Green and Sustainable Chemistry
Vol.08 No.02(2018), Article ID:84271,17 pages
10.4236/gsc.2018.82010
Henry Reaction between Benzaldehyde and Nitromethane over Solid Base Catalysts: A Green Protocol
Magda H. Abdellattif1*, Hany Mahmoud Mohamed2
1Pharmaceutical chemistry department, Deanship of Scientific research Taif University, Taif, KSA
2Chemistry Department, Faculty of Science, Taif University, Taif, KSA

Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 18, 2018; Accepted: April 27, 2018; Published: April 30, 2018
ABSTRACT
The development of environmentally benign solid base catalysts instead of the soluble bases for C-C bond formation in organic reactions especially Henry reactions with nitroalkanes compounds is of intense research activity in the bulk and fine chemical chemistry in order to achieve the selectivity of the desired product and the reduction of the salts formed due to soluble bases neutralization. While using of LDHs catalysts in the synthesis of nitro alcohols is of great interest because LDHs (double layered hydroxides) is of unique properties and an excellent catalytic property. The nitroalcohols are obtained in a very good yield while using catalyst either by conventional at 90˚C in liquid phase, microwave or sonoenergy without solvent methods, and the results yields are compared. A series of different nitro alcohols from (a - o) were prepared, the catalytic test reaction were carried out using benzaldehyde and their derivatives with nitromethane and their derivatives. A series of LDHs catalysts were prepared also and studying of the catalytic effect on the reactions was carried out. Properties of the compounds prepared were characterized by IR, MNR, and GC-MS.
Keywords:
C-C Bond Formation, Henry Reaction, Base Catalyst

1. Introduction
The fine chemical industry has experienced remarkable interest over the past few years due to the high requirements for products like pharmaceuticals, pesticides, fragrances, flavorings and food additives [1] .
The classical methods for the C-C coupling in Henry reaction using soluble bases such as alkali metal hydroxides, carbonates, bicarbonates, alkoxides, alkaline earth metal hydroxide, and aluminium ethoxides, complexes, and also organic bases such as primary, secondary and tertiary amines usually resulted in dehydrated products [2] . Therefore, careful control of the basic properties of the reaction medium is vital to obtain better yields of β-nitroalcohols. However, the efforts done by the researchers in the literature required longer reaction times and produced moderate yields [3] [4] . The stoichiometric organic synthesis largely applied so far resulted in large quantities of inorganic salts as byproducts; the disposal of such material causes a serious problem due to the important environmental issues [5] .
The remarkable progress in the competition in the industry has pushed the researchers to develop more effective catalytic processes in the synthesis of fine chemicals.
The products of the Henry reaction, representing C-C bond formation, are important materials extensively used in many organic syntheses [6] . The greatest challenge in the selective synthesis of 2-nitroalkanols in the multiple product options such as aldol olefin and its polymer and Cannizaro products is the selection of the right type of base [7] .
Significant improvements to the Henry reaction have been achieved by using silyl nitronates in the presence of fluoride ion or altematively α-α doubly deprotonated primary nitroalkanes [8] .
Both of these procedures have proved to be useful for the stereo selective preparation of vicinal amino alcohols under drastic conditions, which reduced diastereoselectivity with aromatic aldehydes. In order to obtain better yields and diastereoselectivity of 2-nitroalcohols, it is necessary to develop new procedures employing heterogeneous catalysts with basic character [9] .
The homogenous catalytic methodologies reported in the literature have many disadvantages, such as disposal of waste and difficulty to recover the catalyst from the products. In the last decade, there were notable improvements in the development of heterogeneous catalyst for Henry reaction [10] .
Heterogeneous catalysis induced by solid catalysts such as basic alumina [11] , alumina-KF [12] and homogeneous phase transfer catalysis with surfactants [13] in bi-phase system, the two divergent approaches being explored are aimed at achieving higher atom selectivity. The solid base catalysts provide an alternative to the classical soluble bases with emphasize to avoid the environmental problems caused by salt formation and hazardous conditions [14] . Previous wok in the synthesis of fine chemicals using layered double hydroxides revealed the importance of such materials and discovered its environmentally favorable routes in comparison to the other catalysts [15] - [20] .
2. Results and Discussion
Henry reaction is a base-catalyzed C-C bond-forming reaction between nitroalkanes and aldehydes or ketones. It is similar to the Aldol Addition, and also referred to as the Nitro Aldol Reaction.
If acidic protons are available (H), the products tend to eliminate water to give nitroalkenes. Therefore, only small amounts of base should be used if the isolation of the β-hydroxy nitro-compounds is desired, Scheme 1.
To generalize this reaction methodology three experiments were carried out using different LDHs catalysts which may also be called hydrotalcite, and their thermally activated form (calcined) were very active and very useful in many different organic synthesis reactions. Henry reaction was carried out under three conditions by these catalysts, by conventional method, and microwave method [21] .
