CFU-F in bone marrow and spleen of immunized and intact mice were practically the same. Expression of pro-inflammatory cytokines genes in primary cultures was not observed (Table 3).
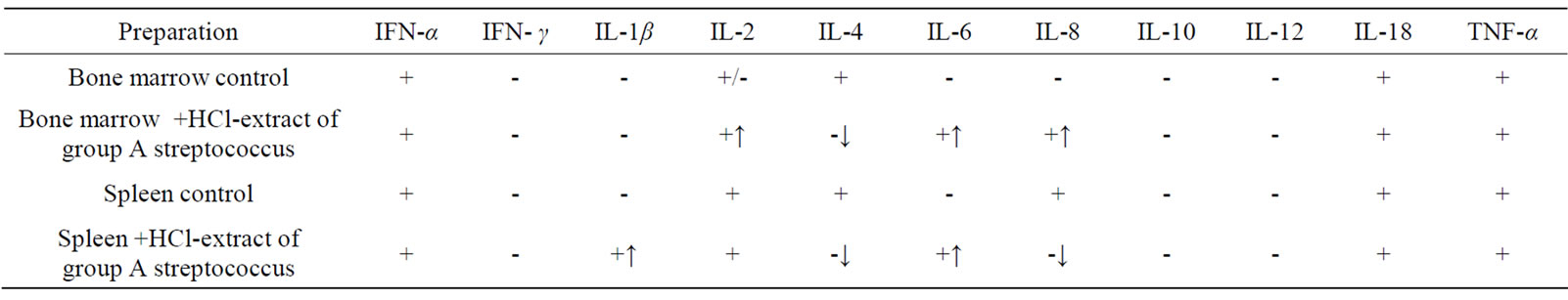
Table 1. Effect of HCL-extract of group A streptococcus on synthesis of cytokine mRNA in primary cultures of bone marrow and spleen of CBA mice in vitro (↑appearance of mRNA, ↓disappearance of mRNA).
