Journal of Environmental Protection
Vol. 4 No. 7A (2013) , Article ID: 34107 , 9 pages DOI:10.4236/jep.2013.47A001
Investigation of Rotavirus Survival in Different Soil Fractions and Temperature Conditions
![]()
1Waterborne Environmental, Inc., Champaign, USA; 2Department of Pathobiology, University of Illinois, Urbana, USA; 3Department of Agricultural and Biological Engineering, University of Illinois, Urbana, USA.
Email: *pkalita@illinois.edu
Copyright © 2013 Paul C. Davidson et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 17th, 2013; revised June 12th, 2013; accepted July 5th, 2013
Keywords: Water Quality; Pathogens; Public Health; Fate and Transport
ABSTRACT
Rotavirus is a leading cause of gastrointestinal illness worldwide. Rotavirus transmission occurs fecal-orally, and becomes a critical water quality issue when soil and water resources are contaminated with feces. Transport of pathogens to surface water sources depends on their survival in the soil, especially considering the fact that large amounts of fecal material are often applied to agricultural lands as fertilizer. In this study, rotavirus survival was investigated in three different soil fractions and at three different temperatures (4˚C, 25˚C and 37˚C). A rotavirus suspension was mixed with whole soil, sand, and clay and allowed to incubate for up to 18 days. Samples were collected daily to investigate virus survival over time, which was quantified using a tissue-culture infectivity assay. Results indicated, in the absence of any soil particles, rotavirus survival was highest at 4˚C, with survival decreasing as temperature increased. These data also indicated whole soil had some protective effect, allowing rotavirus to survive better in soil for the entire range of temperatures and for more than a week at 37˚C. The results also showed that sand fractions were the most effective media for reducing rotavirus recovery at all temperature conditions tested. Although the mechanism responsible for the low recovery from sand is unknown, there is little or no infective rotavirus extracted from sand fractions. This finding strongly supports the use of sand as a filtering material to remove rotavirus from both point and nonpoint sources of water pollution.
1. Introduction
Preventing pathogens from entering drinking water supplies is of great importance worldwide. Eighty-eight percent of diarrheal disease in humans is attributed to unsafe water supply, inadequate sanitation and hygiene. In addition, it is estimated that 1.8 million people die every year from diarrheal diseases worldwide, 90% of whom are children under the age of five [1]. Prior to 2000, it was believed that rotaviruses caused approximately 22% of childhood diarrhea hospitalizations worldwide [2]. However, from 2000 to 2004, this proportion increased to 39% [3]. Application of this proportion to the recent World Health Organization estimates of diarrhea-related childhood deaths gave an estimate of 611,000 rotavirus-related deaths, and at least 100 deaths each year in the US [4]. Rotavirus infections are also of high agricultural importance because of the impact diarrheal disease can have on neonatal and post-weaning animals, especially pigs and calves. There is a lack of recent literature on the economic burden of rotavirus on livestock, but studies have found that morbidity due to rotavirus infections in pigs and calves can be as high as 80% with mortality reaching 60% [5,6]. High morbidity and mortality in livestock animals leads to economic losses from the loss of animals, treatment costs, and reduced growth rates.
Rotaviruses are transmitted mostly by the fecal-oral route: particles are passed in the feces of one host and ingested orally by another. A high degree of resistance to physical inactivation, the large number of virus particles shed, and the very low infectious dose required ensure that transmission easily occurs through environmental sources. For example, during a multi-island outbreak in the islands of Truk Atoll in the Pacific, rotavirus infections spread from the individuals initially infected to at least 31% of the population on one island within one week [7]. More recent rotavirus outbreaks include the outbreak in Nicaragua during February and March of 2005 and the outbreak in India between October 14 and December 17, 2005. The Nicaragua outbreak is one of the largest recorded outbreaks of severe acute gastroenteritis, affecting more than 64,000 individuals and causing at least 56 deaths, with children under the age of five being most affected [8]. Between October 14 and December 17, 2005, a total of 1783 cases of acute diarrheal disease were reported in Tangdar, India (population 65,000). The overall attack rate was 20% in children under 4 years of age following an earthquake. During that time, drinking stream water or tap water without boiling or chlorination may lead to a common source waterborne outbreak of rotavirus gastroenteritis. Other possible contributing factors include overcrowding, poor sanitation, open-air defecation, poor hygiene, and living in makeshift camps near streams [9].
Rotaviruses are highly infectious when transmitted within the same species. Replication within the intestinal tract can result in shedding of 1010 plaque forming units (PFU) per milliliter of feces. Rotaviruses are very durable in the environment and can survive for weeks in potable and recreational water [10]. The infectious dose for the human small intestine has been calculated as approximately 10 PFU per milliliter [11]. Rotaviruses show loss of infectivity at a pH less than 4, so the number that must be ingested to ensure passage of an infectious dose through the stomach is not known.