Cu LDHs series was used in this research which were: uncalcined HT-Solgel Cu:Mg:Al (1:2:1), calcined Cu:Mg:Al (1:2:1), clacined Cu:Mg:Al (2:1:1) and finally calcines Cu:Al (3:1). Table 1 represents the nitroalkanes and the aldehydes used.
To compare the three types of this condensation reaction yield, it should be taken in consideration from literature the most optimum temperature for this reaction by conventional method was 90˚C. While in this research temperature in conventional method decreased by using catalysts, Table 1 represented the yield of this nitro aldol condensation reaction using three methods.
The use of uncalcined HT was not efficient enough like the other catalyst used and the reaction yields and time were near to the reaction yield and time in the absence of any catalyst and solvents in spite of temperature change and decreased to 60˚C, as shown in Table 4. Using of different calcined catalysts at fixed weight 0.5 g, temperature 50˚C for conventional method, fixed microwave temperature many different results but in a good and acceptable yield % for all of the catalysts used as illustrated in Tables 5-7. These good results directed us to make a comparison between the catalysts themselves and their activities toward the nitroaldol reaction.
The higher activity of LDHs catalysts were observed because of their structure flexibility as a host compound during the formation of final nirtoalcohol. It was observed also at fixed amount of catalyst used, fixed temperature, and fixed M.W conditions, changing of the catalyst type led to decrease in time of the reaction required to be finished and increase of the reaction yield.

Scheme 1. Henry reaction and its mechanism.
Table 1. Compounds Structures.
Table 2. Comparison between different methods time and yield without any catalysts.
Table 3. Comparison between different methods time and yield using uncalcined HT-Solgel (0.5 g).
Table 4. Comparison between different methods time and yield using Cu:Mg:Al (1:2:1) (0.5 g).
Table 5. Comparison between different methods time and yield using calcined Cu:Mg:Al (1:2:1) (0.5 g).
Table 6. Comparison between different methods time and yield using clacined Cu:Mg:Al (2:1:1) (0.5 g).
Table 7. Comparison between different methods time and yield using clacined Cu:Al (3:1) (0.5 g).
For Cu:Al 3:1 and Cu:Mg:Al 2:1:1 the reaction tended to be completed in a short time. This can be explained by the catalyst which has higher copper content was more active in this serious. One also could observe that aldehyde which has little electron withdrawing group influence and enhance the reaction activity. By the way microwave yields are the highest every time.
By the way from Table 3 the reaction tented to be finished between 4 - 6.5 min using M.W and 300 - 480 min using conventional method by maximum yield 87% and 63% respectively. While using solvated catalyst reaction Table 2 completed at 3 - 4 min M.W and 300 min. by using conventional method in a max. Yield % 94% and 71% respectively. The 99% yield percentage obtained by using clacined Cu:Al 3:1, using M.W at 1.5 - 2 min. But using of calcined Cu:Mg:Al (2:1:1) gave 98% yield as a highest yield percentage this may attributed to the crystalline structure of Cu:Al 3:1 [22] . The difference in reaction catalysts, yield and time was illustrated in Figures 1-5.
Where:
Figure 1: Compare between different methods time and yield without any catalysts.
Figure 2: Compare the yield % change during use of different catalysts, by MW irradiation method.
Figure 3: Compare the time change during use of different catalysts, MW irradiation method.
Figure 4: Compare time change during use of different catalysts, by conventional method.
Figure 5: Compare the yield % change during use of different catalysts, conventional method.
Figure 1. Comparison between different methods time and yield without any catalysts.
Figure 2. % Yield change during use of different catalysts, MW.
Figure 3. Time change during use of different catalysts, MW.
Figure 4. Time change during use of different catalysts, conventional.
Figure 5. % Yield change during use of different catalysts, conventional.
XRD of the catalysts were represented in Figure 6, indicating the diffraction patterns of HT-1, HT-2, HT3, HT-4 and HT-5, which gave information about the crystalline nature of these five catalysts. Catalyst showed diffraction pattern at 2θ 35.6˚, 38.8˚, 48.8˚, 53.6˚, 58.5˚, 61.5˚, 66.3˚, 68.1˚ and 75.4˚. The catalyst was also analyzed after reuse by XRD and it showed no significant change. XRD patterns catalysts layered structure was obtained (Ref. Pattern 22-0700, JCPDS) [23] .
FTIR (Fourier transform infrared spectroscopy) of HT catalyst series sample (Figure 7) showed a broad band at nearly 3500 cm−1 belonged to stretching vibration OH. This might be because of the water interlayer and hydroxyl groups in HT series [24] . Small weak band appeared at 1650-1700. This might be related to the bridging mode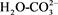 . Because of the vibrational mode of water a weak peak at 1590 cm−1 appeared in the infrared spectrum which might be related to interlayer of water [25] . The peak around 1340 cm−1 belonged to carbonate. All of carbonate bands of HT catalysts were removed completely during calcinations at 450˚C [26] . This was as a result of complete thermal decomposition of hydrated catalysts phase into mixed metal oxides, which gave a good indication of the XRD data [27] [28] .
