World Journal of Vaccines
Vol. 1 No. 1 (2011) , Article ID: 3917 , 4 pages DOI:10.4236/wjv.2011.11001
Perinatal Outcome of Inadvertent Immunization with the Measles-Rubella Vaccine in Pregnant Mexican Women during the Campaign for the Eradication of Congenital Rubella in 2008
![]()
1National Center for the Health of the Childhood and the Adolescence, Ministery of Health, Mexico; 2Epidemiological Diagnostics and Reference Institute, Ministry of Health, Mexico.
Email: jesusreynaf@prodigy.net.mx
Received January 1st, 2011; revised January 25th, 2011; accepted February 10th, 2011.
Keywords: Congenital Rubella, Pregnancy, Perinatal Repercussion
ABSTRACT
Objective: To investigate maternal and neonatal complications resulting from inadvertent immunization against rubella-measles during the first trimester of pregnancy. Methods: A prospective and descriptive study was carried out, including a total of 1,924 pregnant women, 175 (9.1%) of which were classified as non responding to infection by the rubella virus. They underwent clinical and ultrasonographic follow-up to dismiss maternal or fetal complications and complications at the time of delivery. The infant was checked to determine demographic, anthropometric, serological and clinical features at the time of birth. Results: No women had complications during the pregnancy, including exanthematic symptoms. 174/175 newborns were studied; one pregnancy was interrupted based on non-medical arguments. The findings in terms of the analyzed patients suggest a benign evolution after inadvertently immunizing the pregnant women, which support studies with similar results. No complications during the course of the pregnancy or phenotypic alterations of the infant at the time of birth are suggested.
1. Introduction
One of the main worries when massive strategies of immunization are carried out with vaccines containing live attenuated viruses is the possibility of administering them without knowing a woman is pregnant, which would counter-indicate its application. Considering the theoretical risk of producing congenital defects attributable to the administration of immunogens constitutes the main argument to take into account such recommendation [1,2].
One of the components of the measles-rubella (MR) vaccine is the RA 27/3 strain live attenuated rubella virus; the vaccine is considered to be secure, effective and relatively cheap [3]. Each dose of the vaccine is calculated to contain at least 1.000 TCID50 (tissue culture infectious dose). It induces the production of IgM and IgG humoral antibodies, as well as IgA secretory antibodies in the nasopharynx. The infection with the rubella vaccine virus has been detected by means of an IgM (+) serological study, approximately 12 days after the administration of the vaccine or by means of the isolation of the vaccine virus in the case of pregnant women reported to the Centers for Disease Control and Prevention (CDC). None of the newborns displayed clinical manifestations compatible with the congenital rubella syndrome (CRS) [3-6].
In Mexico during 2008, following the Pan American Health Organization’s (PAHO) recommendations, a national strategy was implemented to eradicate the CRS through the application of more than 22 million doses of the MR vaccine to men and women between 19 and 29 years of age. It was feasible to anticipate that some pregnant women would inadvertently receive the vaccine, due to the unawareness of their state during its early stages. This is why the follow-up of these women and their newborns was carried out intentionally in order to describe the perinatal and serological outcomes post-immunization.
2. Methods
A descriptive study of epidemiological surveillance was carried out following up on pregnant women who were inadvertently immunized with the MR vaccine and afterwards on their newborns. Clinical data of CRS or serological evidence of infection were pursued.
The primary study by means of which immunized pregnant women were identified was carried out through the documented reports at the different vaccination facilities they attended once the pregnancy was evident. The follow-up began by looking for human chorionic gonadotropin in urine or its beta fraction in blood once the pregnancy was medically confirmed. Ultrasonographic evidence was also taken into account. The reports were codified according to the type of the applied vaccine, the allotment number, the application date and the clinical follow-up. The follow-up and the evaluation of cases were performed according to PAHO’s recommendations [7].
3. Operative Definitions [7-9]
(a) Inadvertent immunization during pregnancy: Administration of the MR vaccine during the first trimester of pregnancy, according to the date of the last menstruation, or 1-2 weeks previous to or 4-6 weeks after conception.
(b) Immune patients: Those who displayed negative IgM results or positive IgG results by the ELISA method performed during the 30 days post-immunization or after this period.
(c) Non-responding patients: Those who displayed negative IgM and IgG results after the immunization or those who displayed positive IgM results 12 days after the immunization coupled with negative IgG results during a period no longer than 30 days.