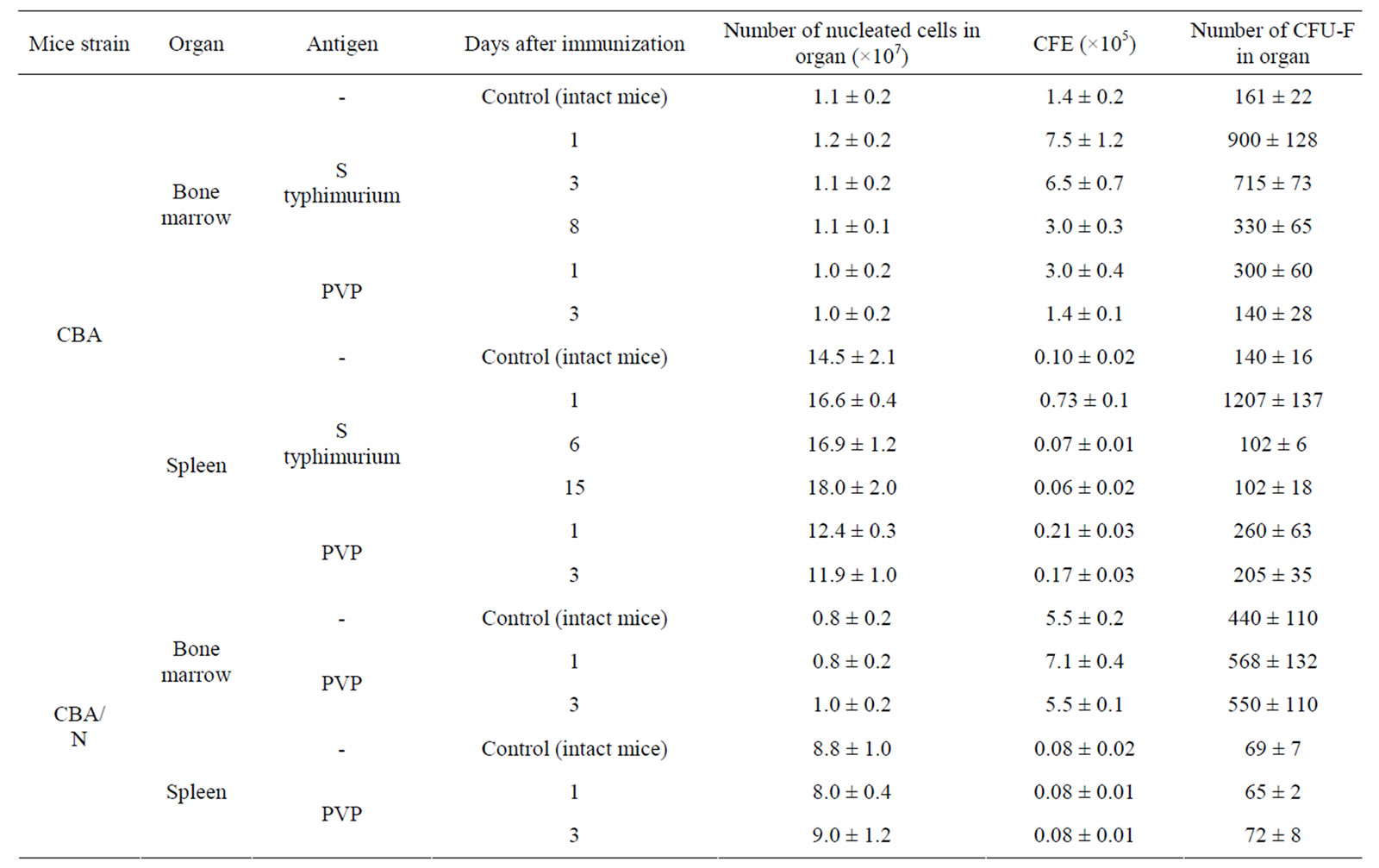
Table 2. CFE in cell cultures of bone marrow and spleen in mice immunized with S typhimurium antigens or PVP (M ± m).
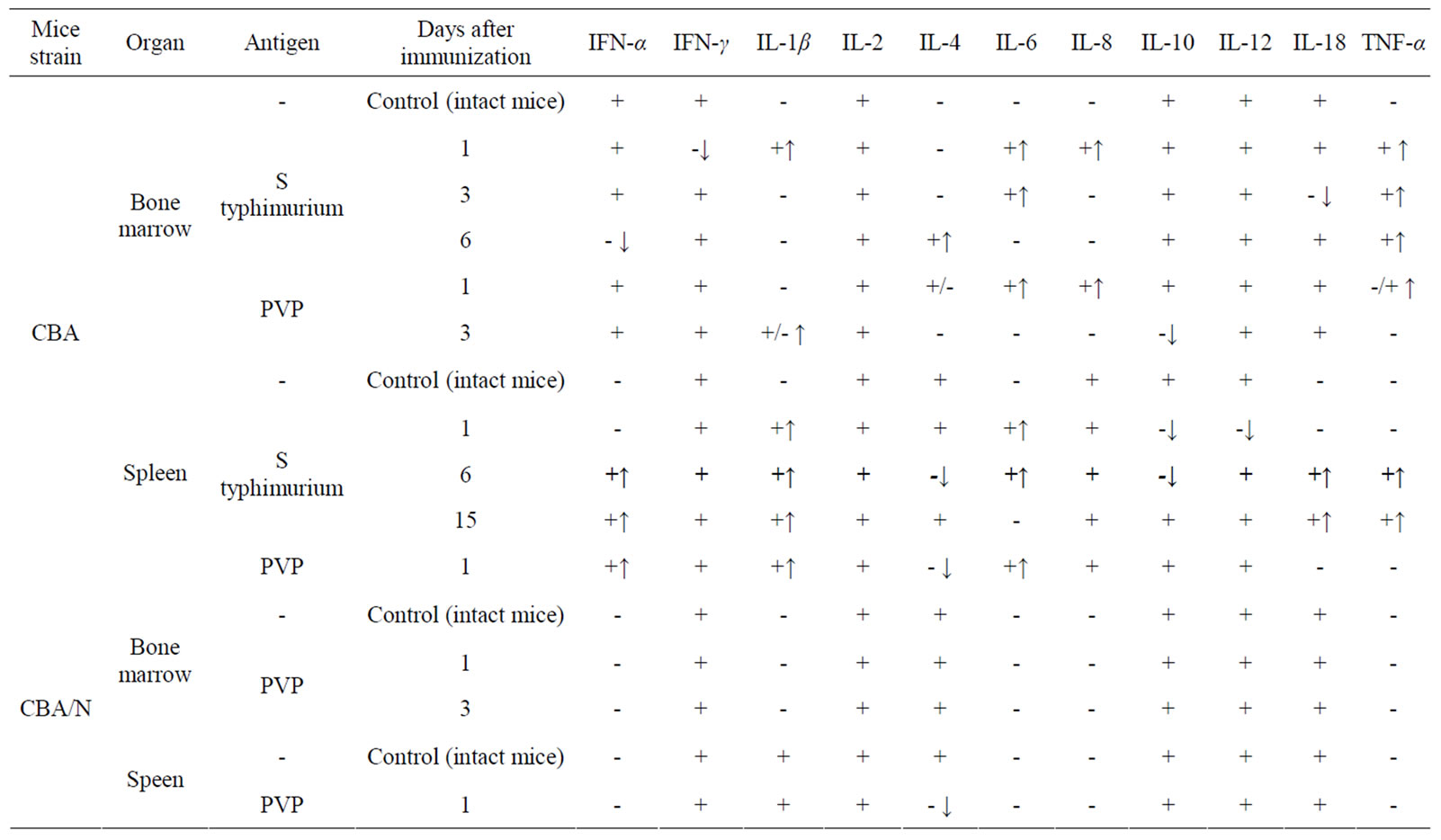
Table 3. Effect of immunization of mice CBA and СВА/N with S typhimurium antigens or PVP on synthesis of cytokines mRNA in primary cultures of bone marrow and spleen (↑appearance of mRNA, ↓disappearance of mRNA).