Rotaviruses can be transmitted to both humans and animals in various ways, including the use of manure as a fertilizer for food crops and by stormwater runoff that contains manure. Therefore, it is necessary to determine the survival and fate of viruses after the application of wastewater on land since secondary treatment of sewage does not remove all viruses present in domestic sewage [12,13]. Virus adsorption to soil is highly dependent on the virus strain, with differences in adsorption due to variability in the configuration of proteins in the outer capsid of the virus, since this can influence the net charge on the virus [14]. The net charge on the virus would affect the electrostatic potential between virus and soil, and thereby could influence the degree of interaction between the two particles.
Some studies have shown that soils with high clay content have a high adsorption capacity for viruses [12,15]. The association of viruses with clay minerals has been attributed to the large surface area and high cation exchange capacity (CEC) of clays. The mechanisms and sites of adsorption differ for different viruses and are influenced by characteristics of the clays such as anion exchange capacity (AEC), CEC, and AEC to CEC ratios [16]. Some researchers believe both positively and negatively charged sites on clay minerals are involved in virus adsorption.
In contrast, however, Blanc and Nasser (1996) found that, in general, virus adsorption to sandy soil was greater than to loamy soil [17]. They attribute the lower adsorption of viruses to loamy soil to the high content of organic material in the soil. Natural organic matters are notoriously heterogeneous because they contain different amounts and types of functional groups, and therefore, vary in their hydrophilic and hydrophobic properties. Organic matter sorbed on soil particles can provide additional negative charges that repulse viruses or cover positively charged sites, which may decrease the electrostatic interactions between viruses and soil particles. Similar finding was reported by Davis et al. (2006) [18]. They performed batch and column studies and found that dairy manure wastewater (high organic matter content) decreased viral adsorption to sandy soil. Furthermore, they found that dairy manure wastewater increased the release of viruses attached to soil. Zhuang and Jin (2003) found that as a general trend, the effect of organic matter was dominated by electrostatic rather than hydrophobic interactions. In no case did changes in pH influence virus adsorption or desorption [19].
The objective of this study was to understand the fate and transport of rotavirus in the environment. Since the survival of rotavirus in soil and soil components has not been previously studied, this research focused on developing the necessary methods for extracting and analyzing rotavirus in the soil-water environment. Since it is not clear which soil fraction (sand of clay) plays more important role for adsorption of rotaviruses in soil, our investigation focused on the kinetics of survival of infective rotavirus in these two fractions as well as whole soil at three temperatures ranging from 4˚C to 37˚C. The results of this research are important because they contribute 1) to an improved understanding of the risks of rotavirus transmission due to environmental contamination and survival, 2) to the design of systems to control its overland transport in natural animal agricultural environments, and 3) to providing sustainable best management practices for animal producers.
2. Materials and Methods
2.1. Soil Particle Separation
A moderately drained silt-loam (Catlin series, mesic Oxyaquic Argiudolls: 24% sand, 50% silt, 26% clay) was collected from Champaign, IL. The soil was oven-dried at 105˚C for 24 hours to reduce microbial contamination and to allow for easier separation of soil particles. The soil was sifted using a 3 mm screen to eliminate large organic matter and rocks and provide a more uniform soil.
To prepare specific soil fractions, the 3 mm sifted soil sample was then passed through a series of finer sieves. The soil was placed in the top sieve and left on an auto sifter for 30 minutes. The bottom collection pan contained the combination of silt and clay. The sieve openings correspond to the USDA classification of soil fractions according to the particle diameter. None of the samples contained any particles that were classified as gravel. Soil samples were separated into six categories using the sieves; very course sand (1 mm), coarse sand (500 µm), medium sand (250 µm), fine sand (106 µm), and very fine sand (50 µm). Everything that passed through the 50 µm sieve was classified as silt and clay. For all experiments in this study, the sand fraction was purified sand (combination of fine and very fine sand fractions) obtained from a local source. The purified sand was used in place of the Catlin sand fractions to reduce variations in the size of sand fractions being examined, and eliminate organic matter that may have been attached to the Catlin sand fractions.
The silt and clay particles were then separated using the principles of Stokes’ Law based on the settling time of the clay particles (diameter of 2 µm or less). The silt and clay mixture was placed in a beaker with distilled water and stirred for 30 seconds using a standard blender to dissociate the particles as much as possible. The clay and silt mixture was then allowed to settle for approximately seven hours, since this time is in excess of the necessary time for silt to settle, but less than the time needed to settle out clay particles. Since the settling time of clay is longer than that of silt, the clay was suspended in distilled water while the silt had already settled to the bottom. The suspended clay was transferred to another container, and both the clay and the silt were oven-dried. This process was repeated as necessary to obtain sufficient material for the experiments.
2.2. Rotavirus Preparation
Group A Porcine rotavirus OSU strain (P9 [7], G5), obtained from the American Type Culture Collection catalog # VR-892, was passaged two additional times in cultured Ma104 cells [20]. Infectious units of activity were determined in a focus forming assay (FFU) as described by Rolsma et al. (1998) [21]. Three colostrum-deprived newborn piglets were inoculated with 1 mL (3 × 106 FFU) each and the feces were pooled and collected over a two day period. The combined fecal material was homogenized with a Dounce homogenizer with an equal volume of Vertrel XF organic cleaning agent (Miller-Stephenson) to enhance rotavirus separation from fecal debris. After centrifugation to separate layers, the aqueous top layer was centrifuged at 13,000 × g to pellet debris. The supernatant was centrifuged at 180,000 × g to pellet the rotavirus. The pellet containing the rotavirus was suspended in TNC (50 mM TRIS, 150 mM NaCl, and 10 mM CaCl2, pH 7.4) buffer and diluted to approximately 1 - 2 × 105 FFU per ml.
