Journal of Environmental Protection
Vol. 3 No. 2 (2012) , Article ID: 17439 , 6 pages DOI:10.4236/jep.2012.32017
Polybrominated Diphenyl Ethers and Polychlorobiphenyls in Fish from the Ionian Sea (Western Mediterranean)
![]()
Department of Environmental Sciences “G. Sarfatti”, University of Siena, Siena, Italy.
Email: silvanofocardi@alice.it
Received December 11th, 2011; revised January 10th, 2012; accepted February 4th, 2012
Keywords: PBDEs; PCBs; Fish Muscle; Western Mediterranean
ABSTRACT
This study reports on the accumulation of polybrominated diphenyl ethers (PBDEs) and polychlorobiphenyls (PCBs) in the muscle tissue of 11 species of fish from the Ionian Sea (Western Mediterranean). The results are consistent with previous studies that have reported now-generalized contamination by PCBs of the Mediterranean marine environment, as well as widespread diffusion of emerging contaminants such as polybrominated diphenyl ethers (PBDEs). The Western Mediterranean Sea, from which the 11 fish species were collected, receives a heavy pollutant input from the urban and industrial areas along its coasts. Higher values of PCBs (average over 1.5 mg/kg f.w.) were observed in pelagic top predators (little tuna, bluefin tuna and swordfish). These same species also showed higher levels of PBDEs, averaging above 0.5 ng/g f.w. This puts a few fish species at the top of the food chain—such as bluefin tuna and swordfish—at particular risk, and the importance of these species in the human diet suggests the need for particular care in our food choices.
1. Introduction
The Mediterranean Sea receives a heavy pollutant input from the urban and industrial areas along its coasts, and from cultivated land through rivers. The United Nations Environment Programme has estimated that in recent decades, 650 million tons of sewage, 129,000 tons of mineral oil, 100,000 tons of polychlorobiphenyls, 60,000 tons of mercury, 3800 tons of lead and 36,000 tons of phosphates have been dumped into the Mediterranean basin each year. Meanwhile, 70 per cent of the wastewater dumped into the Mediterranean is untreated. The sea is also a major oil transportation route, and up to one million tons of crude oil are discharged annually as a result of accidental spills, illegal bunkering and tank cleaning practices, as well as inadequate harbour facilities. Pollution also reaches the Mediterranean through its major river systems—the Po, the Ebro, the Nile, and the Rhone —which carry substantial amounts of agricultural and industrial wastes. As the Mediterranean is almost entirely landlocked, its waters have a very low renewal rate of 100 years [1]; this limited water exchange increases the chemical residence time and allows bioaccumulation, making the organisms excessively sensitive to pollution [2-7].
Among the contaminants that have caused serious problems in recent years for Mediterranean ecosystems, there are certainly a few persistent organic pollutants (POPs), in particular the polychlorobiphenyls (PCBs) [8- 10]. The Stockholm Convention recognized that POPs are chemical substances that persist in the environment, bioaccumulate through the food web, and pose a risk of causing adverse effects to human health and the environment. They include emerging pollutants like brominated flame retardants (polybrominated diphenyl ethers, PBDEs) which also show bioaccumulation, biomagnification, and toxic properties that have already been reported by numerous authors [7,11-13]. As a consequence of their increasing use, environmental levels of PBDEs have risen since their first application, and recent studies have reported that PBDE concentrations are increasing in the environment [14] and in animal and human tissues [3, 7,15-17]. In some areas PBDE levels in wildlife and humans have surpassed the levels of PCBs [18]. Rising concentrations of PBDEs have also been found in polar regions [19,20]. The most significant contributor to the dietary PCB and PBDE intake in humans is fish and seafood [13]. For this reason, this study reports on the accumulation of nineteen congeners of PBDEs and 43 congeners of PCBs in several species of fish from the Ionian Sea (Western Mediterranean).
2. Materials and Methods
2.1. Study Area and Samples
Various samples of 11 fish species (Mullus barbatus, Merluccius merluccius, Solea vulgaris, Helicolenus dactylopterus, Polyprion americanum, Lophius piscatorius, Scorpaena scrofa, Euthynnus alletteratus, Seriosa dumerili, Xiphias gladius and Thunnus thynnus) caught in May and November 2007 off the coasts of Sicily in the Ionian Sea (Figure 1) were analysed. Samples were wrapped in aluminum foil previously cleaned with solvents (acetone and n-hexane), and stored at –20˚C until assay.