(d) Neonatal rubella virus infection: New-born patients who displayed positive IgM serology with or without negative IgG results in samples taken at the moment of birth or newborns with an IgG determination four times higher than the mother’s at the time of birth.
(e) Congenital rubella syndrome: Patients with serological indication of active infection plus structural/ morphological alterations, hearing or visual alterations.
4. Specific Procedures of the Study
After identifying the inadvertently immunized pregnant women, the case study form was filled out; it included the following variables: age, number of pregnancies, gestational age at the time of the immunization (Table 1), date when the sample was taken, number of days lapsed between the immunization and the serology test (ELISA) to assess the mother’s immunological state, determining
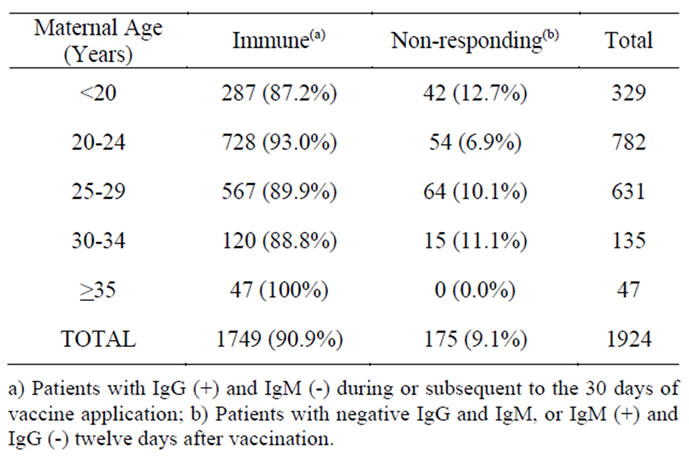
Table 1. Maternal ages and classification of the serologic state at the time of the inadvertent immunization.
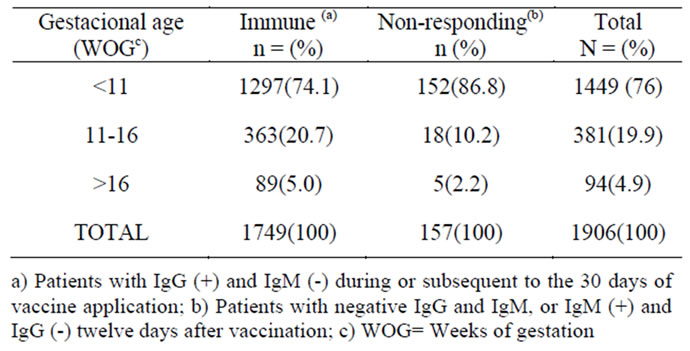
Table 2. Gestational ages and classification of the serologic state at the time of the inadvertent immunization.
immunoglobulin M (IgM) and immunoglobulin G (IgG) levels against the rubella virus (Table 2). Pregnant women classified as non-responding were monitored and assessed until the end of their pregnancy. Women considered as immune were not followed up, since it was considered that they and their fetuses were at no risk of infection neither by the wild virus nor by the vaccine virus given the presence of IgG antibodies.
Determination of antibodies in serum samples from the umbilical cord of all the children of susceptible mothers was carried out at birth. Later on, they were assessed by subspecialists in order to detect clinical manifestations suggestive of CRS. The case study was performed and it included the following variables: gestational age, Apgar score, sex, weight at birth, cephalic perimeter, complications during delivery.
Serology for rubella was carried out at the of the Health Ministry’s Diagnosis and Epidemiological Reference Institute (Instituto de Diagnóstico y Referencia Epidemiológicos – InDRE).
The statistical analysis was carried out by means of descriptive statistics, determining frequencies, means and application rates.
5. Results
5.1. Maternal Demographic and Serological Characteristics
From the estimated 22 million doses of the MR vaccine applied during the campaign to Mexican population of both sexes between 19 and 29 years of age, a total of 1,924 pregnant women were inadvertently immunized (8.7 cases per 100,000 applied doses). One hundred and seventy five (9.1%) of them were identified as non responding to the infection at the time of immunization, while 1,749 (90.9%) were classified as immune. None of the pregnant women, in either group, developed clinical symptoms of rubella during their pregnancy.
