Journal of Diabetes Mellitus
Vol.1 No.1(2011), Article ID:3869,5 pages DOI:10.4236/jdm.2011.11001
Early postprandial Insulin secretion: its relation toInsulin sensitivity
Address for Correspondence Udaya M. Kabadi, FACP, FRCP[c], FACE; Clinical Professor of Medicine, University of Iowa Chief, Endocrinology VAMC 3600, 30th Street, Des Moines, IA 50310, USA; udaya.kabadi@va.gov
Received 10 January 2011; revised 13 February 2011; accepted 20 February 2011.
Keywords: Insulin Secretion; Insulin Resistance; Impaired Glucose Tolerance; Type 2 Diabetes Mellitus
ABSTRACT
Background: Lack of first phase insulin secretion during oral glucose tolerance test [OGTT] in Type 2 Diabetes Mellitus (DM) is attributed to glucose toxicity. Alternatively, the role of insulin resistance in impaired insulin release secondary to lack of glucose entry into β cells may be responsible, but is not examined. Aim: The role of insulin sensitivity in 1st phase insulin secretion was assessed. Material and Methods: Plasma glucose (G) and insulin (I) concentrations were determined after an overnight fast (F) and upto 60 minutes during OGTT with glucose 75g in 12 normal (N), 14 with impaired glucose tolerance (IGT) and 41 subjects with Type 2 DM. First phase insulin secretion (Δ Insulin) was determined as a percentage rise from baseline 100x (Peak-Basal)/Basal. Insulin sensitivity was determined as FI x FG (mUxmM/L). Results: FG were normal (< 5.5 mM/L) in both N and IGT; and >7.0 mM/L in Type 2 DM. FI x FG and ∆ insulin were 35 ± 4 and 389 ± 89% in N; 77 ± 5 and 254 ± 65% in IGT; and 235 ± 19 and 95 ± 15% in Type 2 DM. Significant negative correlations were noted between ∆ insulin one hand and FI x FG on the other amongst all subjects [p < 0.0001 for all correlations]. Conclusion: Decline of 1st phase insulin secretion in IGT and Type 2 DM may be attributed to inhibited release of depleted insulin stores in the β Cells induced by impaired glucose entry due to insulin resistance, and is unlikely to be caused by glucose toxicity in IGT in presence of fasting euglycemia.
1. INTRODUCTION
The presence of post prandial hyperglycemia as well as impaired glucose tolerance [IGT] is well documented to be earlier stages in evolution of Type 2 Diabetes Mellitus [1-4]. Moreover, the role of first phase post prandial insulin secretion in controlling post prandial glycemia is well established [4-6]. Finally, inhibition of first phase post prandial insulin secretion in onset of post prandial hyperglycemia or IGT in early stages of evolution of Type 2 DM is well defined [4-7]. This decline in first phase post prandial insulin secretion in subjects with Type 2 DM is often attributed to ‘glucose toxicity’ in presence of fasting hyperglycemia [8,9]. However, the decline in first phase insulin secretion in presence of fasting euglycemia and post prandial hyperglycemia or IGT can not be explained by ‘glucose toxicity’. Moreover, raising plasma glucose in normal subjects during a hyperglycemic clamp study failed to inhibit first phase post prandial insulin rise in normal subjects [10]. Thus, the concept of ‘glucose toxicity’ may neither be totally accurate nor adequate in explaining the inhibition of first phase post prandial insulin secretion, in IGT or Type 2 DM. Therefore, alternative mechanisms need to be explored for the decline in 1st phase insulin secretion in presence of fasting euglycemia and postprandial hyperglycemia in Type 2 DM or in IGT. Insulin resistance at the level of ß cells themselves may be such an alternative mechanism which is likely to inhibit glucose entry and in turn decrease either the synthesis or release of the stored insulin resulting in decreased insulin secretion in response to oral or IV administration of glucose or a meal. Therefore, this study assessed the influence of insulin sensitivity on the magnitude of the first phase insulin secretion.