4. DISCUSSION
In our previous work on passage human bone marrow stromal fibroblasts it was shown that presence of microbial cells preparations in culture medium during 24 hours suppressed pro-inflammatory cytokines (IL-1β, IL-6, IL-8 and IL-2) genes expression, and stimulated expression of anti-inflammatory cytokine genes IL-4 in these cells [10]. These facts confirm the data about immunosuppressive effect of stromal cells on lymphoid cells. Cultures of human bone marrow cells consisted of stromal fibroblasts only, cells of other categories were absent. [11]. On the contrary in primary cultures of bone marrow and spleen cells (where besides a layer of fibroblasts formed as result of CFU-F colony confluence, a small number of macrophages, hemopoietic and lymphoid cells were present) the addition of HCL—extract of group A streptococcus in these cultures induced expression of pro-inflammatory cytokines genes. The data exist (received in short-term cultures in vitro), that activation of TLR 4 in human MSC leads mostly to synthesis of proinflammatory cytokines by these cells, while activation of TLR 3— anti-inflammatory ones [12]. It was also shown in vitro, that activation of TLR 3 and 4 in MSC abolished the suppression of T-cell immune response [13]. The results of our experiments could hardly be explained by the fact that contrary to human bone marrow passage fibroblasts, in cells of primary cultures of mouse bone marrow and spleen bacterial preparations activated different TLR, as we used the same preparations of microbial cells. Besides, according to our data presence of TLR 4 ligand—LPS in cultures of human bone marrow MSC during 24 hours induced in these cells suppression of pro-inflammatory cytokines genes [10]. So it may be supposed that expression of these cytokine genes in primary cultures of bone marrow and spleen can be a consequence of interactions of stromal and immunocompetent cells present in the cultures.
Thus, in this work we have not observed any signs of immune response suppression from the part of stromal cells, which are predominant cells category in primary cultures. The data received indicate the possibility of positive participation of stromal cells in the development of immune response in an organism. It should be mentioned that expression of a certain set of pro-inflammatory cytokines genes was tested in cultures of cells aged 10 - 12 days. Spectrum of m-RNA changed significantly in dependence of the term after immunization of animals after 1, 3 and 6 days (Table 3). Thus, it seems like stromal cells are “programmed” for expression of genes of a certain spectrum of cytokines and removal or change of this “program” is not realized in cultures in vitro, but only in organism. This fact also argues for assumption that both number of stromal cells of respective organs and synthesis of cytokines in primary cultures are under control of immunocompetent cells (macrophages, lymphocytes) activated by antigens.
Respectively slight increase of CFE and CFU-F number in bone marrow and especially in spleen of CBA mice immunized by PVP in comparison with results of immunization by S. Typhimurium antigens, apparently reflect a lower degree of stromal tissue activation at relatively weak immune response to PVP. Really, it is known that the number of specific antibody forming cells in response to PVP in CBA mice increased 2 times only [14]. This assumption was confirmed in experiments with CBA/N mice not responding on PVP because of absence of CD5+B-1a cells as a result of xid mutation in gene of Bruton tyrosine kinase (Btk). These mice injected by PVP showed neither increase in number of CFU-F in the corresponding organs, nor the expression of pro-inflammatory cytokines genes in primary cultures. The received data testify that the degree of activation of stromal tissue in immunized mice correlates with the degree of manifestation of immune response to respective antigens.