2.2. PCBs
Aliquots of tissues stored at –20˚C from a pool of several samples were analysed, following the method described above, with some modifications [3,21]. PCBs were identified and quantified using a gas chromatograph (Perkin Elmer mod. Autosystem) equipped with 63Ni electron capture detector (GC-ECD); capillary column coated with DB-5 (Supelco Inc.). Blanks were analyzed throughout the analytical procedure to check for interference and laboratory contamination. Recoveries and detection limits were described in advance and validated. PCBs were calculated as the sum of the principal congeners identified (43 congeners). Results are given in ng/g on a fresh weight basis (f. w.).
Some PCB congeners were confirmed by GC/MS (ThermoFinnigan TraceTM GC 2000/GCQ plus with ion trap detector). The gas chromatograph was equipped with an AS 2000 autosampler (ThermoFinnigan) and fitted with an Rtx-5MS capillary column (30 m × 0.25 mm i.d., 0.25 m) from Restek. GC conditions and information on target/qualifier ions are described elsewhere [22]. All compounds were analytical standards of >99% purity. Total PCBs represent the sum of all the congeners (including coeluters) analysed.
The procedures described above were checked for recoveries and reproducibility. Procedural blanks and reference material, purchased from the National Institute of Standards and Technology (NIST), were analysed for quality assurance/quality control (QA/QC) purposes. Prior to extraction, six analytical blanks were prepared using the same extraction and clean-up procedure. A solvent blank was analysed every 15 samples to check the response of the gas chromatograph (GC). A recovery standard was also evaluated by spiking samples with PCB-30, with an average recovery >80%. Concentrations were not recovery-corrected. Analysis of the NIST reference material showed a mean PCB recovery of 93.5%. PCBs 70 + 76, 95, 60 + 56 and 180 had significant blank interference. Limit of detection (LOD) was defined as the aver-

Figure 1. Sampling area.
age blank (n = 4) plus three standard deviations (SD). When target compounds were not detected in blanks, 2/3 of the instrumental detection limit was used as the method detection limit (MDL). All qualified data (i.e. exceeding the MDL) has been blank corrected. The MDL was approximately 0.05 ng/ml for most individual components. Reported PCB concentrations were adjusted by subtracting blank values and calculated on an ng/g fresh weight (f.w.) basis.
2.3. PBDEs
As far as polybromo diphenyl ethers, nineteen BDE congeners (IUPAC numbers BDE3, BDE7, BDE5, BDE17, BDE28, BDE49, BDE71, BDE47, BDE66, BDE77, BDE100, BDE119, BDE99, BDE85, BDE126, BDE154, BDE153, BDE138, BDE156) were identified and quantified using a GC/MS (ion trap mass spectrometer) from ThermoFinnigan (Trace GC 2000/GC Polaris), equipped with an AS2000 autosampler (Rtx-5MS capillary column, 30 m × 0.25 mm i.d., film thickness 0.25 μm; Restek). A 2 μL aliquot of sample in isooctane was injected in splitless injection mode with helium as the carrier gas. The injector temperature was 275˚C. The ramp program was as follows: the initial temperatureof the oven was 80˚C, held for two minutes; it was increased to 200˚C at a rate of 25˚C/min, then to 300 at 4˚C/min and held for 10 min. The excitation voltages were 4.75 V for tri and tetra-BDEs, 4.60 V for penta-BDEs, and 4.70 V for hexaBDEs. The internal standard was CB1413C in isooctane, from Cambridge Isotope Laboratories; the PBDE calibration standard solution was from Wellington Laboratories, Inc. The compounds detected in the blanks were PBDEs 99 and 154. Detection limits, calculated as the mean blank +3 SD, were 4 pg/g tissue. Throughout this manuscript PBDEs are represented by their IUPAC numbers.
3. Results and Discussion
The presence of PCBs and PBDEs was detected in several samples of the eleven fish species analyzed, and the results are shown in Table 1. The values of the standard deviation is high in most cases, and this may be due to the natural variability usually found when analyzing organisms belonging to different sex and age classes; feeding habits and ecological behaviour may also contribute to very different accumulation levels of these pollutants in tissues.