2.3. Experimental Setup
Methods developed by Rolsma et al. (1994; 1998) for measuring rotavirus infectivity were modified to allow assessment of active rotavirus survival in soil [20,21]. The Catlin soil was the aggregate used for the rotavirus survivability experiments, with each of the three soil fractions (whole soil composition, sand fractions, and clay fractions) being incubated at three different temperatures (4˚C, 25˚C and 37˚C). The aggregate Catlin soil is referred to as “whole soil” from this point forward. The whole soil, sand, or clay was saturated to allow for maximum contact between rotavirus and the solid particles. In each experimental setup, a series of samples was collected over time. Individual incubation tubes were uniformly prepared, and the rotavirus was extracted from a single tube each time a sample was collected, including a time zero point taken immediately after addition of the virus suspension.
2.4. Soil and Sample Preparation
A series of tubes, approximately 2.2 g each (corresponding to 2 cm3) of whole soil was weighed and placed into a 15 mL polystyrene conical tube. Then, the dry whole soil was saturated with 1 mL of virus suspension in aqueous buffer and mixed thoroughly, producing an almost viscous uniformly moist form with no excess water above the soil. The virus suspension is a combination of the partially purified rotavirus and a TNC solution, simulating conditions typically present in manure. The soil and virus were mixed thoroughly by stirring with a wooden applicator stick to allow the virus suspension to fully infiltrate the soil. After preparation, the tubes were capped and stored at the appropriate temperature until collection time point.
The sample tube preparation for the sand and clay fractions was similar to the method as described above for whole soil, except that 3.4 g (corresponding to 2 cm3) of sand and 750 µL of virus suspension, which resulted in a uniformly moist near viscous condition as described for the whole soil were used for the sand experiment. Because of limiting amounts of pure clay obtainable, the clay tube set up was scaled down to use a polypropylene microfuge tube, containing 0.1 g pure clay and 75 µL of virus suspension which gave a uniformly moist near viscous condition as described for clay and whole soil.
2.5. Extraction of Rotavirus
At the time of sampling, 1 mL of TNC was added to the tube containing whole soil and virus suspension and the contents mixed thoroughly. The tube was then centrifuged at 4 degrees at 1260 × g. The aqueous supernatant was then removed from the tube. This extraction was repeated twice for a total of 3 mL of extracted virus. Similarly, the sand and clay virus mixtures were extracted except that 0.75 mL and 0.1 mL respectively were used for each of the three extractions.
2.6. Focus Forming Unit (FFU) Assay
The focus forming unit (FFU) virus infectivity assay was performed following previously published methods [21]. Briefly, virus extracts from whole soil, sand, or clay, and virus alone (in the absence of any soil components) time point samples were pretreated at a final concentration of 10 µg/mL trypsin for 30 min at 37˚C to increase attachment to and infection of the test Ma104 cells. Confluent monolayers of Ma104 cells in either 24-well (for whole soil or sand) or 96-well (for clay) plates were rinsed twice with phosphate-buffered saline (PBS). One-tenth milliliter (25 µL for clay) of appropriately diluted trypsin-treated extract or virus alone sample was added to each well. Following a 30 minute attachment incubation period at 37˚C, the cells were rinsed once and 1 mL of serum free MEM (24 well plates) or 250 µL serum free MEM (96 well plates). The plates were incubated at 37˚C in a 5% CO2 incubator overnight.
Following overnight incubation and removal of media, the infected cells in the wells were fixed with methanol: acetic acid and processed for immunochemistry as described [21]. Immunochemically stained focus forming units (FFU) resulting from virus infection were quantified as described below.
2.7. Quantification of Rotavirus Infectivity
The rotavirus FFUs were quantified by counting the number of FFUs present in a given sample. The FFUs (stained viral-antigen-positive cells) were quantified at a magnification of × 100 using a Nikon TS 100 inverted microscope equipped with a computer-controlled electronic stage and Spot RT-slider CCD camera. Twenty five digital images were automatically collected from each plate well using Metamorph software (Molecular Devices, Inc) to develop and capture images within a scan grid which covered >80% of the well surface area. The number of FFU in each of these images were then counted either by hand or automatically using integrated morphometric parameters within the software to recognize FFU. The ability to automatically count FFU was dependent on the degree of background staining which varied between different antibody lots.
2.8. Statistical Analysis
A series of laboratory experiments were conducted to study rotavirus survival in three soil fractions (Catlin soil, sand and clay) for three temperature conditions (4˚C, 25˚C, and 37˚C). An open source package R (R Project, http://www.r-project.org/) was used to perform the statistical analyses for rotavirus survival. The main effects and possible interactions between the variables were considered. In order to determine the most significant parameters that affect rotavirus survival, analysis of variance (ANOVA) was conducted using the general linear model. The parameters were evaluated using a 5% (p = 0.05) significance level.