2. SUBJECTS & METHODS
The study was approved by the Research and Development Committee and the Human Studies Subcommittee at the medical center. 12 lean [BMI, 24 ± 2 kg/m2] male employees, (mean age 46 ± 4 yrs) with normal glucose tolerance [NGT], 14 obese [BMI 35 ± 3 kg/m2] male employees (mean age 48 ± 3 yrs) with impaired glucose tolerance [IGT] and 41 men [BMI, 35 ± 4 kg/m2] newly diagnosed with Type 2 Diabetes (mean age 49 ± 5 yrs) were randomly selected to participate in a study following signing informed consent documents. The diagnosis of NGT, IGT and Type 2 DM was documented by previous OGTT according to criteria established by ADA [11]. Subjects with hospitalization in previous 6 months, elevated liver enzymes greater than twice normal values and serum creatinine > 1.5 mg/dl were excluded. None of the subjects were consuming any medications at the time of the study. They were advised to abstain from alcohol for at least a period of two weeks. Subjects presented at the laboratory between 0800-0900 hours after an overnight fast. 3 ml blood samples were drawn prior to and at 15,30, 45 and 60 minutes after oral ingestion of glucose 75 g. Blood samples were immediately centrifuged at 4˚C and serum was extracted and stored at -20ºC for later determination of glucose and insulin concentrations. Serum glucose and insulin were determined by well established commercial kits. Interassay and intraassay coefficients of variation for these determinations ranged between 4-10% in our laboratory.
First phase insulin secretion was determined as a percentage rise (Δ% insulin 0-30) from baseline [100xPeak insulin level at 30 minutes-basal level/Basal Level.], as well as by insulinogenic index calculated as (Δ% insulin 0-30/Δ% glucose 0-30) as described previously (Table 1) [12]. Insulin sensitivity was determined as a Product, Fasting Insulin x Fasting Glucose (FI x FG) used in HOMA method as well as its modifications, as described previously (Table 1). [13-16]. Moreover, it has been documented recently to be a reliable index of insulin sensitivity [16]. Finally, insulin secretion/insulin resistance index [IS/IR] was determined by a calculation [(Δ%I 0-30/Δ % G 0-30)/ FIxFG] as determined previously [17] because this index also expresses relationship between first phase insulin secretion and insulin sensitivity (Table 1). Statistical analysis was conducted by analysis of variance and by students’ ‘t’ test for comparisons between individual groups. Simultaneously, a relationship between first phase insulin secretion and insulin sensitivity was assessed, by a linear regression method with calculation of a correlation coefficients between Δ% insulin and insulinogenic index on one hand and FI x FG as well as IS/IR on the other. Finally, correlations were also determined between indices of 1st phase insulin secretion on one aspect and both fasting plasma glucose and insulin concentrations on the other.
3. RESULTS
Fasting plasma glucose levels were < 5.5 mM/L in both normal subjects and subjects with IGT whereas HbA1c concentrations were < 5.7% in normal subjects and 5.7-6.4% in subjects with IGT. In comparison, fasting plasma glucose levels and HbA1c concentrations were >8.0 mM/L and > 7.5% in subjects with Type 2 DM. As expressed by both the % rise [Δ%Insulin] as well as insulinogenic index, 1st phase insulin rise was lowest in subjects with Type 2 DM with highest FI x FG, whereas maximum insulin rise was noted in subjects with NGT with lowest FI x FG and intermediate values were observed in subjects with IGT [Table 2]. Moreover, insulin secretion/insulin resistance indices were markedly reduced in subjects with IGT with even further lowering in subjects with Type 2 DM in comparison to values noted in subjects with NGT [Table 2]. A significant negative correlation was noted between % insulin rise on one hand and FI x FG on the other amongst all subjects (Figure 1). Similar significant negative correlation was also noted between insulinogenic index on one hand and index of insulin sensitivity [FPxFG] on the other [Table 2]. Finally, significant positive correlations were also noted between % insulin rise [ΔI] and insulinogenic indices on one aspect and IS/IR indices on the other [Table 3]. However, no significant correlations were noted between Δ % Insulin and insulinogenic indices on one hand and either fasting glucose or fasting insulin concentration on the other. [P > 0.05 for all comparisons].
