World Journal of Condensed Matter Physics
Vol.06 No.04(2016), Article ID:71727,7 pages
10.4236/wjcmp.2016.64027
IR Spectroscopic Study of Silicon Nitride Films Grown at a Low Substrate Temperature Using Very High Frequency Plasma-Enhanced Chemical Vapor Deposition
Shin-ichi Kobayashi
Department of Electronics and Mechatronics, Tokyo Polytechnic University, Atsugi, Japan

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 23, 2016; Accepted: October 30, 2016; Published: November 2, 2016
ABSTRACT
Hydrogenated amorphous silicon nitride (a-SiNx:H) films have been grown from a SiH4–N2 gas mixture through very high frequency (VHF) plasma-enhanced chemical vapor deposition (PECVD) at 50˚C. The films are dense and transparent in the visible region. The peak frequency of the Si–N stretching mode in the IR absorption spectrum increases with increasing N–H bond density, which is similar to the behavior of a-SiNx:H films grown from SiH4–NH3 gas. During storage in a dry air atmosphere, the Si–O absorption increases. A large shift in the peak frequency of the Si–N stretching mode in the initial stage of oxidation, which is higher than the shift expected from the increase in the N–H bond density, is mainly caused by the change in the sum of electronegativity of nearest neighbors around the Si–N bond due to the increase in the Si–O bond density.
Keywords:
Silicon Nitride, PECVD, VHF, FTIR

1. Introduction
Hydrogenated amorphous silicon nitride (a-SiNx:H) films are useful as barrier layers to prevent the diffusion of oxygen and water into optical devices. To protect optical devices against thermal damage during the deposition of an a-SiNx:H film, a plasma- enhanced chemical vapor deposition (PECVD) system is used at a low substrate temperature. However, these a-SiNx:H films generally have high densities of N-H bonds and Si-H bonds; consequently, the aerial oxidation of H-terminated or dangling bonds can easily occur. Therefore, it is important to clarify the mechanism of the initial oxidation process during air exposure to protect optical devices, such as organic photovoltaic devices, which require high performance barrier films.
A-SiNx:H films are usually grown from a SiH4–NH3 gas mixture. However, in order to obtain low-hydrogen-content a-SiNx:H films, it is preferable to use N2 instead of NH3 as the nitrogen source because N2 does not contain N–H bonds. Usually, a-SiNx:H films are deposited using a PECVD system at a 13.56 MHz excitation frequency. Shifting the discharge frequency into the very high frequency (VHF) region results in a higher deposition rate for PECVD in a conventional diode-type reactor for a-Si:H [1] , μc-Si [2] , and a-SiNx:H films deposited from a SiH4–NH3–N2 gas mixture [3] . Thus far, a-SiNx:H films have not been produced through VHF–PECVD from a SiH4–N2 gas mixture without NH3 gas, except for one example of atmospheric-pressure (AP) PECVD at 150 MHz [4] . Thus, in a previous study, the VHF excitation of plasma was attempted to decompose the N2 gas diluted SiH4 gas in a conventional PECVD reactor under reduced pressure at 350˚C and we revealed that VHF plasma can create a transparent film with lower excitation power compared to that resulting from conventional RF plasma [5] .
In this paper, the deposition of a-SiNx:H films at 50˚C is presented. The effects of power density on the deposition rate, optical bandgap (Eopt), and Si–H and N–H bond densities (NSi–H and NN–H) in the resulting a-SiNx:H films are investigated. The changes in the local structure of the films that occurred during storage in dry air, as detected using Fourier transform infrared spectroscopy (FTIR), were also studied to understand the stability mechanism in the film properties.
2. Experimental
The a-SiNx:H films were grown through the VHF–PECVD of a SiH4–N2 gas mixture in a diode-type reactor, in which each electrode had a diameter of 60 mm and the electrode separation was 10 mm. The ultimate vacuum pressure of the reactor was ~10–6 Pa and the total pressure of 2% SiH4 diluted with N2 gas was 200 Pa with a flow rate of 30 sccm. Fused silica and Si(100) wafers were used as the substrates and the substrate temperature was maintained at 50˚C during the deposition. The VHF frequency was 150 MHz and the VHF power density was varied from 70 to 385 mW/cm2. The typical film thickness was 300 nm. The Tauc optical bandgap Eopt was obtained from the optical transmittance spectrum. The Si–H bond density NSi–H and N–H bond density NN–H were calculated from the FTIR absorption spectrum in transmission mode through the expression:
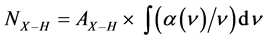 (1)
(1)
where X = Si or N, 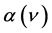 is the absorption coefficient and ν is the wavenumber [6] . The calibration factor
is the absorption coefficient and ν is the wavenumber [6] . The calibration factor 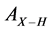 values of the Si–H (~2100 cm−1) and N–H (~3300 cm−1) stretching modes are 1.7 × 1020 and 2.8 × 1020, respectively [7] .
values of the Si–H (~2100 cm−1) and N–H (~3300 cm−1) stretching modes are 1.7 × 1020 and 2.8 × 1020, respectively [7] .