3.1. Polychlorobiphenyls
With regard to polychlorobiphenyls, it is possible identify two groups of fish. The first group (Figure 2) shows average values between 289 ng/g f.w. in M. barbatus and 639 ng/g in L. piscatorius, and also includes M. merluccius (mean 373 ng/g), Solea vulgaris (289 ng/g) and P. americanum (426 ng/g f.w.). In the second group, the average values are higher, between 1000 and 2000 ng/g f.w. (Figure 3); in particular, the average value of PCBs in benthic fish H. dactylopterus and S. scrofa should be 1148 and 1153 ng/g f.w respectively according to Table 1, and pelagic fish show average values of 1145 ng/g f.w. in S. dumerili, 1434 ng/g in little tunny (E. alletteratus), 1510 ng/g in bluefin tuna (T. thynnus) and 2134 ng/g f.w. in swordfish (X. gladius).
If we compare these results with those in the literature on the Mediterranean, the indication that the species with the greatest accumulations of PCBs are the bluefin tuna and the swordfish is confirmed. Swordfish and tuna feed on mackerel and thus show comparatively higher POP levels due to biomagnifications [23]. The swordfish’s opportunistic feeding habits [21] may affect POP accumulation, together with specific metabolic processes. Swordfish eat quite often and have a high gastric digestion rate [24], which may affect the amount of contaminants ingested. This species is becoming very sensitive to parasites, and this may be due to the interference of endocrine disruptor chemicals such as PCBs. Recently, a new species of nematode, Huffmanela paronai, has been discovered in swordfish from the Western Mediterranean [25]. This is also emerging as a commercial problem since certain parasites can damage the edible parts of fish. The bluefin tuna is one of the fastest pelagic swimmers
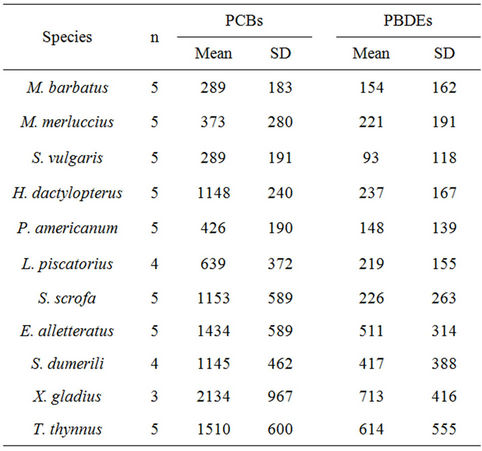
Table 1. Levels of PCBs (ng/g f.w.) and PBDEs (pg/g f.w.) in muscle of fish; n, number of pool analysed; SD, standard deviation.
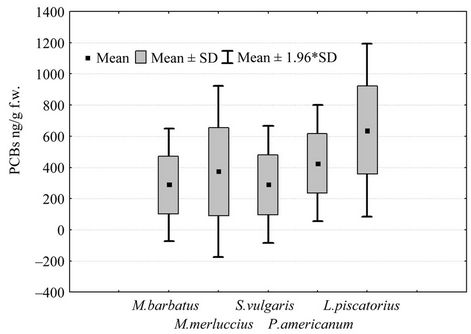
Figure 2. Boxplot of PCBs concentrations (ng/g f.w.) in muscle of 5 species of fish.
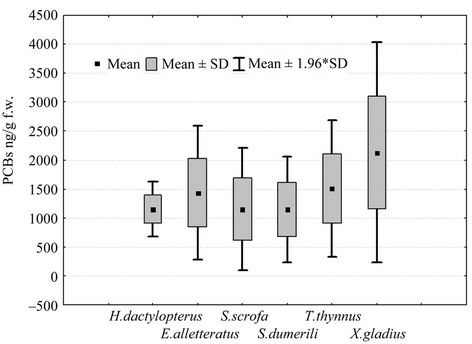
Figure 3. Boxplot of PCBs concentrations (ng/g f.w.) in muscle of 6 species of fish.
and may reach speeds of up to 70 km/h during migrations over thousands of kilometers. Its metabolism is high, and it is a voracious animal that feeds primarily on schooling fishes [23].