. Because of the vibrational mode of water a weak peak at 1590 cm−1 appeared in the infrared spectrum which might be related to interlayer of water [25] . The peak around 1340 cm−1 belonged to carbonate. All of carbonate bands of HT catalysts were removed completely during calcinations at 450˚C [26] . This was as a result of complete thermal decomposition of hydrated catalysts phase into mixed metal oxides, which gave a good indication of the XRD data [27] [28] .
Figure 6. XRD for HT catalysts series.
Figure 7. FTIR of HT-catalyst series.
3. Methodology
All chemicals used were purchased from Sigma Alderich, analytical trade. 1H-NMR spectra, 13C-NMR spectra were recorded on Bruker AM250 NMR spectrometer using CDCl3 as solvent for the samples. Mass spectra were recorded on Shimadzu LCMS-QP 800 LC-MS, IR for the synthesised compounds were recorded in potassium bromide discs on Shimadzu FT IR 8101 PC infrared spectrophotometer. Elemental analysis was obtained using PerkinElmer 2400 II series CHN Analyser. Thin-layer chromatography (TLC) was carried out on pre-coated Merck silica gel F254 plates to 80˚. Melting points of the prepared compounds were measured on a Gallenkamp melting point apparatus.
1) Catalysts
a) Synthesis
Catalysts were prepared by co-precipitation methods as literature [29] [30] , LDHs have the structures [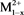 ,
, 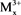 (OH)2]x+ [Ax/y]y−. mH2O, where the divalent may be (Mg2+, Cu2+, Fe2+, Co2+, Ni2+, or Zn2+), and the trivalent are metal cation (e.g., Al3+, Cr3+, Ga3+, Ln3+, Mn3+ or Fe3+) which forms the positively charged layers, where Ay− are called inorganic or organic anions (e.g.,
(OH)2]x+ [Ax/y]y−. mH2O, where the divalent may be (Mg2+, Cu2+, Fe2+, Co2+, Ni2+, or Zn2+), and the trivalent are metal cation (e.g., Al3+, Cr3+, Ga3+, Ln3+, Mn3+ or Fe3+) which forms the positively charged layers, where Ay− are called inorganic or organic anions (e.g., 


i- HT-1 (LDH-As)
Mixing of magnesium nitrate Mg (NO3)2・6H2O 0.2213 mol, and aluminium nitrate Al (NO3)3 0.0885 mol, in a 0.2213 L of dist. H2O, mix (A). Mixing of 0.7162 mol of NaOH, and 0.2084 mol of Na2CO3, in a 0.221 L, mix (B). Drop using a burette drops from Mix (A) and Mix (B) to a Round bottom flask 1 L containing 0.5 L distilled water under vigorous stirring and heating at 60˚C and measure pH during precipitation to be 10-11. Keep the temperature at 60˚C overnight (16 h) in water bath. Filter using (Whatmann 1 filter paper) and washing the cake by hot distilled water till pH = 7. Dry the filtrate at 80˚C in an oven for 16 h.
ii- Cu:Mg:Al (1:2:1) HT-2, Clacined Cu:Mg:Al (1:2:1) HT-3, and Clacined Cu:Mg:Al (2:1:1) HT-4
Cu metal modified HT Mg:Al was prepared by memory effect [30] , where typically, 1.0 g the as-prepared HT-As was treated with an aqueous Cu2+ nitrate or sulfate solution (0.1 mol/L, 100 mL) with stirring at room temperature for 48 h to reconstruct into the layered hydrotalcite structure. The resulting products were filtered, washed several times with distilled water and dried at 100˚C for 12 h, while the HT-Cu catalyst was obtained.
Then Cu-modified Mg-Al catalysts were prepared in oven at 500˚C for 7 h under a flowing stream of pure N2.
iii- Clacined Cu:Al (3:1) HT-5
It was prepared also by coprecipitation methods as mentioned in literature, where mixing of magnesium nitrate Cu SO4・5H2O 0.028 mol, and aluminium nitrate Al (NO3)3 0.01 mol, in a dist. H2O, mix (C), mixing of 0.7162 mol of NaOH, and 0.2084 mol of Na2CO3, in a dist. H2O, mix (D). Drop using a burette drops from Mix (C) and Mix (D) to a round bottle flask 1L containing 0.5 L distilled water under vigorous stirring and heating at 60˚C and measure pH during precipitation to be 10-11. Keep the temperature at 60˚C overnight (16 h) in water bath. Filter using (Whatmann 1 filter paper) and washing the cake by hot distilled water till pH = 7 gives (HT-5). Dry the filtrate at 80˚C in an oven for 16 h. Heat 2 g of the above paste product in oven at 450˚C for 6 h in N2 atmosphere, then dissolve in adequate amount of NaOH (0.1 M) to form a paste. Dry the Paste at 80˚C in an oven for 16 h gives HT-5.