4. DISCUSSION
Lack of early or first phase insulin secretion during oral glucose tolerance test [OGTT] or following a meal in Type 2 Diabetes Mellitus (DM) is attributed to fasting hyperglycemia, a concept of glucose toxicity [8,9]. However, raising fasting glucose concentration failed to
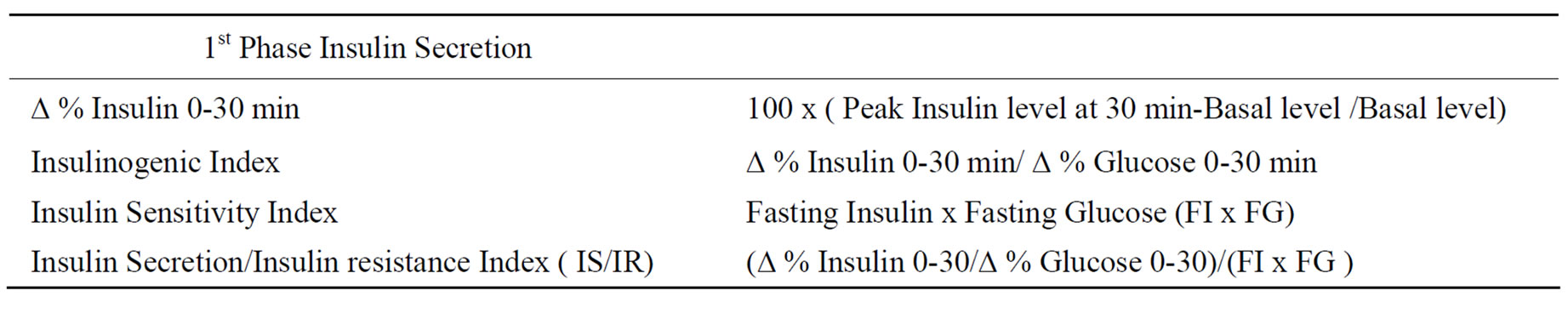
Table 1. Indices of 1st phase insulin secretion and insulin sensitivity.
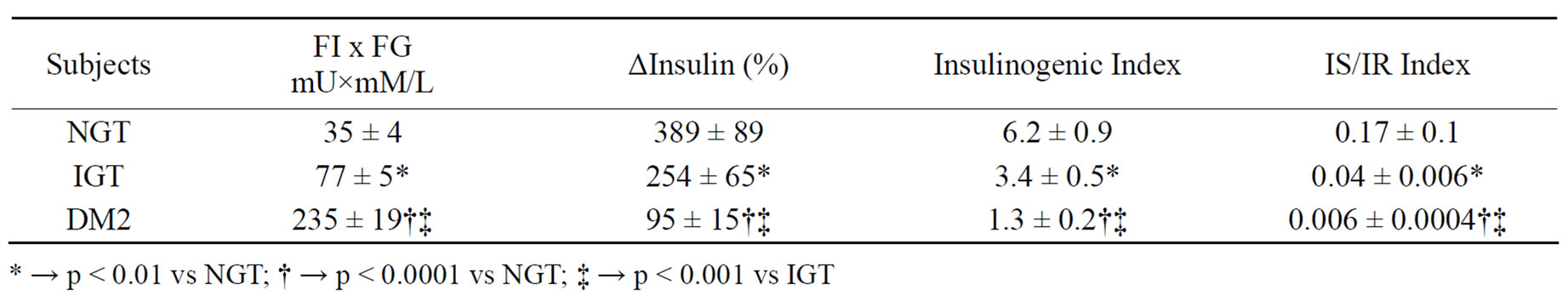
Table 2. Fasting serum insulin x fasting serum glucose (FI x FG), % rise (∆) of insulin at 30 minutes from fasting level, insulinogenic index [Δ I%0-30/Δ% Glucose0-30] and insulin secretion [IS]/insulin resistance [IR] index [(ΔI0-30/ΔG0-30)/(FIxFG)] in participating subjects.
