Journal of Biophysical Chemistry
Vol.05 No.03(2014), Article ID:46663,7 pages
10.4236/jbpc.2014.53010
Thermodynamics of the Second Stage Dissociation Step (pK2) of Buffer Monosodium 1,4-Piperazinediethanesulfonate from (278.15 to 328.15) K
Rabindra N. Roy, Lakshmi N. Roy, John J. Dinga, Matthew R. Medcalf, Katherine E. Hundley, Eric B. Hines, Ryan R. Parmar, Jamie A. Veliz, Clark B. Summers, Lucas S. Tebbe
Hoffman Department of Chemistry, Drury University, Springfield, USA
Email: rroy@drury.edu
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 April 2014; revised 28 May 2014; accepted 4 June 2014
ABSTRACT
Values of the second thermodynamic dissociation constant pK2 of the protonated form of monosodium 1,4-piperazinediethanesulfonate (PIPES) have been determined at twelve different temperatures in the temperature range from (278.15 to 328.15) K including the body temperature 310.15 K by measurement of the electromotive-force for cells without liquid junction of the type: Pt (s), H2 (g), 101.325 kPa|Na-PIPES (m1) + Na2-PIPES (m2) + NaCl (m3)|AgCl (s), Ag (s), where m1, m2 and m3 indicate the molalities of the corresponding species at 1 atm = 101.325 kPa in SI units. The pK2 values for the dissociation of Na-PIPES are represented by the equation: pK2 = −1303.76/T + 48.369 - 6.46889 lnT with an uncertainty of ± 0.001. The values of pK2 for Na-PIPES were found to be 7.1399 ± 0.0004 at 298.15 K and 7.0512 ± 0.0004 at 310.15 K, respectively, and indicate that this buffer would be useful as pH standard in the range of physiological application. Standard thermodynamic quantities ∆G˚, ∆H˚, ∆S˚ and ∆Cp˚ for the acidic dissociation process of Na-PIPES have been derived from the temperature coefficients of the pK2. These values are compared with those of structurally related N-substituted PIPERAZINE and TAURINE at 298.15 K.
Keywords:
Dissociation Constant, Zwitterion, Ionic Strength, pK2, Buffer Compound, e.m.f, Thermodynamic Quantities

1. Introduction
The control of pH in the physiologically important pH range is difficult because of the limitation in the number of weak acid-base systems whose pH values fall within the pH range of 6 - 8. Second-stage dissociation process and the acid-base behavior of the simplest zwitterionic amino acids, 2-aminoethanesulfonic acid (TAURINE) [1] and (PIPERAZINE) [2] , have been studied. The pK2 values of these two buffers at 298.15 K are 9.061 and 9.731, respectively. Careful thermodynamic investigation of the dissociation of other amino acids, (MES) [3] , (MOPS) [4] , (BES) [5] , (TES) [6] , and (CHES) [7] , has contributed significantly to an understanding of this important subject. Biological buffers are of great importance for research in biomedicine, pharmaceutical chemistry, clinical chemistry, and oceanography. Good et al. [8] recommended several biological buffer substances which are useful in the physiological pH region of 6 - 8. One of the buffer compounds selected was monosodium 1,4-pi- perazine diethanesulfonate (PIPES) whose deprotonation constant is about 10−8 at 298.15 K. The compound PIPES is, therefore, a better buffer for pH control in the buffer region of greatest physiological interest than its parent compound, (PIPERAZINE) [2] .
In order to calculate pH values with the accuracy needed for a pH buffer standard, it is essential to determine very accurate values of the thermodynamic dissociation constant pK2 for the second-stage dissociation equilibria of PIPES. Roy et al. [9] determined pK2 values and related thermodynamic quantities of PIPES at 12 temperatures using the galvanic cell without liquid junction:
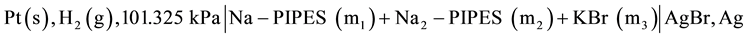 [A]
[A]
In this cell, Ag, AgBr electrode and KBr solid were used. Because of the importance of the chloride ion Cl− in clinical samples such as blood, plasma and cerebrospinal fluids, we have undertaken to study PIPES at 12 temperatures from (278.15 to 328.15) K employing the cell:
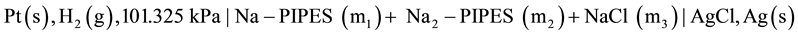 [B]
[B]
in which NaCl salt and Ag, AgCl were used. Thus, there is a great difference between cell A and cell B in terms of the use of the salt and the electrode. No thermodynamic data on pK2 of PIPES is available in the literature using Ag-AgCl electrode. This is the original research work. The monosodium salt of the (PIPES) 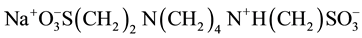 is designated as P±−, and the deprotonation process is represented by
is designated as P±−, and the deprotonation process is represented by
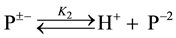 (1)
(1)
where K2 is the thermodynamic equilibrium constant. The structure of the monosodium 1,4-piperazinedietha- nesulfonate (PIPES) is shown below.