2) Synthesis of β-Nitroalcohol, via Henry Reaction
a) Microwave Irradiation Method
i- Without Catalyst
A mixture of an aldehyde series (10 mmol) and nitroalkane series (10 mmol) were added to a vesicle, and the mixture was mixed strongly with a glass-rod at room temperature. The mixture was added in a Teflon vial and irradiated under 350 W microwave till the reaction temperature raised to 120˚C with fixed microwave pressure. The vial was exposed to microwaves for a required time to complete the reaction. Check the reaction and monitor it using TLC using (eluent; Diethyl ether: chloroform) every 1 min. until the reaction reached the end. Then the product mixture was cooled and extracted using ethanol analytical grade. The process were repeated using different aldehyde and different to form compounds from a - o.
The product compounds were purified by crystallization using EtOH/DMF solvent mixture to afford the pure crude β-nitroalcoohls a - o in an excellent yield.
ii- Using catalyst
Catalysts 0.5 g from HT-1 up to HT-5 were used and added by repeated adequate sequence to a reaction mixture as mentioned above for the series a - o till the reaction completed as before.
Each time and each run the catalysts were removed by filtration and washing by hot ethanol after that the excess solvent was removed by vaporization to get the final solid product. The product compounds were purified by crystallization using EtOH/DMF solvent mixture to afford the pure crude β-nitroalcoohls a - o in an excellent yield.
b) Conventional Method
The reaction were carried on electrical heating hotplate with stirrer and the methods as mentioned above in microwave irradiated were applied without catalyst at 90˚C and with the catalysts series at 60˚C for the series of catalysts and series of the aldehydes and nitro alkanes. The reactants aldehydes, nitroalkanes and catalyst were added together in a round flask bottle, closed with rubber septum connected to a condenser, after the completion of the reaction, the mixture cooled room air temperature then separated and purified as above mentioned and also the catalyst was treated as the above method.
i- p-2-Nitroethenylchlorobenzene (C8H6ClNO2)
mp. 262˚C, IR (KBr) υ max/cm−1: 1515 - 1560 (NO2), 1H NMR (DMSO): δ 5.1 (d, 1H, -C=C), 5.6 (d, 1H, -C=C) δ 7.27 - 7.4 (dd, 4H, Ar), 13C NMR (CDCl3): δ 77.8, 111.22, 127.4, 128.2, 131.8, 134.3, 134.7, MS (m/z): 183.01 (M+), Anal. Calcd for C8H6ClNO2 (183.6), C-52.61; Cl-19.31; H-3.3; N-7.65% Found: C-52.66; Cl-19.3; H-3.3; N-7.63; %.
ii- 1-(p-Chlorophenyl)-2-nitro-1-propene (C9H8ClNO2)
mp. 386˚C, IR (KBr) υ max/cm−1: 1525 - 1570 (NO2), 1H NMR (DMSO): δ 1.7 (d, 3H, CH3), δ 4.9 (q, 1H, -C=CH), δ 7.2 - 7.45 (dd, 4H, Ar), 13C NMR (CDCl3): H, CH), δ 7.27 - 7.4 (dd, 4H, ArH’s), 13C NMR (CDCl3): δ 25.5, 39.5, 77.82, 81.1 126.66, 130.8, 134.5, MS (m/z): 230.01 (M+), Anal. Calcd for C10H12ClNO3 (229), C-52.30; Cl-15.44; H-5.27; N-6.10% Found: C-52.2; Cl-15.44; H-4.01; N-6.8; %.
iii- 1-(4-chlorophenyl)-2-methyl-2-nitropropan-1-ol (C10H12ClNO3)
mp. 465˚C, IR (KBr) υ max/cm−1: 1530 (NO2), 1H NMR (DMSO): δ 1.1 (s, 3H, CH3), δ 5.1 (s, H, CH), δ 7.27 - 7.4 (dd, 4H, ArH’s), 13C NMR (CDCl3): δ 25.5, 39.5, 77.82, 81.1 126.66, 130.8, 134.5, MS (m/z): 230.01 (M+), Anal. Calcd for C10H12ClNO3 (229), C-52.30; Cl-15.44; H-5.27; N-6.10% Found: C-52.2; Cl-15.44; H-4.01; N-6.8; %.
iv- 2-Nitroethenyl-3,5-dibromobenzene C8H5Br2NO2
mp. 491,˚C, IR (KBr) υ max/cm−1: 1480 (NO2), 670 (C-Br), 1H NMR (DMSO): δ δ 5.2 (d, 1H, -C=CH), 6.2 (d, 1H, -C=CH), 7.7 - 7.9 (m, 3H, ArH’s), 13C NMR (CDCl3): δ 77.8, 112.33, 123, 128, 131.1, 135.4, 139.5, MS (m/z): 306.5 (M+), Anal. Calcd for C8H5Br2NO2 (307), Br-52; C-31.5; H-1.7; N-4.58% Found: Br-51.7; C-30.57; H-1.6; N-4.46%.