In order to investigate the local structural changes that occurred in a-SiNx:H films during storage, the samples were stored in an FTIR system containing dry air for 100 days with the temperature kept at 25˚C and the relative humidity (RH) maintained below 0.5 %.
3. Results and Discussion
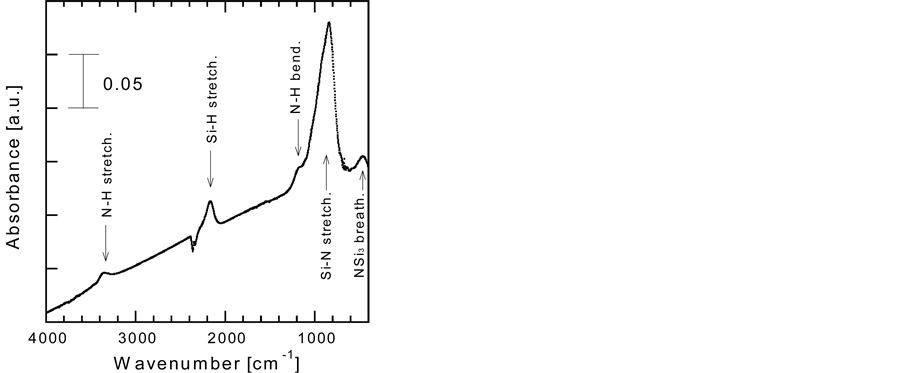
Figure 1 shows the VHF power density dependence of Eopt of the a-SiNx:H films. Eopt has a lower value of 3.7 eV at a VHF power density of 70 mW/cm2; it is suggested that the electron-impact dissociation of N2 is insufficient to obtain a transparent film at a low VHF power density. Eopt becomes ~5 eV when the VHF power density is greater than 140 mW/cm2. At these values, a-SiNx:H films are transparent in the visible region and the deposition rate is ~1 nm/s. The surface morphology and structure of the films were studied using a scanning electron microscope (SEM) and a transmission electron microscope (TEM). The films appear to be homogeneous and there is no evidence of a micro columnar structure which observed in [8] . A typical FTIR absorption spectrum of an a-SiNx:H film is shown in Figure 2. The bands assigned to the Si–N stretching vibration centered at ~850 cm−1, N–H bending mode at 1170 cm−1, Si–H stretching mode at 2100 cm−1, N–H stretching mode at 3340 cm−1, and Si–N breathing mode at 470 cm−1 can be clearly identified.
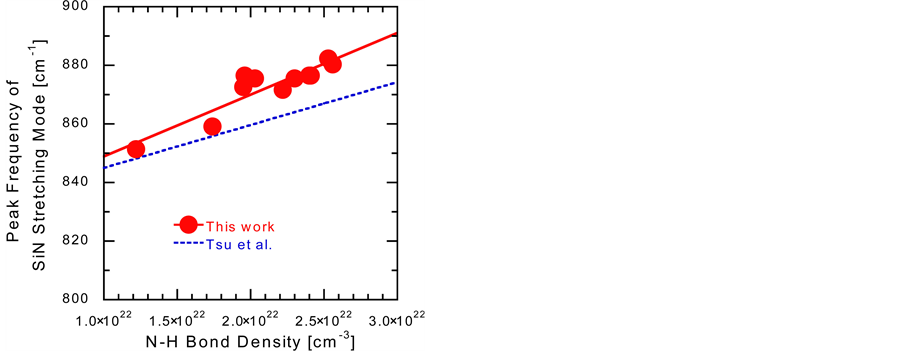
Figure 3 shows the VHF power density dependence of NSi–H and NN–H in the a-SiNx:H films. At a VHF power density of 70 mW/cm2, at which Eopt is 3.7 eV, indicating a Si-rich a-SiNx:H film, NSi–H is higher than NN–H. As the VHF power is increased, NN–H slightly increases and then saturates, while NSi–H exponentially decreases, because an increase in the VHF power enhances the decomposition of SiH4 and N2 gases. Although N2 does not contain N–H bonds itself, the NN–H value of the a-SiNx:H films with an Eopt of ~5 eV is of the order of 1022 cm−3. In Figure 4, it can be observed that the Si–N stretching frequency increases with increasing NN–H. This shift in the Si–N stretching frequency is induced by the back-bonding of the H atom, which is more electronegative than Si, to the N atom of the Si–N bonds. The peak frequencies of the Si–N
Figure 1. VHF power dependence of the optical bandgap of a-SiNx:H film.