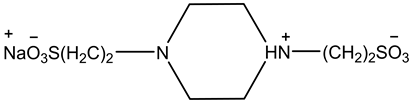
2. Experimental
The commercial sample of monosodium 1,4-piperazinediethanesulfonate (PIPES) was obtained from Research Organics (Cleveland, Ohio). The technique to determine the assay of PIPES has been previously reported [9] . It assayed 99.98% when titrated with a standard solution of NaOH. From the preliminary measurements, the difference in e.m.f (cell voltage) between a purified and unpurified sample was well within the experimental error ±0.05 mV. Thus, the commercial sample was used as received. It was always dried and stored in a desiccator. The preparation of all 16 experimental buffer solutions of Na-PIPES were made by mixing accurate amounts of the commercial sample of PIPES, CO2-free NaOH, Fisher ACS certified grade NaCl, and CO2-free double distilled water. The molality range of the buffer solutions varied from 0.002 to 0.04 mol∙kg−1, and the ionic strength range was from I = 0.006 to 0.12 mol∙kg−1. Vacuum corrections were applied to all masses with accuracy better than ±0.02 mass percent. The precise e.m.f measurement technique was originally used by Harned and Ehlers [10] . The e.m.f method used in the present study was modified by Gary et al. [11] and was successfully used by Sankar and Bates [12] as well as Roy et al. [13] . The following galvanic cell without liquid junction of the type was used:
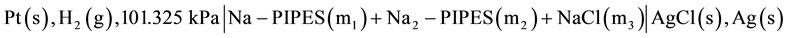 (A)
(A)
In all previous publications from this laboratory, the preparation of the platinum black hydrogen electrode and the silver-silver chloride electrode (thermal electrolytic type [14] ), the cell design of all glass cells, purification of the hydrogen gas, and other experimental details have been described in detail elsewhere [15] [16] . The bias potential of the (silver + silver chloride) electrodes was within ±0.05 mV. The e.m.f measurements were made at intervals of 5 K from (278.15 to 328.15) K for all 16 buffer solutions at 12 temperatures in the molality range 0.002 to 0.04 mol∙kg−1. The e.m.f at 298.15 K was recorded two times (sometimes three times), namely at the beginning, in the middle, and at the end of the run. These e.m.f values, on average, agreed to within ±0.04 mV, demonstrating excellent stability and high reproducibility of the silver-silver chloride in the presence of nitrogen base. The constant temperature of the water bath was regulated to ±0.005 K within a digital thermometer (Guildline model 9540). All e.m.f measurements were made by employing Hewlett-Packard 2000 multimeter.
3. Methods and Results
The equation for the calculation of the “apparent” thermodynamic dissociation constant  for the second dissociation step of PIPES is expressed:
for the second dissociation step of PIPES is expressed:
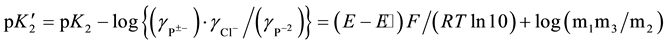 (2)
(2)
where P±− is the zwitterionic species for Na-PIPES; m1, m2, and m3 are stoichiometric molalities of the corresponding species; P−2 stands for Na2-PIPES; pK2 the thermodynamic dissociation constant; γ the activity coefficient of the respective species; E the e.m.f corrected to a hydrogen partial pressure of 101.325 kPa; F is the Faraday constant (96487 C・mol−1); RT ln10/F = k = 0.059156 at 298.15 K; and E˚ is the standard electrode potential of the (silver + silver chloride) electrode. The cell voltage (E) values are listed in Table 1. The values of (E˚) [17] were obtained in the authors’ laboratory by using 0.01 mol・kg−1 HCl solution with a value of 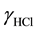 = 0.904 at 298.15 K. The activity coefficient of Equation (2) is unknown. In such situations, the simple form of Equation
= 0.904 at 298.15 K. The activity coefficient of Equation (2) is unknown. In such situations, the simple form of Equation
(2) involves assumptions that a) the activity coefficient term 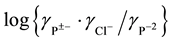 will, because of charge
will, because of charge
type, be small and is expected to become linear function of the ionic strength I, and b) the nearly neutral pH of the buffer solutions which make hydrolysis correction of the ratio m1/m2 unnecessary. The ionic strength of the solution of cell B is indicated by:
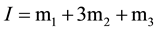 (3)
(3)
and the simplified version of Equation (2) becomes:
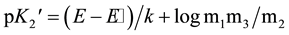 (4)
(4)
As expected, 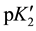 varies linearly with I at each temperature and is represented by the following equation:
varies linearly with I at each temperature and is represented by the following equation:
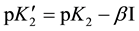 (5)
(5)
where the intercept at I = 0 yields the value of pK2 and β is the slope parameter. These values of pK2 together with the standard deviation are listed in Table 2. The experimental values of pK2 are fitted as a function of the thermodynamic temperature T from (278.15 to 323.15) K by the method of least squares to an equation of the form suggested by Ives and Mosely [18] . The final equation takes the form
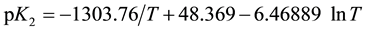 (6)
(6)
The standard deviation of regression for Equation (6) is 0.0014. The values of the standard changes of Gibbs energy (∆G˚), enthalpy (∆H˚), entropy (∆S˚) and the heat capacity (∆Cp˚) for the dissociation process indicated by Equation (1) have been derived from the constants of Equation (6) by using simple thermodynamic relationships. The values of these thermodynamic functions, along with the estimates of the standard deviations calculated by the method of Please [19] are summarized in Table 3. It is of interest to compare the values of the pK2 and thermodynamic functions of Na-PIPES with some structurally related N-substituted zwitterionic compounds of (TAURINE) [1] and (PIPERAZINE) [2] . At 298.15 K, these values are compiled in Table 4.
Table 1. Cell potential of cell B (in volts).
Table 2. Second dissociation constant of Na-PIPES.
aStandard deviation of pK2. bSlope parameter.
Table 3. Thermodynamic quantities for the dissociation of Na-PIPES from (278.15 - 328.15) K.
Units: ∆G˚. ∆H˚, J∙mol−1; ∆S˚, ∆Cp˚, J∙K−1∙mol−1.
4. Discussion
For each of the structurally related compounds in Table 4, two methylene groups separate positive and negative charge centers. The comparison of the thermodynamic properties of some substituted compounds and the parent compound (TAURINE) [1] and the derivative of TAURINE such as (MOPS) [4] , (BES) [5] , (TES) [6] , (CHES) [7] , (MOBS) [20] and (PIPERAZINE) [2] and its N-substituted PIPERAZINE such as (HEPBS) [20] , (HEPES) [21] , (TAPS) [22] and PIPES [this investigation] reveal useful information in terms of acidic strength, steric and inductive effects. The parent compound PIPERAZINE has a pK2 of 9.731 at 298.15 K whereas that of PIPES in the present work is 7.140. The interpretation is that the substitutions of methylene-(CH2)2 and hydroxymethyl- (HOCH2)2 groups on the nitrogen atom of (TAURINE) [1] and (PIPERAZINE) [2] enhance the acidic strength for the dissociation of (BES) [5] and PIPES [this study], respectively.
Table 4 clearly shows a decrease in pK2 value (increase in acidic strength) for both N-substituted PIPERAZINE and TAURINE compounds. The alterations in acidic strength for the dissociation process of PIPES are attributable to the inductive effects of the oxygen atom from both 
It is interesting to discuss the trend of the standard thermodynamic properties from Table 4. Usually, the decrease in pK2 value is paralleled by the decrease in values of ∆H˚, which are observed for PIPERAZINE and PIPES. Table 4 shows the value of ∆H˚ at 298.15 K for (PIPERAZINE) [2] is 53,390 J・mol−1 whereas for PIPES ∆H˚ = 11,964 J・mol−1. The explanation is that in addition to lowering the pK2, the hydroxymethyl and hydroxyethyl substitutions lower the value of ∆H˚ for isoelectric dissociation process [23] [24] . This pattern is
Table 4. Thermodynamic quantities for the dissociation of a series of structurally related compounds of Taurine, Morpho- line and Piperazine in water at 298.15 K.
Units: ∆H˚, J∙mol−1; ∆S˚, ∆Cp˚, J∙K−1∙mol−1.
also consistent for (TAURINE) [1] and its derivative (BES) [5] . The changes of entropy ∆S˚ from Table 4 are −33.9 J・K−1・mol−1 for (PIPERAZINE) [2] , whereas that for PIPES is −96.55 J・K−1・mol−1. The more negative values of ∆S˚ for PIPES compared to PIPERAZINE might indicate that for the dissociation process, the stabilization of the solvent structure causes an increased order (orientation) of polar water molecule in the proximity of the charged species (P±−, H+, P−2, Na+). The values of ∆Cp˚ for the dissociation of (PIPERAZINE) [2] is 88 J・K−1・mol−1 and that found for PIPES is 124 J・K−1・mol−1. Since the value of ∆Cp˚ for PIPES is more positive compared to PIPERAZINE, this represents a substantial change in the solvation patterns (water structure) for the dissociation process of PIPES. The charge type (electrostatic effects) appears to be the primary factor in determining the large positive P±− values of ∆Cp˚ [13] . The quantitative explanations for the interactions of Na+, Cl−, P±−, H+ and P−2 with water are complex.