3.2. Polybrominated Diphenyl Ethers
Average PBDE concentrations—here indicated as the sum of nineteen BDE congeners—vary from 93 pg/g f.w. in S. vulgaris to 713 pg/g f.w. in swordfish. Most of the average values are between 100 and 200 pg/g f.w. (Figure 4), confirming the penetration of these contaminants into the Mediterranean marine food web. Higher average values are observed in pelagic fish (>500 pg/g f.w.), which also explains the higher endocrine disrupting chemical (EDC) levels observed in tuna fish and swordfish [3,7,17]. In the muscle tissue of 9 bluefin tuna caught in 2003 in the Southern Tyrrhenian Sea, the average concentration found was 15 ng/g f.w. [17], and in the same area in 17 swordfish caught in 2005, the average concentration found was 612 pg/g f.w. [7]. In human fat samples collected between 2005 and 2006 in Siena (Tuscany, Italy), average concentrations of 11 ng/g on a lipid base were reported [20]. In three wild swordfish fillets purchased from markets in northern California (U.S.), the sum totals of 31PBDEs were 282, 974, and 4955 pg/g f.w. [26]. In our swordfish samples, the sums of 19 congeners ranged from 150 to 1320 pg/g f.w.
If we consider PBDE congeners, the abundance in the muscle tissue of all the species analyzed was tetra-BDEs > penta-BDEs > hexa-BDEs. It is interesting to note that tetra-BDEs are under 50% of total PBDEs in pelagic fish (Figure 5), and over 50% in benthonic fish (Figure 6); this could be attributed both to the different feeding habits of fish and to the different chemical properties of PBDEs.
Among the congeners analyzed, BDE47 was the predominant one in all cases. In swordfish muscle, the abundance followed the pattern BDE47 > BDE100 > BDE154 > BDE49. In bluefin tuna muscle, the abundance followed the pattern BDE47 > BDE100 > BDE49 > BDE- 154. This profile has been described in many aquatic spe-
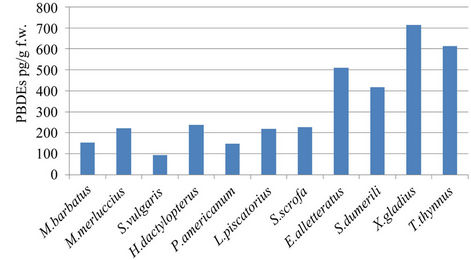
Figure 4. Concentrations (pg/g f.w.) in muscle of fish.
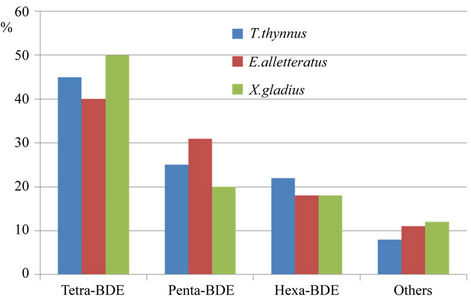
Figure 5. PBDE congeners in muscle of pelagic fish.
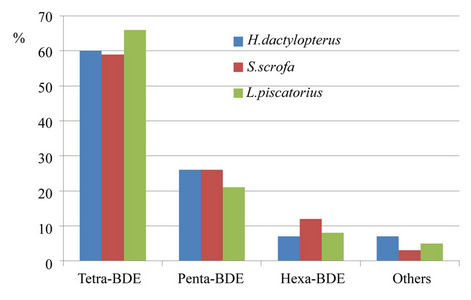
Figure 6. PBDE congeners in muscle of benthonic fish.
cies by several authors [27-29]. PBDE47 is the congener most used in penta-brominated commercial mixtures, which have been banned in Europe since 15 August 2004. Previous uses, debromination processes [28], or long-range transport [30] from countries where pentabrominated mixtures are still in use may be responsible for this pattern. The predominante of BDE47 may also be due to preferential elimination or metabolic degradation of BDE99 [30], following the debromination pattern [27].