3. Results
The recovery of infective rotavirus from three different types of soil fractions at varying times following incubation at different temperatures was measured. The range of temperatures (4˚C - 37˚C) selected spans typical conditions under which rotavirus overland transport occurs. The variation in the data from this study is represented by error bars. Variations can be attributed to sampling and handling, but most likely to the variation in the FFU assay. While the Ma104 cells are grown following uniform methodology each time, there may be differences in the confluence of the cells from one assay to the next. At 4˚C, the best-fit line is shown as a horizontal line at 100% because the percent recovery values of greater than 100% are assumed to be due to aforementioned variations in the preparation of the assay, and the assay itself. Unless otherwise stated, the survival of rotavirus refers to the percent recovery of viable, infective rotavirus particles and thus measures the percent of infective rotavirus that can be extracted from the soil or soil components. Rotavirus that remains in the soil may or may not remain viable. However, if the rotavirus cannot be extracted using the extraction process detailed above, it is unlikely that it would be transported in a natural soil environment, and therefore is not considered to be a threat to human or animal health.
3.1. Statistical Analysis Result
Statistical results indicate that the primary source of variation was sand % (p < 2e−16), followed by clay % (p = 4.38e−12). Among the interaction terms, Sand % × Clay %, Sand % × Temperature, Clay % × Time, Clay % × Temperature and Temperature × Temperature were found to be other sources of variation in rotavirus infectivity. The individual effects of time and temperature did not play a considerable role in rotavirus infectivity. Even though true replications were not possible in this study, this general statistical analysis clearly indicates that soil type plays a significant role in rotavirus infectivity. The results of the statistical analysis are presented in Table 1.
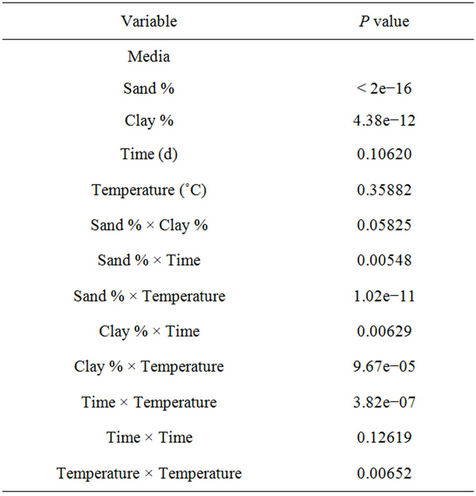
Table 1. Statistical analysis for rotavirus recovery for different soil and vegetation conditions.
3.2. Rotavirus Survival at 4˚C
The lowest temperature examined in this study was 4˚C, since soil infiltration and thin-layer mixing (mixing of surface runoff with the top layer of soil) at lower temperatures is unlikely due to freezing. Rotavirus survives well at 4˚C in relatively concentrated suspensions of both pure and non-pure (feces) forms. Therefore, the first experiments compared the survival of rotavirus in TNC solution versus in soil at 4˚C (Figure 1). Survival in TNC serves as the control for all soil or soil component survival experiments.
At 4˚C, rotavirus infectivity remained nearly constant at 100% recovery over 18 days when incubated in the absence of any soil (in TNC). When combined with whole soil, recovery of infective virus was initially very low, but increased over time until reaching approximately 90% at day 10 and then beginning to decline from day 15 - 18. The recovery of viable rotavirus from sand is initially relatively high (60%), but then decreases gradually with time. The percent recovery plateaus at approximately 15%, which suggests that some rotavirus particles survived for at least two weeks in sand at 4˚C. Clay initially had a low recovery for rotavirus at 4˚C, but the percent recovery increased over time before stabilizing at approximately 50%.
Both the whole soil and clay showed a reduction in recovery of viable virus when compared to incubation in TNC. When mixed with whole soil, the rotavirus recovery peaked at approximately 90%, while the rotavirus recovery in clay peaked at approximately 40%. However, the recovery in whole soil and clay was similar in that it
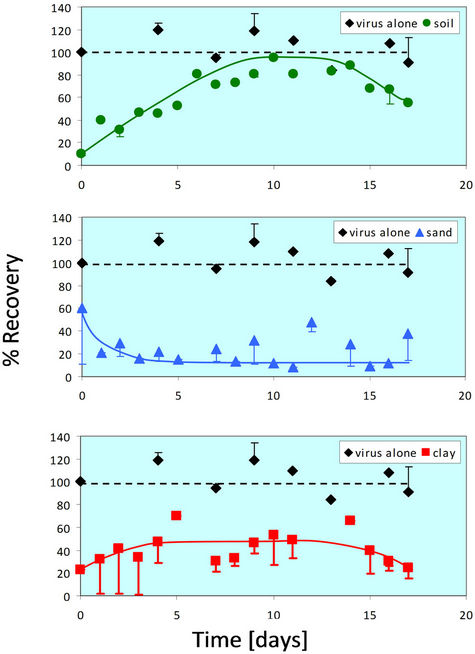
Figure 1. Percent recovery of virus alone and in the presence of soil particles at 4˚C. The virus alone trend is shown as a dotted line because a greater than 100% recovery is not possible, and any error present was attributed to the FFU assay itself. The 4˚C experiment was done in duplicate for all four conditions (virus alone, soil, sand, and clay). Error bars represent +/− one standard deviation from the mean.
shows an initial increase, plateaus, and then begins a slow decrease after about 14 days. This is in contrast to the recovery of rotavirus in sand, which shows an initial high recovery, but quickly decreases until plateauing at approximately 15% after 5 days.