5.2. Characteristics of the Newborns
Out of 175 pregnancies considered non-responding to infection, 174 produced live-born infants; one pregnancy was voluntarily terminated. Nine (5.1%) pregnancies were considered pre-term, 163 (93.6%) were full-term, and 3 (1.7%) were post-term. The nine pre-term patients had to be hospitalized due to this condition. None of the newborns displayed congenital malformations and their serology was similar to the mother’s in all cases. No positive IgM results were reported in any of them. Audiometric and visual tests showed no alterations.
6. Discussion
The findings in this study are in conformity with the results of follow-up studies carried out with pregnant women inadvertently immunized with the rubella vaccine RA 27/3 strain in countries such as the United States, Canada, the United Kingdom, Germany and Iran. These studies did not detect cases of CRS, thus supporting the evidence of the absence of fetal risk [3-5], [10-12].
Theoretically, arguments consider that infection risk for the product of pregnant women inadvertently immunized is less than 1%, in comparison with the 60% established for the primo infection with the wild rubella virus in its systemic manifestation [12-14].
Arguments for the vaccine virus’s diminished capacity to hurt the fetus have been based on a biological point of view [10-14]:
1) The vaccine virus requires several weeks to go through the placenta and it finds it difficult to grow in cell cultures enriched with amniotic fluid. This gives the pregnant woman enough time to give rise to an appropriate inflammatory response before the virus is able to makes its way through the placenta.
2) There is no replication of the vaccine virus in the respiratory system; therefore a minimum or practically null phase of viremia is established. This implies that the virus is not able to reach the different tissues, the placenta included.
3) It has been argued that the reactivation and dissemination of the rubella vaccine virus is accomplished by direct cell-to-cell contact, through the fusion of the virus with the membranes of the adjacent cells. Even so, viremia is a more important condition in the case of placental infection, which would be followed by fetal infection.
4) Finally, the basic molecular mechanism responsible for the success of vaccines must be taken into account: The individual receives a dead or attenuated pathogen and establishes a primary response, free of the expression of virulence and of the pathological conditions that would enable the agent to hurt the tissues. When the infection occurs, the memory cells react rapidly eliminating the aggressive agent [9-14].
In the face of the benign evolution of the pregnancies complicated with the inadvertent immuni-zation with rubella, we do not consider a therapeutic intervention to be necessary at any point during the pregnancy, and much less should the interruption of the gestation be contemplated. Even though no real risk of CRS associated to vaccine against rubella has been documented, the recommendation not to immunize pregnant women has been established to avoid associating the vaccine to complications that may eventually take place during the pregnancy, including the possibility of a miscarriage or of the newborn exhibiting alterations not related to the vaccine [15]. Majority inadvertent immunizations were made before 11 weeks of gestational age, at the time of organogenesis; however, structural organic alterations were not present.
On the other hand, from a public health approach, the goal of massive immunization for the prevention of con genital rubella in Mexico is to have an impact on the disease considering that the benefit of such strategy is much higher than the risk of the procedure. In the case of women in their reproductive stage, the benefit is clear because if they were not to be immunized, then they would be at risk of getting infected by the wild virus and, hence, there would be a 60% chance of their children having CRS.
The rate of non responding pregnant women in this study corresponded to 9.1% of all the women inadvertently immunized and this represents a number of lost opportunities that similar campaigns worldwide want to have an impact on. This rate corresponds to the figures reported in seroepidemiological surveys in Mexico and they constitute the rationale for the immunization for all women in reproductive age during adolescence: Protecting 9.1% of women of reproductive age against the infection means that one susceptible woman out of every nine women of reproductive age will be protected. Financially, this seems more feasible than performing a serological test on every woman of reproductive age to identify those who are susceptible and would benefit from the vaccine. What has been demonstrated up to date is that the benefit is greater than the risk [16,17,18].
We should continue encouraging the public immunization of all adolescent women in the country at 12 years of age, before their first pregnancy, and then carry out serological measurements that will enable us to determine if the duration of the immunity will protect them and their children during their reproductive years. Adult women between 19 and 39 years of age that did not receive the recommended doses of the MR vaccine during adolescence should be encouraged to do it before thinking about getting pregnant. On the other hand and according to the results, the risk of infection and complications for the mother or the child due to an inadvertent immunization is practically null, so terminating the pregnancy is not justified. Even so, due to the theoretical risk of infection of the fetus, MR immunization should still be avoided during pregnancy.