v- 1-(3,5-Dibromophenyl)-2-nitro-1-propene (C9H7Br2NO2 )
mp. 538˚C, IR (KBr) υ max/cm−1: 1545 (NO2), 672 (C-Br), 1H NMR (DMSO): δ 1.1 (d, 3H,CH3) δ 3.45 (m, 2H, CH2), δ 5.15 (d, 1H, -CH), δ 7.27 - 7.8 (m, 3H, ArH’s), 13C NMR (CDCl3): δ 18.9, 44.5, 77.8, 84.75, 126.66, 129.8, 131.77, 134.7, MS (m/z): 338.9 (M+), Anal. Calcd for C9H9Br2NO3 (339), Br-47.2; C-31.85; H-2.65; N-6.85% Found: Br-47.14; C-31.89; H-2.68; N-4.13; %.
vi- 1-(3,5-dibromophenyl)-2-methyl-2-nitropropan-1-ol C10H11Br2NO3
mp. 440.3˚C, IR (KBr) υ max/cm−1: 3550 (OH), 1523-1550 (NO2), 670 (C-Br), 1H NMR (DMSO): δ 3.15 (d, 2H, CH2), δ 7.27 - 7.4 (dd, 4H, ArH’s), δ 5.2 (t, 1H, -CH), 13C NMR (CDCl3): δ 25.5, 39.8, 77.8, 88.7, 124.4, 129.8, 131.1, 135.1 , MS (m/z): 353.3 (M+), Anal. Calcd for C10H11Br2NO3 (353), Br-45.27; C-34.02; H-3.14; N-3.97% Found: Br-45.3, C-34.01; H-3.15; N-3.95; %.
vii- 1-(3,5-Dibromophenyl)-2-nitro-1-propene (C9H7Br2NO2)
mp. 362˚C, IR (KBr) υ max/cm−1: 3600 (OH), 1520 - 1580 (2NO2), 675 (C-Br), 1H NMR (DMSO): ): δ 1.75(d, 3H, CH3) , 4.95 (m, H, C=CH), δ 7.7 - 7.9 (m, 3H, ArH’s), 13C NMR (CDCl3): δ 24.9, 77.8, 119.83, 123, 128, 130, 131.5, 139.5, MS (m/z): 320.91 (M+), Anal. Calcd for C9H7Br2NO2 (321), Br-50; C-33.5; H-2.30; N-4.4% Found: Br-49.79; C-33.68; H-2.20; N-4.36%.
viii- 4-bromo-2-(1-hydroxy-2-nitropropyl)-6-nitrophenol
mp. 609˚C, IR (KBr) υ max/cm−1: 3650 (OH), 1540 (2NO2), 675 (C-Br), 1H NMR (DMSO): δ 1.75(d, 3H, CH3) , 4.95 (m, H, C=CH), δ 6.5 - 7.2 (dd, 2H, ArH’s), 13C NMR (CDCl3): δ 18.5, 44.37, 77.82, 84.1, 110.86, 112.4, 117.4, 118.3, 144.3, 146.27, MS (m/z): 319.5 (M+), Anal. Calcd for C9H9BrN2O6 (321), Br-24.89; C-33.67; H-2.83; N-8.72 Found: Br-24.89; C-33.8; H-2.87; N-8.6%.
ix- 4-bromo-2-(1-hydroxy-2-methyl-2-nitropropyl)-6-nitrophenol
mp. 765˚C, IR (KBr) υ max/cm−1: 3500 - 3700 (2OH), 1520 - 1580 (2NO2), 690 (C-Br), 1H NMR (DMSO): δ 1.09, (s, 3H,CH3), δ 5.2 (s, 1H, -CH), δ 6.5 - 7.2 (m, 2H, ArH’s), 13C NMR (CDCl3): δ 25.1, 39.48, 88.7, 110.86, 112.36, 117.5, 118.3, 145.3, 147.9, MS (m/z): 334.9 (M+), Anal. Calcd for C10H11BrN2O6 (335), Br-23.84; C-35.9; H-3.3; N-8.4% Found: Br-Br-23.84; C-35.75; H-3.35; N-8.36%.
x- 2-nitro-1-(2-nitrophenyl) ethanol
mp. 428.8˚C, IR (KBr) υ max/cm−1: 1510 - 1555 (2NO2), 1H NMR (DMSO): δ 3.15 (d, 2H, CH2), δ 5.15 (t, 1H, -CH), δ 6.66 - 7.3 (m, 4H, ArH’s), 13C NMR (CDCl3): δ 49.5, 77.8, 81.1, 112.87, 116.8, 126.66, 129.4, 150.97, MS (m/z): 212 (M+), Anal. Calcd for C8H8N2O5 (212), C-45.29; H-3.80; N-13.20% Found: C-45.29; H-3.80; N-13.20; %.