3.3. Rotavirus Survival at 25˚C
In the absence of any soil particles at 25˚C, the survival of rotavirus is initially 100% (similar to that at 4˚C), but after two days, the survival begins to decline (Figure 2). The recovery of virus alone (in the absence of any soil components) appears to level off at approximately 25%. When combined with whole soil, rotavirus percent recovery is initially very low as seen in the 4˚C case. Over time, the rotavirus again is more easily extracted, remaining constant at a recovery rate of approximately 50% after five days. After about 6 days, it can be seen that rotavirus has a higher percent recovery in whole soil than alone. Rotavirus responds nearly identically in sand at 25˚C as it did at 4˚C. Rotavirus percent recovery re-
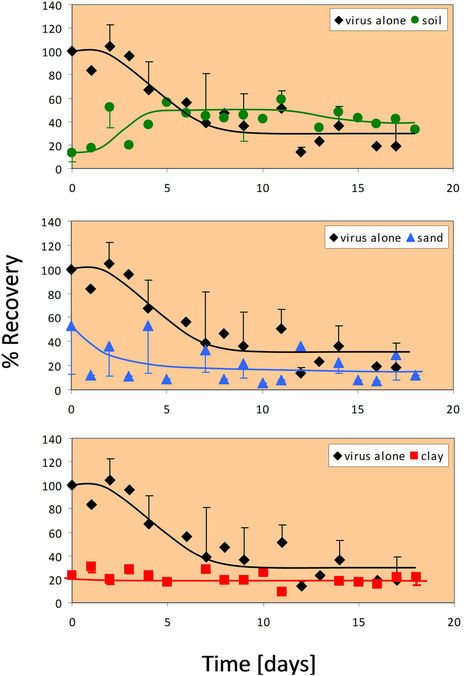
Figure 2. Percent recovery of virus alone and in the presence of whole soil at 25˚C. The 25˚C experiment was done in duplicate for all four conditions (virus alone, whole soil, sand, and clay). Error bars represent +/− one standard deviation from the mean.
mains nearly constant in clay at 25˚C. Only about 20% of the initial rotavirus is recovered from any one day up to eighteen days in the presence of clay fractions. At 25˚C, rotavirus recovery in clay is more similar to the recovery in sand, rather than whole soil as seen at 4˚C. However, after two days the recovery is higher in clay than in sand, which agrees with the findings of the 4˚C experiment.
3.4. Rotavirus Survival at 37˚C
The maximum temperature tested in this study was 37˚C for the rotavirus survival experiments. This temperature is close to the upper limit of temperatures experienced in the Midwestern United States during hot summer months. As expected, the die-off rate for rotavirus at this temperature was much quicker than at the other two temperatures.
Figure 3 shows that at 37˚C, rotavirus by itself dies off rather quickly. The graph is similar to an exponential decay. After only seven days, the percent recovery is negligible at approximately 2% or less. At 37˚C in the presence of whole soil, rotavirus again appears to survive
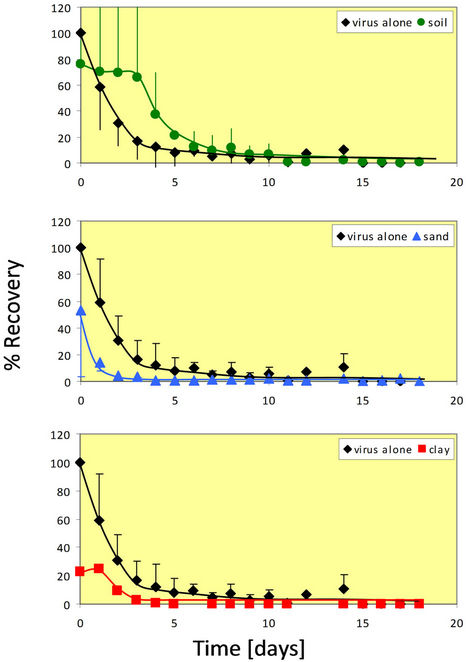
Figure 3. Percent recovery of virus alone and in the presence of whole soil particles at 37˚C. The 37˚C experiment was done in duplicate for all four conditions (virus alone, whole soil, sand, and clay). Error bars represent +/− one standard deviation from the mean.
better than alone. For almost one week, rotavirus in whole soil has a higher percent recovery than the rotavirus alone.