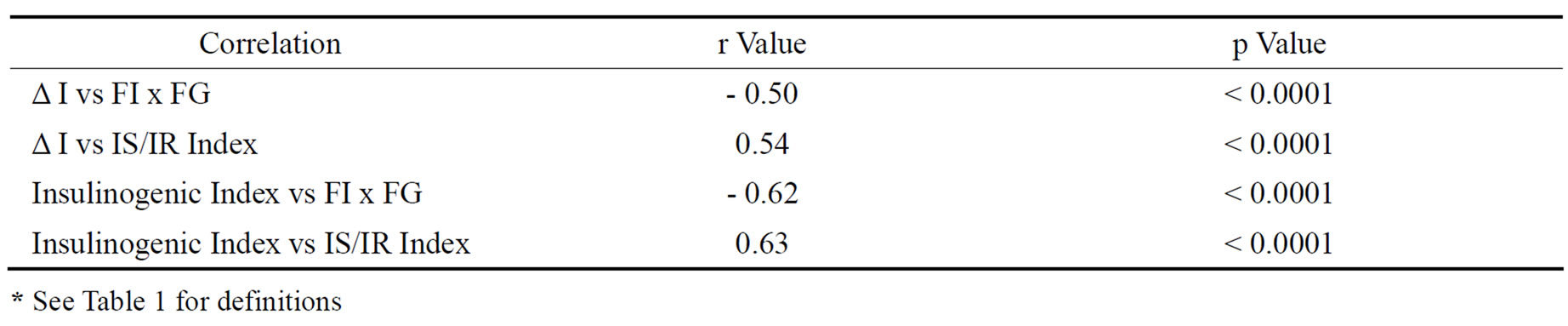
Table 3. Correlations between parameters of insulin secretion [IS] and insulin resistance [IR].
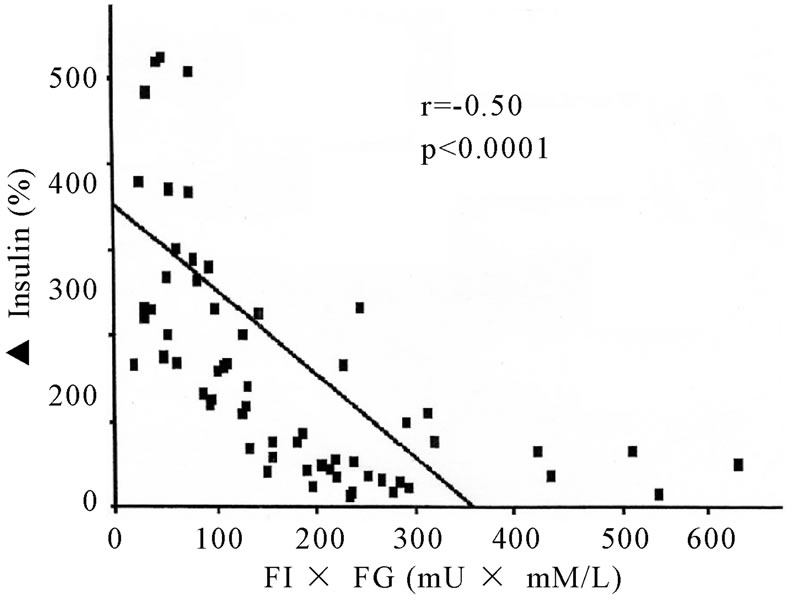
Figure 1. Correlation between1st Phase Insulin Secretion (▲ Insulin) and Index of Insulin Sensitivity (FIxFG).
inhibit first phase insulin secretion in normal subjects [9].
Moreover, the concept of glucose toxicity can not explain the decline in 1st phase insulin secretion in subjects with impaired glucose tolerance (IGT) because of presence of fasting euglycemia. This study demonstrates that first phase or early insulin response to oral glucose load is distinctly dependent on the degree of insulin resistance as expressed by markedly significant correlations between the indices of insulin secretion and insulin resistance [Table 2]. This finding is consistent with previous studies conducted in subjects with varying degrees of glucose tolerance ranging from normal glucose tolerance to Type 2 diabetes [1,17-25]. Some of these reports used glucose clamps technique whereas others have utilized HOMA or its modifications to quantify insulin resistance, but with identical conclusions that in subjects with IGT, first phase or early insulin secretion is related to insulin resistance [17-25]. It is plausible that the decline in 1st phase insulin secretion during the stages of IGT, and postprandial hyperglycemia with fasting euglycemia or the decrease in both the 1st and 2nd phase insulin rise with both fasting and postprandial hyperglycemia in Type 2 DM may be caused by worsening insulin resistance at the level of pancreatic β cell itself [4-7,21,24, 26-29]. However, normal glucose tolerance in some obese subjects despite presence of insulin resistance as documented in our previous study [16] is attributed to a prompt and a brisk response to ingestion of glucose or a meal [1,17-25]. In this population of obese subjects with normal glucose tolerance, mild insulin resistance with an intermediate values of FI x FG between normal lean subjects and obese subjects with IGT have been documented [16]. Therefore, it is apparent that a critical degree of severity of insulin resistance may be required for inhibition of glucose entry into pancreatic beta cells causing decline in 1st phase insulin secretion. Furthermore, progressively increased inhibition of entry of glucose secondary to a progressive rise in insulin resistance appears to be responsible for a progressive decline in 1st phase insulin secretion with progression from normal glucose tolerance to type 2 Diabetes as documented in several studies [1,17-25].