5. Conclusion
The e.m.f data are stable and highly reproducible. The results of the second dissociation constant pK2 and associated thermodynamic quantities are very accurate and reliable. The pK2 value lies in the physiological region of pH 6 - 8. Thus buffer solutions of Na-PIPES and Na2-PIPES can be considered as a pH buffer standard for the physiological use. In a separate communication, pH values of buffer solutions without and with NaCl (isotonic saline media) from (278.15 - 328.15) K at an ionic strength I = 0.16 mol∙kg−1 will be reported as what was done for the physiological buffer (TAPSO) [25] .
Acknowledgements
The authors are grateful for the funding from the National Institutes of Health (NIH-AREA) under the grant 2R15GM66866-3. The content of this paper is the sole responsibility of the authors and does not necessarily represent the official views of the NIH of the National Institutes of the General Medical Science. R.N. Roy is indebted to the Hoffman Endowment Research Fund.
References
- King, E. (1952) The Ionization Constants of Taurine and Its Activity Coefficients in Hydrochloric Acid Solutions from Electromotive Force Measurements. Journal of the American Chemical Society, 75, 2204-2209. http://dx.doi.org/10.1021/ja01105a053
- Hetzer, H., Robinson, R. and Bates, R. (1968) Dissociation Constants of Piperazinium Ion and Related Thermodynamic Quantites from 0 to 50˚C. The Journal of Physical Chemistry, 72, 2081-2086. http://dx.doi.org/10.1021/j100852a034
- Vega, C. and Bates, R. (1976) Buffers for the Physiological pH Range: Thermodynamic Constants of Four Substituted Aminoethansulfonic Acids from 5 to 50˚C. Analytical Chemistry, 48, 1293-1295. http://dx.doi.org/10.1021/ac50003a010
- Roy, R.N., Mrad, D.R., Lord, P.A., et al. (1998) Thermodynamics of the Second Dissociation Constant and Standards for pH of 3-(N-Morpholino) Propanesulfonic Acid (MOPS) from 5 to 55˚C. Journal of Solution Chemistry, 27, 73-87. http://dx.doi.org/10.1023/A:1022692629289
- Roy, R.N., Gibbons, J.J., Krueger, C., et al. (1977) Second-Stage Dissociation of N,N-Bis(2-Hydroxyethyl)-2-Ami- noethane-Sulfonic Acid (BES) in Water and in 50 Mass% Methanol + Water from 278.15 to 328.15 K. Journal of Chemical Thermodynamics, 9, 325-332. http://dx.doi.org/10.1016/0021-9614(77)90053-2
- Roy, R.N., Moore, C.P., Carlsten, J.A., Good, W.S., et al. (1997) Second Dissociation Constants of Two Substituted Aminoethanesulfonic Acids (MES) and (TES) in Water from 5 to 55˚C. Journal of Solution Chemistry, 26, 1209-1216. http://dx.doi.org/10.1023/A:1022937324983
- Roy, R.N., Bice, J., Greer, J., et al. (1997) Buffers for the Physiological pH Range: Acidic Dissociation Constants of Zwitterionic Compounds (ACES and CHES) in Water from 5 to 55˚C. Journal of Chemical Engineering Data, 42, 41-44. http://dx.doi.org/10.1021/je960279s
- Good, N.E., Winget, G.D., Winter, W., et al. (1966) Hydrogen Ion Buffers for Biological Research. Biochemistry, 5, 467-477. http://dx.doi.org/10.1021/bi00866a011
- Roy, R.N., Gibbons, J.J., Padron, J.L. and Moeller, J. (1980) Second-Stage Dissociation Constants of Piperazine-N,N’- Bis(2-Ethanesulfonic Acid) Monosodium Monohydrate and Related Thermodynamic Functions in Water from 5 to 55˚C. Analytical Chemistry, 52, 2409. http://dx.doi.org/10.1021/ac50064a040
- Harned, H.S. and Ehlers, R.W. (1932) The Dissociation Constant of Acetic Acid from 0 to 35˚ Centigrade. Journal of the American Chemical Society, 54, 1350-1357. http://dx.doi.org/10.1021/ja01343a013
- Gary, R., Bates, R.G. and Robinson, R.A. (1964) Thermodynamics of Solutions of Deuterium Chloride in Heavy Water from 5 to 55˚C. Journal of Physical Chemistry, 65, 1186-1190. http://dx.doi.org/10.1021/j100787a037
- Sankar, M. and Bates, R.C. (1978) Buffers for the Physiological pH Range: Thermodynamic Constants of 3-(N-Mor- pholino)Propanesulfonic Acid from 5 to 55˚C. Analytical Chemistry, 50, 1922-1924. http://dx.doi.org/10.1021/ac50035a048
- Roy, R.N., Robinson, R.A. and Bates, R.G. (1973) Thermodynamic of the Two Dissociation Steps of N-Tris(Hydro- xymethyl)Methylglycine (“Tricine”) in Water from 5 to 55˚C. Journal of the American Chemical Society, 95, 8231- 8235. http://dx.doi.org/10.1021/ja00806a004
- Bates, R.G. (1973) Determination of pH. Wiley, New York, Chapter 10.