7. Acknowledgements
We are grateful to Irma Lopez Martinez of the Epidemiological Diagnostics and Reference Institute for her assistance with this investigation.
REFERENCES
- Centers for Disease Control & Prevention. Notice to Readers: Revised ACIP Recommendation for Avoiding Pregnancy After Receiving a Rubella-Containing Vaccine. MMWR 50 (No.49); 1117, 2001.
- F. T Cutts, “Lucha contra la rubéola y el síndrome de rubéola congénita (SRC) en los países en desarrollo,” Bulletin of the World Health Organization, 1997, pp.55-68.
- CDC, “Achievements in Public Health: Elimination of Rubella and Congenital Rubella Syndrome-United States 1969-2004,” MMWR, Vol. 54, No. 11, 2005, pp. 279-282.
- NM Gregg, “Congenital cataract following German measles in the mother,” Epidemiology and Infection Vol. 107, No.1, Aug., 1991, pp. 3-14.
- S. Sheppard, R.W. Smithells, A. Dickson and H. Holzel, “Rubella vaccination and pregnancy: preliminary report of a national survey,” British Medical Journal (Clinical Research Ed), Vol. 292, No. 6522, 1986, p. 727. doi:10.1136/bmj.292.6522.727
- Centers for Disease Control and Prevention, “Measles, mumps and rubella vaccine use and strategies for elimination of measles, rubella and congenital rubella syndrome and control of mumps. Recommendation of the Advisory Committee on Immunization Practices,” MMWR, Vol. 47, No. 8, 1998, pp. 1-57.
- Organización Mundial de la Salud, “Documento de posición de la OMS,” Vacuna contra la rubeola, http://www.who.int/immunization/PP_rubella_SP.pdf.
- SSA, “NOM-017-SSA2-1994, para la vigilancia epidemiológica,” Norma Oficial Mexicana, 1994.
- L. Dontigny, M. Arcenault and M. Martel, “Rubella in Pregnancy,” SOGC Clinical Practice Guidelines, 2008, pp. 153-154.
- G Enders, “Rubella Antibody Titers in Vaccinated and Nonvaccinated Women and Results of Vaccination during Pregnancy,” Reviews of Infectious Disease, Vol. 7, No. 1, 1985, pp. 103-107. doi:10.1093/clinids/7.Supplement_1.S103
- Centers for Disease Control & Prevention, “Current Trends Rubella Vaccination during Pregnancy–United States,” MMWR, Vol. 38, No.17, 1971-1988, pp. 289-293.
- J. Banatvala, “Rubeola,” The Lancet, 2004, pp. 1-10.
- E. Miller, J. E. Cradock-Watson and T. H. Pollock, “Consequences of confirmed maternal rubella at successive stages of pregnancy,” Lancet, Vol. 2, 1982, pp. 781-784. doi:10.1016/S0140-6736(82)92677-0
- R. Hamkar and S. Jalilvand, “Inadvertent rubella vaccination of pregnant women: Evaluation of possible transplacental infection with rubella vaccine,” Vaccine, Vol. 24, No. 17, 2006, pp. 3558-3563. doi:10.1016/j.vaccine.2006.02.001
- Morice A, Ulloa-Gutierrez R; Ávila-Agüero M. Congenital Rubella Syndrome: Progress and Future Challenges. Expert Rev Vaccines. 2009; 8(3): 323-331. doi:10.1586/14760584.8.3.323
- Secretaria de Salud (México), “Anuarios de morbilidad,” 1984-2008. http://www.dgepi.salud.gob.mx/anuario/index.html.
- Díaz-Ortega JL, Meneses-Reyes CD, Palacios-Martínez M. Incidencia y patrones de transmisión de rubeola en México. Salud Pública Mex 2007; 49: 337-344.
- G. Gutiérrez, O. Muñoz, R. Tapia, M. E. Bustamante, M. T. Álvarez and J. P. Guiscafré, et al, “Seroepidemiología de la rubéola en mujeres mexicanas. Encuesta Nacional Probabilística,” Salud Publica Mex, Vol. 32, 1990, pp. 623-631.
- J. S. Remington, J. O. Klein, C. B. Wilson and C. J. Beker, “Infectious diseases of the fetus and newborn infant,” Elseviers Saunders, 2006, Vol. 6, pp. 464-73.