The results for sand at 37˚C were similar to the results of the whole soil. The percent recovery is initially quite high, but decreases very quickly before reaching zero by the second day. In sand, the virus is no longer infectious after only two days. In addition, for sand, it appears that the extractability is better at 37˚C than at 4˚C or 25˚C. This can also be said for clay. For the lower temperatures, the rotavirus percent recovery started out low and increased over time. However, it can be seen that rotavirus percent recovery in clay is initially 20% and then decreases to zero in three days. The percent recovery is always lower with the clay than the virus alone.
At 37˚C, whole soil reacts quite differently than at the two lower temperatures. In the other two cases, there was a lower percent recovery initially, with it increasing and eventually leveling off. In this case, however, the percent recovery is very high initially (78%) before gradually decreasing and eventually approaching zero. In the presence of sand, the rotavirus recovery is very similar to that seen at 4˚C and 25˚C. At no point does the rotavirus percent recovery in sand exceed that of the virus alone. The virus in the presence of clay reacts similar to that in the presence of sand, but the viability in sand diminishes more quickly (at least one day sooner). These findings are in contrast to those found with the whole soil in which rotavirus viability remains for more than a week.
4. Discussion
Prior to this study, we hypothesized that rotavirus would not survive in soil considering the lack of a suitable host and the potential harsh environmental conditions the soil may present to the virus. The results, however, show that viable rotavirus is capable of surviving in different soil conditions.
These results indicate that rotavirus by itself may survive for extended periods of time at 25˚C. At 37˚C, rotavirus infectivity, in the absence of any soil particles, declines rather quickly. After only seven days, the percent recovery is negligible (£2%). This is in contrast to results obtained at the other two temperatures where recovery of rotavirus infectivity was 100% at 4˚C and approximately 25% after eighteen days at 25˚C.
The initial low recovery in whole soil at 4˚C and 25˚C is most likely due to inability to extract the virus at these early times. The whole soil may bind or adsorb rotavirus very tightly during an initial equilibrium period before gradually allowing it to dissociate from the soil particles and be extracted. At 25˚C, the percent recovery remains constant at approximately 50%. This indicates that the soil may have a thermal protective effect as the virus survives better in the whole soil than by itself following the equilibration period. This result is in contrast to those obtained for sand, in which rotavirus is either inactivated or unable to be extracted from the sand particles. At 37˚C, rotavirus in whole soil survives better than rotavirus in the absence of soil particles for an entire week. In addition, at 37˚C, rotavirus recovery is very high initially for the whole soil before gradually decreasing and approaching zero. This is in contrast to trends at 4˚C and 25˚C, where rotavirus recovery was initially low and increased over time before leveling off. Therefore, rotavirus may not adsorb as tightly to the whole soil as seen previously at lower temperatures.
While the virus appears to survive much better when incubated in the absence of soil components than when mixed with sand, it is unclear if the sand is directly inactivating the virus. If so, the virus would likely not remain constant at 15%, but would decrease to zero. However, since sand has relatively large individual pores, it could be proposed that the 15% of the rotavirus that remains viable is never actually interacting with the sand particles: this fraction of virus may be recovered only because the sand never directly contacts it after the initial stirring. It is important to consider, though, that the viruses may not be homogeneous, with some more resistant than others. It is also possible that the amount of rotavirus surviving in sand is actually higher than 15%. The virus could be getting trapped between the sand particles; not adsorbed, but physically trapped. The rotavirus particles contain spikes (VP 4) on its outer capsid and the rough surface of the sand particles may cause the particles to become tangled, preventing extraction and producing a lower percent recovery. The decrease in recovery from 25˚C to 4˚C in sand may be because rotavirus is more easily trapped in the particles at 4˚C than at 25˚C. It is possible that VP 4 could bind directly to the sand particles, but unlikely considering the neutral surface charge of the sand particles. For 25˚C, sand continues to hinder the recovery of viable rotavirus particles. At 37˚C and in the presence of sand, the percent recovery is initially quite high, but decreases very quickly before reaching zero by the second day. At no point does the rotavirus percent recovery in sand exceed that of the virus alone. These findings are in contrast to those found with the whole soil in which rotavirus viability remains for more than a week. In sand, the virus is no longer infectious after only two days. For all three temperatures and in the presence of sand, it is unclear whether the virus is inactivated, being trapped amongst sand particles, or if this trend is an effect of the viruses not being homogeneous, with some more resistant than others. Regardless, this finding could have important implications for removal of rotavirus from both municipal wastewater and runoff from animal feeding operations.
For the recovery of rotavirus in clay, it can be argued that the data follows a biphasic trend at 4˚C. This could be due to the rotavirus not being uniformly equilibrated in each sample, causing differences in the extractability of rotavirus from one time point to the next. Clay has a higher overall porosity than sand, but much smaller individual pores, possibly hindering the percolation of water into the clay samples. The reason for this fluctuation will be investigated further in future studies. In addition, the low initial recovery could be similar to that seen in whole soil, with a high initial adsorbance and then desorption of the virus as time progresses. At 25˚C and in the presence of clay, only about 20% of the initial rotavirus is recovered from any one day up to eighteen days. Initially, it appears that clay is inhibiting the survival of rotavirus, as the virus alone survives much better than in clay. However, as in the 4˚C case and with the whole soil, the rotavirus seems to be adsorbing to the clay particles. Therefore, there may be a threshold (~20% for this case) at which rotavirus cannot be extracted from clay particles. At 37˚C and in the presence of clay, it can be seen that rotavirus percent recovery is initially 20% and then decreases to zero in three days. The percent recovery is always higher for the virus alone than the virus in the presence of clay. The virus in the presence of clay reacts similar to that in the presence of sand, but the viability in sand diminishes more quickly (at least one day sooner).