xi- 2-nitro-1-(2-nitrophenyl) propan-1-ol
mp. 552˚C, IR (KBr) υ max/cm−1: 1515 - 1560 (2NO2), 1H NMR (DMSO): δ 1.04, (d, 3H, CH3), δ 3.45 (m, H, CH), δ 5.15 (d, H, CH), δ 6.7 - 7.3 (m, 4H, ArH’s), 13C NMR (CDCl3): δ 18.1, 44.73, 77.8, 84.74, 112.87, 116.75, 129.3, 150.9, MS (m/z): 226 (M+), Anal. Calcd for C9H10N2O5 (226.2), C-47.79; H-4.46; N-12.39% Found: C-47.79; H-4.46; N-12.39%.
xii- 2-methyl-2-nitro-1-(2-nitrophenyl)propan-1-ol
mp. 453.3˚C, IR (KBr) υ max/cm−1: 3650 (OH), 1520 - 1570 (2NO2), 1H NMR (DMSO): δ 1.04 (s, 3H, CH3), δ 5.15 (s, H, CH), δ 6.7 - 7.2 (m, 4H, ArH’s), 13C NMR (CDCl3): δ 25.12, 39.9, 77.82, 88.65, 112.86, 116.75, 129.36, 150.99, MS (m/z): 240.12 (M+), Anal. Calcd for C10H12N2O5 (240), C-50.00; H-5.04; N-11.66% Found: C- 49.90; H-5; N-11.66%.
xiii- 1-(4-methoxyphenyl)-2-nitroethanol
mp. 443.87˚C, IR (KBr) υ max/cm−1: 1545 (NO2), 1150 (C-O), 1H NMR (DMSO): δ 3.15 (s, H, CH3), δ 5.2 (t, 1H, -CH), δ 6.57 - 7.2 (m, 4H, ArH’s), 13C NMR (CDCl3): δ 49.5, 65.5, 77.8, 114.2, 120.86, 129.56, 159.96, MS (m/z): 197.2 (M+), Anal. Calcd for C9H11NO4 (197), C-54.9; H-5.62; N-7.10% Found: C-54.8; H-5.66; N-7%.
xiv- 1-(4-methoxyphenyl)-2-nitropropan-1-ol
mp. 440˚C, IR (KBr) υ max/cm−1: 1565 (NO2), 1210 (C-O), 1H NMR (DMSO): δ 1.04(d, 3H, CH3), δ 3.14 (s, 3H, CH3), δ 3.5(m, 1H, -CH), δ 5.3 (d, H, CH), δ 7.27 - 7.4 (m, 4H, ArH’s), 13C NMR (CDCl3): δ 18.02, 44.95, 65.01, 77.8, 84.7, 114.16, 120.86, 129.6, 159.99, MS (m/z): 211.2 (M+), Anal. Calcd for C10H13NO3 (211), C-56.86; H-6.20; N-6.63% Found: C-56.86; H-6.20; N-6.63%.
xv- 1-(4-methoxyphenyl)-2-methyl-2-nitropropan-1-ol
mp. 468.8˚C, IR (KBr) υ max/cm−1: 3650 (OH), 1535 (NO2), 1050 (C-O), 1H NMR (DMSO), δ 1.04 (s, 3H, CH3), δ 3.14 (s, 3H, CH3), δ 5.2 (s, H, CH), δ 6.9 - 7.3 (m, 4H, ArH’s), 13C NMR (CDCl3): δ 18.02, 44.95, 65.01, 77.8, 84.7, 114.16, 120.86, 129.6, 159.99, MS (m/z): 225.3 (M+), Anal. Calcd for C11H15NO3 (225), C-58.66; H-6.71; N-6.22% Found: C-58.66; H-6.71; N-6.22; %.
4. Conclusion
In brief, we reported Henry reaction between benzaldehyde and nitromethane over solid base catalysts. Cu:Mg:Al-HT catalyst gave a precious advantage over all solid base catalysts under investigation especially calcied Cu:Al 3:1 (HT-5). Microwave irradiation technique introduced us high yields of β- alcohol derivatives using the prepared series of HT solid catalyst in very short time. Strong Basic characterization of catalyst was responsible for the power of catalytic activity. HT series kept their physical properties (texture and structure) even after many catalytic runs, which facilitated gaining of high yields beta nitro alcohols. These synthesized derivatives are of great importance in industry and medical uses.
Acknowledgements
The authors are very grateful to Taif University, Taif, KSA, because this work was financial supported by Taif University, Taif, KSA, under project number 1/437/4938. The authors express their great thanks to Prof. Dr. Mohamed Mokhtar, Chemistry Department, Faculty of Science, King Abdualziz University, Jeddah, KSA, for help and advices.
The authors presented the abstract as poster at Green and sustainable chemistry conference 13-15 May 2017, Berlin.
Conflict of Interest
The authors declare that, there is no conflict of interest.
Cite this paper
Abdellattif, M.H. and Mohamed, H.M. (2018) Henry Reaction between Benzaldehyde and Nitromethane over Solid Base Catalysts: A Green Protocol. Green and Sustainable Chemistry, 8, 139-155. https://doi.org/10.4236/gsc.2018.82010
References
- 1. Olah, G.A., Krishnamurti, R. and Prakash, G.K.S. (1991) Comprehensive Organic Synthesis. Pergamom Press, Oxford, 293-339. https://doi.org/10.1016/B978-0-08-052349-1.00065-2
- 2. Rosini, G. (1991) Comprehensive Organic Synthesis. Pergamon Press, Oxford, 321.