There are data pointing out that stromal tissue in xid mice is not disturbed. Indeed, adoptive transfer of lymphocytes from normal mice of respective line quickly and fully restores their response to TH-2 antigens [15]. This fact testifies that in itself injection of antigens in organism in absence of lymphocytes able to respond on it, can not necessarily lead to activation of stromal tissue. Also taking in consideration that PVP is a synthetic non-bacterial antigen, the data we received indicate that activation of stromal tissue cells in the development of immune response can occur not only through TLR, but rather through interaction of immunocompetent and stromal cells, at that the immunocompetent cells, apparently, play the leading role in this process.
REFERENCES
- Rasmussen, I., Ringen, O., Sundberg, B., et al. (2005) Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Experimental Cell Research, 305, 33-41. doi:10.1016/j.yexcr.2004.12.013
- Pevsner-Fisher, M., Morad, V., Cohen-Sfady, M. et al., (2007) Toll-like receptors and their ligands control mesenchymal stem cells function. Blood, 109, 1422-1432. doi:10.1182/blood-2006-06-028704
- Kim, D.H., Yoo, K.H., Choi, K.S. et al., (2005) Gene expression profile of cytokine and growth factor during differentiation of bone marrow derived mesenchymal stem cell. Cytokine, 31, 119-126. doi:10.1016/j.cyto.2005.04.004
- Friedenstein, A.Ya., Latzinic, N.V., Gorskaya, Yu.F. et al., (1992) Bone marrow stromal colony formation requires stimulation by haemopoietic cells. Bone and Mineral, 18, 199-213. doi:10.1016/0169-6009(92)90807-P
- Сhomezynski, P. and Sacchi, N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Analytical Biochemistry, 162, 156-159.
- Gelder, C.M., Thomas, P.S., Yates, D.H., et al., (1995) Cytokine expression in normal, atopic and asthmatic subjects using the combination of sputum induction and the polymerase chain reaction. Thorax, 50, 1033-1037. doi:10.1136/thx.50.10.1033
- Lin, Y., Zhang, M. and Barnes, P.F. (1998) Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infection and Immunity, 66, 1121-1126.
- Yamamura, M, Uyemura, K., Deans, R.J., et al., (1991) Defining protecting responses to pathogens: Cytokine profiles in leprosy lesions. Science, 254, 277-279. doi:10.1126/science.1925582
- Gaede, K.I., Mamat, U., Schlaak, M., et al., (1999) Analysis of differentially regulated mRNAs in monocytic cells induced by in vitro stimulation. Journal of Molecular Medicine, 77, 847-852. doi:10.1007/s001099900064
- Gorskaya, Yu.F., Mezentseva, M.V., Shapoval, I.V., Danilova, T.A., et al., (2009) The influence of bacterial cell preparation, IFN-γ, and MIF on cytokine gene expression in passage stromal fibroblasts of human bone marrow in vitro and cells of mouse bone marrow and spleen in primary cultures. New Horizons in Allergy, Asthma & Immunology MEDIMOND International Proceedings Proceedings of the 11 World Asthma & Cord Forum SP, 205-208.
- Chaylakyan, R.K., Gerasimov, Yu.V., Kuralesova, A.I., et al., (2001) Proliferative and differential potentions of individual clones of stromal cell precursors in bone marrow. Izvestiya АS, 6, 682-687.
- Waterman, R.S., Tomchuk, S.L., et al., (2010) A new mesenchymal stem cell paradigm: Polarization into a pro-inflammatory MSC-1 or an immunosupressive VSC- 2 phenotype. PLoS ONE, 5, 1-14. doi:10.1371/journal.pone.0010088
- Liotta, F., Angeli, R., Cosmi, L., et al., (2008) Toll-like receptors 3 and 4 are expressed by human bone marrow derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells, 26, 279-289. doi:10.1634/stemcells.2007-0454
- Sidorova, E.V. (2006) What do we know today about B lymphocytes? Uspehi Sovremennoy Biologii, 3, 227-241.
- Prior, L., Pierson, S., Woodland, R.T. and Riggs, J. (1994) Rapid restoration of B-cell function in xid mice by intravenous transfer of peritoneal cavity B-cells. Immunology, 83, 180-183.