Overall, it appears that rotavirus is better able to survive in whole soil and clay than sand, and survives better at 4˚C than 25˚C or 37˚C. This makes sense since the Catlin soil used in this study contains a high percentage of clay. Sand has the lowest percent recovery of any fraction tested. The reason for the low recovery of viable rotavirus particles is unclear, but regardless, infective rotavirus is not leaving the sand under these conditions. This may have important implications when considering agricultural best management practices (BMP), such as a vegetative filter strip (VFS) for rotavirus control.
The overall goal of this study was to determine if rotavirus could even survive in the presence of whole soil and individual soil fractions. Contrary to initial beliefs, the results show that rotavirus is actually able to survive quite well in some cases, for more than eighteen days at 4˚C and 25˚C, perhaps much longer. On the other hand, the results also indicate that rotavirus is not easily recovered from sand. Even in the cases where rotavirus was recovered, the percent recovery was quite low in comparison with whole soil, clay, and the virus alone.
As previously stated, these findings have implications for agricultural BMPs as well as water treatment processes. The knowledge that sand inhibits rotavirus survival could promote the implementation of a water pretreatment process including the incorporation of sand filtration. Previous research has shown that the clay minerals are most critical for the attachment of viruses to soil [16]. The results of this study clearly show, however, that sand particles reduce the recovery of rotavirus more than the clay particles. The results of this study are in agreement with Blanc and Nasser (1996) who found that, in general, virus adsorption to sandy soil was greater than to loamy soil. They attribute the lower adsorption of viruses to loamy soil to the high content of organic material in the soil. The results of this study are also in agreement with Davis et al. (2006) who performed batch and column studies and found that dairy manure wastewater (high organic matter content) decreased viral adsorption to sandy soil. The findings of this study do not completely eliminate clay particles as a source of rotavirus adsorption, but sand appears to be the dominant soil component responsible for limiting the recovery of infective rotavirus from soil. Although there is occasionally variation in virus recovery in replicate samples, the data demonstrate clear differences in the kinetics of infective virus survival in different soil types and at different temperatures. Therefore, the use of sandy soils to construct vegetated filter strips may be more effective in reducing overland transport from animal agriculture facilities than high clay soils. We are currently testing this assumption by conducting overland transport experiments using soil beds differing in percent clay and sand content as well as surface vegetation types. The results of these experiments, combined with those reported above should help in designing optimal vegetated filter strips to reduce or eliminate rotavirus overland transport.
5. Conclusions
Experimental results have shown that rotavirus remains viable in whole soil, sand, and clay under certain experimental conditions. Whole soil appears to have a protective characteristic that improves rotavirus survival at relatively high temperatures (37˚C). Both whole soil and clay showed a lower percent recovery of rotavirus in early time periods, probably due to an initial tight adsorption of the rotavirus to the clay particles, with the virus being somewhat desorbed as time progresses. After the initial poor recovery, rotavirus was found to survive slightly better in whole soil than by itself at 25˚C, therefore further confirming that whole soil is somewhat thermal protective for rotavirus.
Sand fractions were found to inhibit rotavirus survival in every temperature tested. Rotavirus remains viable in sand only at early time points of the experiment. It is possible that any or all of the following could be occurring: the virus is being inactivated by the sand, is being trapped amongst sand particles, or the viruses are not homogeneous (some viruses more resistant than others). From this study, it can not be concluded that the sand is toxic or killing the virus. However, considering the large individual pore spaces in sand fractions, the 15% of viable rotavirus that is recovered may never actual come into direct contact with the sand particles. Even though the exact reason for the low recovery in sand in unknown, infective rotavirus is incapable of escaping the sand. This finding strongly supports the use of sand as a filtering material for rotavirus from both point and nonpoint sources of water pollution.
6. Acknowledgements
This work has been funded in part by a grant (ILLV-44- 6751) from the USDA NRI CSREES Water and Watersheds Program.
REFERENCES
- World Health Organization (WHO), “Water, Sanitation and Hygiene Links to Health. FACTS AND FIGURES,” Geneva, 2004.
- U. D. Parashar, E. G. Hummelman, J. S. Bresee, M. A. Miller and R. I. Glass, “Global Illness and Deaths Caused by Rotavirus Disease in Children,” Emerging Infectious Diseases Journal, Vol. 9, No. 5, 2003, pp. 565-572. doi:10.3201/eid0905.020562
- U. D. Parashar, C. J. Gibson, J. S. Bresee and R. I. Glass, “Rotavirus and Severe Childhood Diarrhea,” Emerging Infectious Diseases Journal, Vol. 12, No. 2, 2006, pp. 304-306. doi:10.3201/eid1202.050006
- Center for Disease Control (CDC), “Are You RotavirusReady?” The National Healthy Mothers Healthy Babies Coalition, Atlanta, 2005.