- 3. Ballini, R., Bosica, G. and Forconi, P. (1996) Nitroaldol (Henry) Reaction Catalyzed by Amberlyst A-21 as a Far Superior Heterogeneous Catalyst. Tetrahedron, 52, 1677-1684. https://doi.org/10.1016/0040-4020(95)00996-5
- 4. Contantino, U., Curini, M., Marmottini, F., Rosati, O. and Pisani, E. (1994) Potassium Exchanged Layered Zirconium Phosphate as Base Catalyst in the Synthesis of 2-Nitroalkanols. Chemistry Letters, 23, 2215. https://doi.org/10.1246/cl.1994.2215
- 5. Sheldon, R.A. (1997) Catalysis: The Key to Waste Minimization. Journal of Chemical Technology & Biotechnology, 68, 381-388.
- 6. Choudary, B.M., Lakshmi Kantam, M., Venkat Reddy, C., Koteswara Rao, K. and Figueras, F. (1999) Henry Reactions Catalysed by Modified Mg-Al Hydrotalcite: An Efficient Reusable Solid Base for Selective Synthesis of β-Nitroalkanols. Green Chemistry, 1, 187-189. https://doi.org/10.1039/a904075g
- 7. Choudary, B.M., Kantam, M.L. and Kavita, B. (2001) Synthesis of 2-Nitroalkanols by Mg3Al2O-t-Bu Hydrotalcite. Journal of Molecular Catalysis A: Chemical, 169, 193-197. https://doi.org/10.1016/S1381-1169(00)00558-6
- 8. Seebach, D., Beck, A.K., Mukhopdyay, T. and Thomas, E.H. (1982) Diastereoselective Synthesis of Nitroaldol Derivatives. Helvetica Chimica Acta, 65, 1101-1133.
- 9. Bulbule, V.J., Deshpande, V.H., Velu, S., Sudalai, A., Sivasankar, S. and Sathe, V.T. (1999) Heterogeneous Henry Reaction of Aldehydes: Diastereoselective Synthesis of Nitroalcohol Derivatives over Mg-Al Hydrotalcites. Tetrahedron, 55, 9325-9332. https://doi.org/10.1016/S0040-4020(99)00494-9
- 10. de Vries, A.H.M., de Vries, J.G., van Assema, F.B.J., de Lange, B., Mink, D. and Hyett, D.J. (2011) Asymmetric Synthesis of (S)-2-Indolinecarboxylic Acid by Combining Biocatalysis and Homogeneous Catalysis. ChemCatChem, 3, 289-292.
- 11. Rosini, G., Ballini, R. and Sorrenti, P. (1983) Synthesis of 2-Nitroalkanols on Alumina Surfaces without Solvent: A Simple, Mild and Convenient Method. Synthesis, 1983, 1014-1016.
- 12. Melot, J.M., Texier-Boullet, F. and Foucaud, A. (1986) Preparation and Oxidation of α-Nitro Alcohols with Supported Reagents. Tetrahedron Letters, 27, 493-496. https://doi.org/10.1016/S0040-4039(00)85513-6
- 13. Ballini, R. and Bosica, G. (1997) Nitroaldol Reaction in Aqueous Media: An Important Improvement of the Henry Reaction. The Journal of Organic Chemistry, 62, 425-427. https://doi.org/10.1021/jo961201h
- 14. Kloetstra, K.R. and Van Bekkum, H. (1995) Base and Acid Catalysis by the Alkali-Containing MCM-41 Mesoporous Molecular Sieve. Journal of the Chemical Society, Chemical Communications, No. 10, 1005-1006. https://doi.org/10.1039/c39950001005
- 15. Mokhtar, M., Saleh, T.S., Ahmed, N.S., Al-Thabaiti, S.A. and Al-Shareef, R.A. (2011) An Eco-Friendly N-Sulfonylation of Amines Using Stable and Reusable Zn-Al-Hydrotalcite Solid Base Catalyst under Ultrasound Irradiation. Ultrasonics Sonochemistry, 18, 172-176. https://doi.org/10.1016/j.ultsonch.2010.05.001
- 16. Mokhtar, M., Saleh, T.S. and Basahel, S.N. (2012) Mg-Al Hydrotalcites as Efficient Catalysts for Aza-Michael Addition Reaction: A Green Protocol. Journal of Molecular Catalysis A: Chemical, 353-354, 122-131.
- 17. Saleh, T.S., Narasimharao, K., Ahmed, N.S., Basahel, S.N., Al-Thabaiti, S.A. and Mokhtar, M. (2013) Mg-Al Hydrotalcite as an Efficient Catalyst for Microwave Assisted Regioselective 1,3-Dipolar Cycloaddition of Nitrilimines with the Enaminone Derivatives: A Green Protocol. Journal of Molecular Catalysis A: Chemical, 367, 12-22.