The major stimulus for both the insulin synthesis and release by the pancreatic β cells is the entry of glucose. This phenomenon is recently described as ‘β cell glucose sensitivity’ [24]. This study [24] attributed worsening glucose tolerance to progressive β cell dysfunction induced by declining β cell glucose sensitivity. This finding is almost identical to our observation of declining 1st phase insulin secretion with rising insulin resistance with progressive worsening of glucose tolerance. Furthermore, the role of insulin sensitivity in insulin secretion is further evident by studies demonstrating the presence of glucose transporters on β cell membranes with their enhancement by circulatory insulin [30-36]. Finally, it is likely that inhibited entry of glucose into β cells caused by declining β cell glucose sensitivity and insulin resistance decreases insulin synthesis and storage in β cell granule pool leading to a fall in 1st phase postprandial rise as documented in another study [37].
Therefore, the maximum 1st phase insulin secretion in subjects with NGT may be attributed to prompt facilitation of glucose entry into β cells secondary to normal insulin sensitivity to circulating insulin concentration. Alternatively, the least 1st phase insulin rise in subjects with Type 2 DM may be explained by markedly inhibited glucose entry into β cell caused by extreme insulin. Finally, the intermediate 1st phase insulin rise in subjects with IGT is likely to be secondary to subnormal β cell insulin sensitivity but not lowered to the extent noted in subjects with Type 2 DM.
In conclusion, the decline of 1st phase insulin secretion in subjects with IGT, as well as postprandial hypoglycemia in Type 2 DM may be a manifestation of decreased insulin sensitivity at the level of pancreatic β cell itself and not caused by “glucose toxicity” alone.
5. ACKNOWLEDGMENT
The authors are indebted to Mackenzie Pedersen for her secretarial assistance.
REFERENCES
- Weyer, C., Bogardus, C., Mott, D.M. and Pratley, R.E. (1999) The natural history of insulin secretary dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. The Journal of Clinical Investigation, 104, 787-794. doi:10.1172/JCI7231
- Ward, W.K., Beard, J.C., Halter, J.B., Pfeifer, M.A. and Porte, D. (1984). Pathophysiology of insulin secretion in non-insulin-dependent diabetes mellitus. Diabetes Care, 7, 491-502. doi:10.2337/diacare.7.5.491
- Grodsky, G.M. (1989) A new phase of insulin secretion. How will it contribute to our understanding of beta-cell function? Diabetes, 38, 673-678. doi:10.2337/diabetes.38.6.673
- Dinneen, S., Gerich, J. and Rizza, R. (1992) Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. The New England Journal of Medicine, 327, 707- 713.