- K. Dhama, R. S. Chauhan, M. Mahendran and S. V. Malik, “Rotavirus Diarrhea in Bovines and Other Domestic Animals,” Veterinary Research Communications, Vol. 33, No. 1, 2009, pp. 1-23. doi:10.1007/s11259-008-9070-x
- B. Dodet, E. Heseltine and P. Saliou, “Rotaviruses in Human and Veterinary Medicine,” Trends in Microbiology, Vol. 5, No. 3, 1997, pp. 176-178. doi:10.1016/S0966-842X(97)01045-7
- S. O. Foster, E. L. Palmer, G. W. Gary Jr., M. L. Martin, K. L. Herrmann, P. Beasley and J. Sampson, “Gastroenteritis Due to Rotavirus in an Isolated Pacific Island Group: An Epidemic of 3439 Cases,” Journal of Infectious Diseases, Vol. 141, No. 1, 1980, pp. 32-39. doi:10.1093/infdis/141.1.32
- F. Bucardo, B. Karlsson, J. Nordgren, M. Paniagua, A. Gonzalez, J. J. Amador, F. Espinoza and L. Svensson, “Mutated G4P Rotavirus Associated with a Nationwide Outbreak of Gastroenteritis in Nicaragua in 2005,” Journal of Clinical Microbiology, Vol. 45, No. 3, 2007, pp. 990-997. doi:10.1128/JCM.01992-06
- S. Karmakar, A. S. Rathore, S. M. Kadri, S. Dutt, S. Khare and S. Lal, “Post-Earthquake Outbreak of Rotavirus Gastroenteritis in Kashmir (India): An Epidemiological Analysis,” Public Health, Vol. 122, No. 10, 2008, 981-989. doi:10.1016/j.puhe.2008.01.006
- S. A. Ansari, V. S. Springthorpe and S. A. Sattar, “Survival and Vehicular Spread of Human Rotaviruses: Possible Relation to Seasonality of Outbreaks,” Reviews of Infectious Diseases, Vol. 13, No. 3, 1991, pp. 448-461. doi:10.1093/clinids/13.3.448
- D. Y. Graham, G. R. Dufour and M. K. Estes, “Minimal Infective Dose of Rotavirus,” Archives of Virology, Vol. 92, No. 2-3, 1987, pp. 261-271. doi:10.1007/BF01317483
- C. P. Gerba, J. L. Melnick and C. Wallis, “Fate of Wastewater Bacteria and Viruses in Soil,” Journal of the Irrigation and Drainage Division, Vol. 101, No. 3, 1975, pp. 157-174.
- A. W. Hoadley and S. M. Goyal, “Public Health Implications of the Application of Wastewater to Land,” In: R. L. Sanks and T. Asano, Eds., Land Treatment and Disposal of Municipal and Industrial Wastewater, Ann Arbor Science, Michigan, 1976, pp. 101-132.
- S. M. Goyal and C. P. Gerba, “Comparative Adsorption of Human Enteroviruses, Simian Rotavirus, and Selected Bacteriophages to Soils,” Applied and Environmental Microbiology, Vol. 38, No. 2, 1979, pp. 241-247.
- G. Bitton, O. C. Pancorbo, A. R. Overman and G. E. Gifford, “Retention of Viruses during Sludge Application to Soils,” Progress in Water Technology, Vol. 10, 1978, pp. 597-606.
- M. Schiffenbauer and G. Stotzky, “Adsorption of Coliphages T1 and T7 to Clay Minerals,” Applied and Environmental Microbiology, Vol. 43, No. 3, 1982, pp. 590- 596.
- R. Blanc and A. Nasser, “Effect of Effluent Quality and Temperature on the Persistence of Viruses in Soil,” Water Science and Technology, Vol. 33, No. 13, 1996, pp. 237- 242. doi:10.1016/0273-1223(96)00425-8
- J. A. Davis, S. R. Farrah and A. C. Wilkie, “Adsorption of Viruses to Soil: Impact of Anaerobic Treatment,” Water Science and Technology, Vol. 54, No. 3, 2006, pp. 161-167. doi:10.2166/wst.2006.464
- J. Zhuag and Y. Jin, “Virus Retention and Transport as Influenced by Different Forms of Soil Organic Matter,” Journal of Environmental Quality, Vol. 32, No. 3, 2003, pp. 816-823.
- M. D. Rolsma, H. B. Gelberg and M. S. Kuhlenschmidt, “Assay for Evaluation of Rotavirus-Cell Interactions: Identification of an Enterocyte Ganglioside Fraction That Mediates Porcine Group A Porcine Rotavirus Recognition,” Journal of Virology, Vol. 68, No. 1, 1994, pp. 258- 268.
- M. D. Rolsma, T. B. Kuhlenschmidt, H. B. Gelberg and M. S. Kuhlenschmidt, “Structure and Function of a Ganglioside Receptor for Porcine Rotavirus,” Journal of Virology, Vol. 72, No. 11, 1998, pp. 9079-9091.
NOTES
*Corresponding author.