3.3. Toxicological Risk
This results clearly highlight the now consolidated presence of PCBs as well as the more recent appearance of PBDEs in the marine trophic chain in the area of the study and in the Mediterranean in general. This puts a few fish species at the top of the food chain, such as bluefin tuna and swordfish, at particular risk. The first warning about toxicological risk to large Mediterranean pelagic fish due to endocrine disruptors (EDCs) was sounded with regard to swordfish [10]. The author used vitellogenin (Vtg) and zona radiata proteins (Zrp) as diagnostic and prognostic biomarkers. Dramatic induction of these typically female proteins was detected by ELISA and Western blot in adult males of the two species. The importance of this species in the human diet in Italy suggests that we should be making our food choices with particular care.
4. Conclusion
The results of this study are consistent with previous studies that have reported now-generalized contamination by polychlorobiphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in the Mediterranean marine environment. The Ionian Sea (Western Mediterranean), from which the 11 fish species were collected, receives a heavy pollutant input from the urban and industrial areas along its coasts. As the Mediterranean is almost entirely landlocked, its waters have a very low renewal rate of 100 years; this limited water exchange increases the chemical residence time and allows bioaccumulation, making the organisms excessively sensitive to pollution Higher values of PCBs (average over 1.5 mg/kg f.w.) was observed in pelagic top predators (little tuna, bluefin tuna and swordfish). These same species also showed higher levels of PBDEs, averaging above 0.5 ng/g f.w. This puts a few fish species at the top of the food chain, such as bluefin tuna and swordfish, at particular risk, and the importance of these species in the human diet suggests the need for particular care in our food choices. As far as PBDEs are concerned, some clear differences among species emerged in the abundance patterns of congeners; this could be attributed both to the different feeding habits of fish and to the different chemical properties of PBDEs.
5. Aknowledgements
This work was supported from a grant of FMPS (Fondazione Monte dei Paschi di Siena) in the year 2007.
REFERENCES
- W. S. Broecker and R. Gerard, “Natural Radiocarbon in the Mediterranean Sea,” Limnology and Oceanography, Vol. 14, No. 6, 1969, pp. 883-888. doi:10.4319/lo.1969.14.6.0883
- K. Kannan, S. Corsolini, J. Falandysz, G. Oehme, S. Focardi and J. P. Giesy, “Perfluoroctanesulfonate and Related Fluorinated Hydrocarbons in Marine Mammals, Fishes and Birds from Coasts of the Baltic and Mediterranean Seas,” Environmental Sciences and Technology, Vol. 36, No 15, 2002, pp. 3210-3216.
- K. Kannan, S. Corsolini, T. Imagawa, S. Focardi and J. P. Giesy, “Polychlorinated-naphthalenes, -biphenyls, -dibenzop-dioxins and -dibenzofurans in Bluefin Tuna, Swordfish, Cormorants and Barn Swallows from Italy,” Ambio, Vol. 31, No. 3, 2002, pp. 207-211.
- P. Stefanelli, A. Ausili, A. Di Muccio, M. C. Fossi, S. Di Muccio, S. Rossi and A. Colasanti, “Organochlorine Compounds in Tissues of Swordfish (Xiphias gladius) from Mediterranean Sea and Azores islands,” Marine Pollution Bulletin, Vol. 49, No. 11-12, 2004, pp. 938-950. doi:10.1016%2FS0956-7135%2803%2900004-5
- S. Damiano, P. Papetti and P. Menasatti, “Accumulation of Heavy Metals to Assess the Health Status of Swordfish in a Comparative Analysis of Mediterranean and Atlantic areas,” Marine Pollution Bulletin, Vol. 62, No. 8, 2011, pp. 1920-1925. doi:10.1016/j.marpolbul.2011.04.028