- 18. Narasimharao, K., Al-Sabban, E., Saleh, T. and Gallastegui, A.G., Sanfiz, A.C., Basahel, S., Al-Thabaiti, S., Alyoubi, A., Obaid, A. and Mokhtar, M. (2013) Microwave Assisted Efficient Protocol for the Classic Ullman Homocoupling Reaction Using Cu-MG-Al Hydrotalcite Catalysts. Journal of Molecular Catalysis A: Chemical, 379, 152-162. https://doi.org/10.1016/j.molcata.2013.08.013
- 19. Basahel, S.N., Al-Thabaiti, S.A., Narasimharao, K., Ahmed, N.S. and Mokhtar, M. (2014) ChemInform Abstract: Nanostructured Mg-Al Hydrotalcite as Catalyst for Fine Chemical Synthesis. Journal of Nanoscience and Nanotechnology, 14, 1931-1946.
- 20. Celaya-Sanfiz, A., Morales-Vega, N., De Marco, M., Iruretagoyena, D., Mokhtar, M., Bawaked, S.M., Basahel, S.N., Al-Thabaiti, S.A., Alyoubi, A.O. and Shaffer, M.S.P. (2015) Self-Condensation of Acetone over Mg-Al Layered Double Hydroxide Supported on Multi-Walled Carbon Nanotube Catalysts. Journal of Molecular Catalysis A: Chemical, 398, 50-57. https://doi.org/10.1016/j.molcata.2014.11.002
- 21. Phukan, M., Borah, K.J. and Borah, R. (2009) Henry Reaction in Environmentally Benign Methods Using Imidazole as Catalyst. Green Chemistry Letters and Reviews, 2, 249-253. https://doi.org/10.1080/17518250903410074
- 22. Li, T.F., Miras, H.N. and Song, Y.-F. (2017) Polyoxometalate (POM)—Layered Double Hydroxides (LDH) Composite Materials: Design and Catalytic Applications. Catalysts, 7, 260. https://doi.org/10.3390/catal7090260
- 23. Nakhate, A.V., Rasal, K.B., Deshmukh, G.P., Gupta, S.S.R. and Mannepalli, L.K. (2017) Synthesis of Quinoxaline Derivatives from Terminal Alkynes and O-Phenylenediamines by Using Copper Alumina Catalyst. Journal of Chemical Sciences, 129, 1761-1769. https://doi.org/10.1007/s12039-017-1393-0
- 24. Wang, D.F., Zhang, X.L., Liu, C.L., Cheng, T.T., Wei, W. and Sun, Y.H. (2015) Transition Metal-Modified Mesoporous Mg-Al Mixed Oxides: Stable Base Catalysts for the Synthesis of Diethyl Carbonate from Ethyl Carbamate and Ethanol. Applied Catalysis A: General, 505, 478-486.
- 25. Jitianu, M., Gunnes, D.C., Aboghye, D.E., Zaharescu, M. and Jitianu, A. (2013) Nanosized Ni-Al Layered Double Hydroxides—Structural Characterization. Materials Research Bulletin, 48, 1864-1873. https://doi.org/10.1016/j.materresbull.2013.01.030
- 26. Das, D.P., Das, J. and Parida, K. (2003) Physicochemical Characterization and Adsorption Behavior of Calcined Zn/Al Hydrotalcite-Like Compound (HTlc) towards Removal of Fluoride from Aqueous Solution. Journal of Colloid and Interface Science, 261, 213-220. https://doi.org/10.1016/S0021-9797(03)00082-1
- 27. Pil, K., Younghun, K., Heesoo, K., In, K.S. and Jongheop, Y. (2004) Synthesis and Characterization of Mesoporous Alumina with Nickel Incorporated for Use in the Partial Oxidation of Methane into Synthesis Gas. Applied Catalysis A: General, 272, 157-166. https://doi.org/10.1016/j.apcata.2004.05.055
- 28. Renuka, N.K., Shijina, A.V., Praveen, A.K. and Aniz, C.U. (2014) Redox Properties and Catalytic Activity of CuO/γ-Al2O3 Meso Phase. Journal of Colloid and Interface Science, 434, 195-200. https://doi.org/10.1016/j.jcis.2014.08.005
- 29. Sun, Z.X., Zheng, T.T., Bo, Q.B., Du, M. and Forsling, W. (2015) Effects of Calcination Temperature on the Pore Size and Wall Crystalline Structure of Mesoporous Alumina. Journal of Colloid and Interface Science, 319, 247-251,
- 30. Kuma, P.A., Reddy, M.P., Ju, L.K., Hyun-Sook, B. and Phil, H.H. (2008) Low Temperature Propylene SCR of NO by Copper Alumina Catalyst. Journal of Molecular Catalysis A: Chemical, 291, 66-74. https://doi.org/10.1016/j.molcata.2008.05.006