Figure 2. FTIR spectrum of an a-SiNx:H film deposited at 50˚C with a VHF power density of 70 mW/cm2.
Figure 3. VHF power dependence of Si–H (NSi–H) and N–H (NN–H) bond densities.
Figure 4. Peak frequency of the Si–N stretching mode as a function of the N–H bond density (NN–H).
stretching mode obtained in the present study are slightly higher than those obtained from films deposited from a SiH4–NH3 gas mixture reported by Tsu et al. [9] .
Figures 5(a)-(c) show the FTIR spectra of the as-deposited and aged a-SiNx:H films deposited at 210 mW/cm2. The as-deposited a-SiNx:H film has an Eopt of 5.2 eV, NN–H of 2.6 × 1022 cm−3, and NSi–H of 1.6 × 1021 cm−3, respectively. The sample was fixed on the sample holder in the FTIR system containing dry air (25˚C, <0.5% RH) for 100 days, during which the peak frequency of the Si–N stretching mode shifted from 880 cm−1 to 898 cm−1 (Figure 5(c)). Assuming that this shift was caused only by the change in the NN–H value in the same manner observed for the as-deposited film, as shown in Figure 4, a large increase of 2.0 × 1022 cm−3 in the NN–H value is required. To clarify the changes in the absorbance during storage, the difference between the as-deposited film and the same film aged for 100 days are depicted in Figures 5(d)-(f). The small increase of 1.0 × 1021 cm−3 in the NN–H value during storage (Figure 5(d)) cannot explain the large shift of nearly 20 cm−1 in the peak frequency of the Si–N stretching mode during storage.
In Figure 5(f), the spectrum is clearly divided into six Gaussian peaks and valleys labeled as n1–n6. The absorbance at 1050 cm−1 (n5) is assigned to Si–O stretching mode and increases as the exposure time increases, implying that film oxidation occurred. The absorbance at 450 cm−1 (n1), existing in the vicinity of the Si–N breathing mode (470 cm−1), is assigned to the Si–O rocking mode. The increase of 13% in the integrated absorbance at 1170 cm−1 (n6) is due to the Si–O transverse mode [10] rather than the N–H bending mode because the increase in the integrated absorbance at 1050 cm−1 (n5) is 12% and the change in NN–H calculated from the 3340 cm−1 band during storage is small (4%). Since the FWHM values of these peaks are at most 75 - 120 cm−1, increases in the spectrum derived from Si-O bonds do not directly affect to the shift in the Si–N stretching frequency.
For the Si–N stretching vibration at 750 - 1000 cm−1, while the intensities at 780 cm−1 (n2) and 835 cm−1 (n3) obviously decrease, a peak simultaneously appears at 940 cm−1
Figure 5. (a) - (c) FTIR spectra of the as-deposited and aged a-SiNx:H films; (d) - (f) changes in FTIR spectra during storage.
(n4). These peaks and valleys are the main causes for the peak shift of the Si–N stretching mode to a higher value at the initial stage of oxidation. As mentioned before, the increase in NN–H during storage is small. Therefore, the effect of increase in NN–H during storage on the shift in the peak frequency through changes in the sum of electronegativity of the nearest neighbors around the Si–N bond, is small. On the other hand, oxidation of a-SiNx:H films occurred during storage, thus, Si–O bonds increased. This increase in the concentrations of the O atom, which is more electronegative than N, back- bonded to the Si atoms of the Si–N bonds caused a new increase in absorption at the high-wavenumber side (940 cm−1, n4). For the low-wavenumber side, there are some possible causes for the generation of valleys. When a peak appears in a spectrum due to changes in electronegativity of the nearest neighbors, a valley of almost identical area appears necessarily. The valley at the low-wavenumber side (n3) is considered to be caused by such a reduction with the generation of the new peak (n4). Meanwhile, Lucovsky and co-workers pointed out that the low-wavenumber side (840 cm−1) of the Si–N stretching mode reflects the vibration of the Si–N bonds, in which at least one H atom is back-bonded to the Si atom [11] ; however, the change in the NSi–H value of this a-SiNx:H film obtained from Figure 5(e) is too small (<1.0 × 1021 cm−3) to evaluate the effect of bond-breaking of Si-H on the decrease in the absorbance. Furthermore, a reduction due to the Si–N bond-breaking by oxidation is also expected. Although the reported values of the peak frequency in the Si–N stretching mode vary between 835 - 875 cm−1 [7] [10] [11] , the values are slightly higher than n2 in the low-wavenumber side of the Si–N stretching mode.
As shown in Figure 4, the values obtained in this study are slightly higher than those reported by Tsu et al.; this is reasonable if we consider that oxidation already started in the as-deposited film. To clarify the mechanism of the initial oxidation process, including an assignment of n2, further investigations are ongoing using samples having different NSi–H and NN–H values.