- Mitrakou, A., Kelley, D., Mokan, M., Veneman, T., Pangburn, T., Reily, J. and Gerich, J. (1992) Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. The New England Journal of Medicine, 326, 22-29. doi:10.1056/NEJM199201023260104
- Van Haeften, T.W., Pimenta, W., Mitrakou, A., Korytikowski, M., Jenssen, T., Yki-Jarvinen, H. and Gerich, J.E. (2000) Relative contributions of beta-cell function and tissue insulin sensitivity to fasting and postglucose-load glycemia. Metabolism, 49, 1318-1325. doi:10.1053/meta.2000.9526
- Bruce, D.G., Chisholm, D.J., Storlien, L.H. and Kraegen, E.W. (1988). Physiological importance of deficiency in early prandial insulin secretion in non-insulin dependent diabetes. Diabetes, 37, 736-744. doi:10.2337/diabetes.37.6.736
- Rossetti, L., Giaccari, A. and DeFronzo, R.A. (1990) Glucose toxicity. Diabetes Care, 13, 610-630. doi:10.2337/diacare.13.6.610
- Flax, H., Matthews, D.R., Levy, J.C., Coppack, S.W., Turner, R.C. (1991) No glucotoxicity after 53 hours of 6.0mmol/l hyperglycaemia in normal man. Diabetologia, 34, 570- 575. doi:10.1007/BF00400275
- American Diabetes Association, (2006) Diagnosis and classification of diabetes mellitus. Diabetes Care. 29, S43-S48.
- Kabadi, U.M. and Einstein, A.B. (1980). Glucose intolerance in hyperthyroidism: Role of glucagons. The Journal of Clinical Endocrinology & Metabolism, 50, 392- 396. doi:10.1210/jcem-50-2-392
- Matthews, D.R., Hosker, J.P., Rudensji, A.S., Naylor, B.A., Treacher, D.F. and Turner, R.C. (1985). Homeostasis model assessment. Diabetologia, 28, 412-419. doi:10.1007/BF00280883
- Matsuda, M. and Defronzo, R. (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care, 22, 1462-1470. doi:10.2337/diacare.22.9.1462
- Stumvoll, M., Mitrakou, A., Pimenta, W., Jenssen, T., Yki-Jarvinen, H., Van Haeften, T., Renn, W. and Gerich, J. (2000). Use of oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care, 23, 295-301. doi:10.2337/diacare.23.3.295
- Kanauchi, M., Yamano, S., Kanauchi, K. and Yoshihiko, S. (2003). Homeostasis ModelAssessment of Insulin Resistance, Quantitative Insulin Sensitivity CheckIndex and Oral Glucose Insulin Sensitivity Index in Nonobese, Nondiabetic Subjects with High-Normal Blood Pressure. The Journal of Clinical Endocrinology & Metabolism, 88, 3444-3446. doi:10.1210/jc.2002-021641
- Clarke, N., Sivitz, W. and Kabadi, U. (2005) Product of fasting plasma glucose and fasting plasma insulin: A simple and reliable index of insulin sensitivity. Diabetes Research, 39, 25-31.
- Abdul-Ghani, M.A., Tripathy, D., Jenckinson, C., Ritchardson, D. and DeFronzo, R.A. (2006). Insulin secretion and insulin action in subjects with impaired fasting glucose and impaired glucose tolerance: Results from the Veterans Administration Genetic Epidemiology Study (VEGAS). Diabetes, 55, 1430-1435. doi:10.2337/db05-1200
- Kahn, S.E. (2003) The relative contributions of insulin resistance and B-cell dysfunction to the pathophysiology of type 2 diabetes mellitus. Diabetologia, 46, 3-19.
- Wasada, T., Kuroki, H., Katsumori, K., Arii, H,. Sato, A., Aoki, K., Jimba, S. and Hanai, G. (2004). Who are more insulin resistant, people with IFG or people with IGT? Diabetologia; 47, 758-759.
- Festa, A., D’Agostino, R., Hanley, A.J., Karter, A.J., Saad, M.F. and Haffner, S.M. (2004). Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes; 53, 1549-1555. doi:10.2337/diabetes.53.6.1549
- Osei, K., Gaillard, T. and Schuster, D.P. (1997). Pathogenetic mechanisms of impaired Glucose tolerance and type II diabetes in African Americans: The significance of insulin secretion, insulin sensitivity, and glucose effectiveness. Diabetes Care, 20, 396-404. doi:10.2337/diacare.20.3.396
- Pimenta, W.P., Santos, M.L., Cruz, N.S., Aragon, F.F., Padovani, C.R. and Gerich, J.E. (2002). Brazilian individuals with impaired glucose tolerance are characterized by impaired insulin secretion. Diabetes & metabolism, 28, 468-476.