- C. Leonzio, E. Bacci, S. Focardi and A. Renzoni, “Heavy Metals in Organisms from the Northern Tyrrhenian Sea,” Science of the Total Environment, Vol. 20, No. 3, 1981, pp. 131-146. doi:10.1016/0048-9697(81)90059-0
- S. Corsolini, C. Guerranti, G. Perra and S. Focardi, “Polybrominated Diphenyl Ethers, Perfluorinated Compounds and Chlorinated Pesticides in Swordfish (Xiphias gladius) from the Mediterranean Sea,” Environmental Sciences and Technology, Vol. 42, No. 12, 2008, pp. 4344-4349. doi:10.1021/es703057f
- K. Kannan, S. Tanabe, A. Borrell, A. Aguilar, S. Focardi and R. Tatsukawa, “Isomer-Specific Analysis and Toxic Evaluation of Polychlorinated Biphenyls in Striped Dolphins Affected by an Epizootic in the Western Mediterranean Sea,” Archives of Environmental Contamination and Toxicology, Vol. 25, No. 2, 1993, pp. 227-233. doi:10.1007/BF00212134
- A. Borrell, A. Aguilar, S. Corsolini and S. Focardi, “Evaluation of the Toxicity and Sex-Related Variation of PCB Levels in Mediterranean Striped Dolphins Affected by an Epizootic,” Chemosphere, Vol. 32, No. 12, 1996, pp. 2359-2369. doi:10.1016/0045-6535(96)00143-9
- M. C. Fossi, S. Casini, L. Marsili, S. Ancora, G. Mori, G. Neri, T. Romeo and A. Ausili, “Evaluation of Ecotoxicological Effects of Endocrine Disrupters during a Four Year Survey of the Mediterranean Population of Swordfish (Xiphias gladius),” Marine Environmental Research, Vol. 58, No. 2-5, 2004, pp. 425-429. doi:10.1016/j.marenvres.2004.03.026
- J. P. Boon, W. E. Lewis, M. R. Tjoen-A-Choy, C. R. Allchin, R. J. Law, J. de Boer, C. Ten Hallers-Tjabbes and B. N. Zegers, “Levels of Polybrominated Diphenyl Ether (PBDE) Flame Retardants in Animals Representing Different Trophic Levels of the North Sea Food Web,” Environmental Sciences and Technology, Vol. 36, No 19, 2002, pp. 4025-4032. doi:10.1021/es0158298
- S. Voorspoels, A. Covaci, P. Schepens, “Polybrominated diphenyl ethers in marine species from the Belgian North Sea and the Western Scheidt Estuary: Levels, Profiles, and Distribution,” Environmental Sciences and Technology, Vol. 37, No. 19, 2003, pp. 4348-4357. doi:10.1021/es034503r
- A. Bocio, J. M. Llobet, J. L. Domingo, J. Corbella, A. Teixido and C. Casas, “Polybrominated Diphenyl Ethers (PBDEs) in Foodstuffs: Human Exposure through the Diet,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 10, 2003, pp. 3191-3195. doi:10.1021/jf0340916
- C. De Wit, “An Overview of Brominated Flame Retardants in the Environment,” Chemosphere Vol. 46, No. 5, 2002, pp. 583-624. doi:10.1016/S0045-6535(01)00225-9
- M. Lebeuf, B. Gouteux, L. Measure and S. Trottier, “Le-vels and Temporal Trends (1988-1999) of Polybrominated Diphenyl Ethers in Beluga Whales (Delphinapterus leucas) from the St. Lawrence Estuary, Canada,” Environmental Sciences and Technology, Vol. 38, No. 11, 2004, pp. 2971-2977. doi:10.1021/es035187j
- K. S. Sajwan, K. Senthilkumar, S. Nune, A. Fowler, J. Richardson and B. G. Loganathan, “Persistent Organochlorine Pesticides, Polychlorinated Biphenyls, Polybrominated Diphenyl Ethers in Fish from Coastal Waters off Savannah, GA, USA,” Toxicological and Environmental Chemistry, Vol. 90, No. 1, 2008, pp. 81-96. doi:10.1080/02772240701270047
- N. Borghesi, S. Corsolini, P. Leonards, S. Brandsma, J. De Boer and S. Focardi, “Polybrominated Diphenyl Ether Contamination Levels in Fish from the Antarctic and the Mediterranean Sea,” Chemosphere, Vol. 77, No. 5, 2009, pp. 693-698. doi:10.1016/j.chemosphere.2009.07.035