4. Conclusion
The a-SiNx:H films grown in the present study through VHF-PECVD from a SiH4–N2 gas mixture are transparent in the visible region. The peak frequency of the Si–N stretching mode increases with increasing NN–H, which is similar to the behavior of a-SiNx:H films grown from SiH4–NH3 gas. During film storage in dry air, the initial stage of oxidation was observed through in-situ IR measurement. The peak frequency of the Si–N stretching mode also increases in the initial stage of oxidation because of the increase of absorbance at the high-wavenumber side, and the decrease at the low-wavenumber side of the Si–N stretching mode caused by an increase of the concentration of O atoms back-bonded to the Si atoms of the Si–N bonds.
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (C) No. 15K04682 from the Ministry of Education, Culture, Sports, Science and Technology of Japan. I wish to acknowledge helpful discussions and encouragement from Professor Y. Hoshi.
Cite this paper
Kobayashi, S. (2016) IR Spectroscopic Study of Silicon Nitride Films Grown at a Low Substrate Temperature Using Very High Frequency Plasma- Enhanced Chemical Vapor Deposition. World Journal of Condensed Matter Physics, 6, 287-293. http://dx.doi.org/10.4236/wjcmp.2016.64027
References
- 1. Heintze, M., Zedlitz, R. and Bauer, G.H. (1993) Analysis of High-Rate a-Si:H Deposition in a VHF Plasma. Journal of Physics D: Applied Physics, 26, 1781-1786.
- 2. Fukawa, M., Suzuki, S., Guo, L., Kondo, M. and Matsuda, A. (2001) High Rate Growth of Microcrystalline Silicon Using a High-pressure Depletion Method with VHF Plasma. Solar Energy Materials and Solar Cells, 66, 217-223.
http://dx.doi.org/10.1016/S0927-0248(00)00176-8 - 3. Takagi, T., Takechi, K., Nakagawa, Y., Watabe, Y. and Nishida, S. (1998) High Rate Deposition of a-Si:H and a-SiNx:H by VHF PECVD. Vacuum, 51, 751-755.
http://dx.doi.org/10.1016/S0042-207X(98)00284-X - 4. Kakiuchi, H., Nakahama, Y., Ohmi, H., Yasutake, K., Yoshii, K. and Mori Y. (2005) Investigation of Deposition Characteristics and Properties of High-rate Deposited Silicon Nitride Films Prepared by Atmospheric Pressure Plasma Chemical Vapor Deposition. Thin Solid Films, 479, 17-23.
http://dx.doi.org/10.1016/j.tsf.2004.11.104 - 5. Kobayashi, S., Ohrui, N., Chao, Y.C., Aoki, T., Kobayashi, H. and Asakawa, T. (2007) Deposition of Luminescent a-SiNx:H Films with SiH4–N2 Gas Mixture by VHF–PECVD Using Novel Impedance Matching Method. Journal of Materials Science: Materials in Electronics, 18, S29-S32.
- 6. Lanford, W.A. and Rand, M.J. (1978) The Hydrogen Content of Plasma-Deposited Silicon Nitride. Journal of Applied Physics, 49, 2473-2477.
http://dx.doi.org/10.1063/1.325095 - 7. Demichelis, F., Giorgis, F. and Pirri, C.F. (1996) Compositional and Structural Analysis of Hydrogenated Amorphous Silicon-Nitrogen Alloys Prepared by Plasma-Enhanced Chemical Vapour Deposition. Philosophical Magazine B, 74, 155-168.
http://dx.doi.org/10.1080/01418639608240333 - 8. Smith, D.L. (1993) Controlling the Plasma Chemistry of Silicon Nitride and Oxide Deposition from Silane. Journal of Vacuum Science & Technology A, 11, 1843-1850.
http://dx.doi.org/10.1116/1.578436 - 9. Tsu, D.V., Lucovsky, G. and Mantini, M.J. (1986) Local Atomic Structure in Thin Films of Silicon Nitride and Silicon Diimide Produced by Remote Plasma-enhanced Chemical Vapor Deposition. Physical Review B, 33, 7069-7076.
http://dx.doi.org/10.1103/PhysRevB.33.7069 - 10. Serra, J., Parada, E.G., Gonzalez, P., Fernandez, D., Chiussi, S., Pou, J., Leon, B. and Perez-Amor, M. (1996) Modification of Silicon Nitride Films to Oxynitrides by ArF Excimer Laser Irradiation. Surface and Coatings Technology, 80, 211-215.
http://dx.doi.org/10.1016/0257-8972(95)02714-9 - 11. Lucovsky, G., Yang, J., Chao, S.S., Tyler, J.E. and Czubatyj, W. (1983) Nitrogen-Bonding Environments in Glow-Discharge-Deposited a-Si:H Films. Physical Review B, 28, 3234- 3240.
http://dx.doi.org/10.1103/PhysRevB.28.3234