- Carnevale Schianca, G.P., Rossi, A., Sainaghi, P.P., Maduli, E. and Cartoli, E. (2003). The significance of impaired fasting glucose versus impaired glucose tolerance: Importance of insulin secretion and resistance. Diabetes Care; 26, 1333-1337. doi:10.2337/diacare.26.5.1333
- Ferrannini, E., Gastaldelli, A., Miyazaki, Y., Matsuda, M., Mari, A. and DeFronzo, R.A. (2005) B-cell function in subjects spanning the range from normal glucose Tolerance to overt diabetes: A new analysis. The Journal of Clinical Endocrinology & Metabolism, 90, 493-500.
- Abdul-Ghani, M.A., Sabbah, M., Kher, J., Minuchin, O., Vardi, P. and Raz, I. (2006). Different contributions of insulin resistance and beta-cell dysfunction in overweight Israeli arabs with IFG and IGT. Diabetes/Metabolism Research and Reviews, 22, 126-130. doi:10.1002/dmrr.595
- Abdul-Ghani, M.A., Tripathy, D. and DeFronzo, R.A. (2006). Contributions of β-cell dysfunction and insulin reistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care, 29, 1130-1138. doi:10.2337/dc05-2179
- Pfeifer, M.A., Halter, J.B. and Porte, D.J. (1981). Insulin secretion in diabetes mellitus. The American Journal Medicine, 70, 579-588. doi:10.1016/0002-9343(81)90579-9
- World Health Organization (1985). Diabetes Mellitus: Report of a WHO Study Group. Geneva, World Health Organization.
- Bergman, R.N., Finegood, D.T., Kahn, S.E. (2002). The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. The European Journal of Clinical Investigation, 32, 35-45. doi:10.1046/j.1365-2362.32.s3.5.x
- Orci, L., Unger, R.H., Ravazzola, M., Ogawa, A., Komiya, I., Baetens, D., Lodish, H.F. and Thorens, B. (1990). Reduced beta-cell glucose transporter in new onset diabetic BB rats. The Journal of Clinical Investigation, 86, 1615-1622. doi:10.1172/JCI114883
- Brant, A.M., McCoid, S., Thomas, H.M., Baldwin, S.A., Davies, A., Parker, J.C., Gibbs, E.M. and Gould, G.W. (1992). Analysis of the glucose transporter content of islet cell lines: Implications for glucose-stimulated insulin release. Cellular Signaling, 4, 641-650. doi:10.1016/0898-6568(92)90045-A
- Girard, J., Postic, C., Burcelin, R., Guillet, I. and Leturque, A. (1992) Glucose Transporters. Physiology and physiopathology. Presse Medicale, 21, 2053-2059.
- Hughes, S.D., Quaade, C., Johnson, J.H., Ferber, S., and Newgard, C.B. (1993). Transfection of AtT-20ins cells with GLUT-2 but not GLUT-1 confers glucose -stimulated insulin secretion. Relationship to glucose metabolism. The Journal of Biological Chemistry, 268, 15205-15212.
- Valera, A., Solanes, G., Fernandez-Alvarez, J., Pujol, A., Ferrer, J., Asins, G., Gomis, R. and Bosch, F. (1994). Expression of GLUT-2 antisense RNA in beta cells of transgenic mice leads to diabetes. The Journal of Biological Chemistry, 269, 28543-28546.
- Wang, M.Y., Koyama, K., Shimabukuro, M., Mangelsdorf, D., Newgard, C.B. and Unger, R.H. (1998) Overexpression of leptin receptors in pancreatic islets of Zucker diabetic fatty rats restores GLUT-2, glucokinase, and glucose-stimulated insulin secretion. Proceedings of the National Academy of Sciences of the United States of Americ, 95, 11921-11926. doi:10.1073/pnas.95.20.11921
- Lopes Da Costa, C., Sampaio De Freitas, M. and Sanchez Moura, A. (2004) Insulin secretion and GLUT-2 expression in undernourished neonate rats. The Journal of Nutritional Biochemistry, 15, 236-241. doi:10.1016/j.jnutbio.2003.12.004
- Daniel, S., Noda, M., Straub, S.G. and Sharp, G.W. (1999) Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes, 48, 1686-1690. doi:10.2337/diabetes.48.9.1686