- R. Hale, M. Alaee, J. B. Manchester-Neesvig, H. M. Stapleton and M. G. Ikonomou, “Polybrominated Diphenyl Ethers (PBDE) Flame Retardants in the North America Environment,” Environment International, Vol. 29, No. 6, 2003, pp. 841-853. doi:10.1016/S0160-4120(03)00113-2
- M. G. Ikonomou, S. Rayne, R. F. Addison, “Exponential Increases of the Brominated Flame Retardants, Polybrominated Diphenyl Ethers, in the Canadian Arctic from 1981 to 2000,” Environmental Sciences and Technology, Vol. 36, No. 9, 2002, pp. 1886-1892. doi:10.1021/es011401x
- A. Schiavone, K. Kannan, Y. Horii, S. Focardi and S. Corsolini, “Polybrominated Diphenyl Ethers, Polychlorinated Naphthalenes and Polycyclic Musks in Human Fat from Italy: Comparison to Polychlorinated Biphenyls and Organochlorine Pesticides,” Environmental Pollution, Vol. 158, No. 2, 2010, pp. 599-606. doi:10.1016/j.envpol.2009.08.011
- S. Corsolini, A. Ademollo, T. Romeo, S. Greco and S. Focardi, “Persistent Organic Pollutants in Edible Fish: A Human and Environmental Health Problem,” Microchemical Journal, Vol. 79, No. 1-2, 2005, pp. 115-123. doi:10.1016/j.microc.2004.10.006
- I. Corsi, M. Mariottini, A. Badesso, T. Caruso, N. Borghesi, S. Bonacci, A. Iacocca and S. Focardi, “Contamination and Sub-Lethal Toxicological Effects of Persistent Organic Pollutants in the european Eel (Anguilla anguilla) in the Orbetello lagoon (Tuscany, Italy),” Hydrobiologia, Vol. 550, No. 1, 2005, pp. 237-249. doi:10.1007/s10750-005-4392-y
- B. C. Chase, “Differences in Diet of Atlantic Bluefin Tuna (Thunnus thynnus) at Five Seasonal Feeding Grounds on the New England Continental Shelf,” Fishery Bulletin, Vol. 100, No. 2, 2002, pp. 168-180.
- F. Moreira, “Food of the Swordfish, Xiphias gladius Linnaeus, 1758, off the Portuguese Coast,” Journal of Fish Biology, Vol. 36, No. 4, 1990, pp. 623-624. doi:10.1111/j.1095-8649.1990.tb03565.x
- F. Moravec and F. Garibaldi, “Huffmanela paronai sp. n. (Nematoda: Trichosomoididae), a New Parasite from the Skin of Swordfish Xiphias gladius in the Ligurian Sea (Western Mediterranean),” Folia Parasitologica, Vol. 47, No. 4, 2000, pp. 309-313.
- W. Luksemburg, R. Wenning, M. Maier, A. Patterson and S. Braithwaite, “Polybrominated Diphenyl Ethers (PBDE) and Polychlorinated Dibenzo-p-Dioxins (PCDD/F) and Biphenyls (PCB) in Fish, Beef, and Fowl Purchased in Food Markets in Northern California USA,” Organohalogen Compounds, Vol. 66, 2004, pp. 3987-3982.
- M. G. Ikonomou, S. Rayne and M. Fischer, “Occurrence and Congener Profiles of Polybrominated Diphenyl Ethers (PBDEs) in Environmental Samples from Coastal British Columbia, Canada,” Chemosphere, Vol. 46, No. 5, 2002, pp. 649-663. doi:10.1016/S0045-6535(01)00229-6
- J. H. Christensen, M. Glasius, M. Pe’cseli, J. Platz and G. Pritzl, “Polybrominated Diphenyl Ethers (PBDEs) in Marine Fish and Blue Mussels from Southern Greenland,” Chemosphere, Vol. 46, No. 5, 2002, pp. 631-638. doi:10.1016/S0045-6535(02)00009-7
- K. Kannan, K. Ramu, N. Kajiwara, R. K. Sinha and S. Tanabe, “Organochlorine Pesticides, Polychlorinated Biphenyls, and Polybrominated Diphenyl Ethers in Irrawaddy Dolphins from India,” Archives of Environmental Contamination and Toxicology, Vol. 49, No. 3, 2005, pp. 415-420. doi:10.1007/s00244-005-7078-6
- F. Wania and C. B. Dugani, “Assessing the Long-Range Transport Potential of Polybrominated Diphenyl Ethers: A Comparison of Four Multimedia Models,” Environmental Toxicology and Chemistry, Vol. 22, No. 6, 2003, pp. 1252-1261. doi:10.1002/etc.5620220610