- Bates, R.G., Vega, C.A. and White, D.R. (1987) Standards for pH Measurements in Isotonic Saline Media of Ionic Strength I = 0.16. Analytical Chemistry, 50, 1295-1300. http://dx.doi.org/10.1021/ac50031a026
- Roy, R.N., Vogel, K.M., Good, C.E., et al. (1992) Activity Coefficients in Electrolyte Mixtures: HCl + ThCl4 + H2O for 5 - 55˚C. Journal of Physical Chemistry, 96, 11065-11072. http://dx.doi.org/10.1021/j100205a081
- Bates, R.G., Guggenheim, E.A., Harned, S.H., Ives, D.J.G, et al. (1956) Standard Electrode Potential of the Silver, Silver Chloride Electrode. Journal of Chemical Physics, 25, 361. http://dx.doi.org/10.1063/1.1742893
- Ives, D.J.G. and Moseley, P.G.N. (1975) Derivation of Thermodynamic Functions of Ionization from Acidic Dissociation Constants. Journal Chemical Society, 72, 1132-1143.
- Please, N.W. (1954) Estimation of the Variance of the Data Used in the Calculation of Dissociation Constants. Biochemistry Journal, 56, 196-201.
- Roy, R.N., Grant, J.G., Roy, L.N., Cummins, M.P., et al. (2002) Second Dissociation Constants of N-[4-Morpholi- no]Butanesulfonic Acid and N-[2-Hydroxymethyl]Piperazine-N’-4-Butanesulfonic Acid from 5 to 55˚C. Journal of Solution Chemistry, 31, 861-872. http://dx.doi.org/10.1023/A:1021459621374
- Feng, D., Koch, W.F. and Wu, Y.C. (1989) Second Dissociation Constant and pH of N-(2-Hydroxyethyl)Piperazine- N’-2-Ethanesulfonic Acid from 0 to 50˚C. Analytical Chemistry, 61, 1400-1405. http://dx.doi.org/10.1021/ac00188a019
- Roy, R.N., Roy, L.N., LeNoue, S.R., et al. (2005) Thermodynamic Constants of N-[Tris(Hydroxymethyl)Methly- 3-Amino]Propanesulfonic Acid (Taps) from the Temperatures 278.15 K to 328.15 K. Journal of Chemical Thermodynamics, 38, 413-417. http://dx.doi.org/10.1016/j.jct.2005.06.009
- Paabo, M. and Bates, R.G. (1970) Dissociation Constant of Protonated 2,2-Bis(Hydroxymethyl)-2,2’,2”-Nitrilotrie- thanol (Bis-Tris) and Related Thermodynamic Functions from 0 to 50˚. Journal of Physical Chemistry, 74, 702-705. http://dx.doi.org/10.1021/j100699a003
- Timimi, B.A. and Everett, D.H. (1968) The Thermodynamics of the Acid Dissociation of Some Amino-Alcohols in Water. Journal Chemical Society B, 1380-1386. http://dx.doi.org/10.1039/j29680001380
- Roy, L.N., Roy, R.N., Bodendorfer, B., Downs, Z., et al. (2011) Buffer Standards for the Physiological pH of the Zwitterionic Buffer 3-[N-Tris(Hydroxymethyl)Methylamino]-2-Hydroxypropanesulfonic Acid (TAPSO) from (287.15 to 328.15) K. Journal of Biophysical Chemistry, 2, 414-421. http://dx.doi.org/10.4236/jbpc.2011.24048


