Journal of Sensor Technology
Vol.04 No.03(2014), Article ID:48459,7 pages
10.4236/jst.2014.43011
Temperature Effects on Gas Sensing Properties of Electrodeposited Chlorine Doped and Undoped n-Type Cuprous Oxide Thin Films
Nayana Bandara1,2, Charith Jayathilaka1,3, Dhammika Dissanayaka4, Sumedha Jayanetti1
1Department of Physics, University of Colombo, Colombo, Sri Lanka
2Department of Physics, Open University of Sri Lanka, Nawala, Sri Lanka
3Department of Physics, University of Kelaniya, Kelaniya, Sri Lanka
4Department of Chemistry, University of Colombo, Colombo, Sri Lanka
Email: sumedhajayanetti@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 30 May 2014; revised 30 June 2014; accepted 31 July 2014
ABSTRACT
As one of the most widely used domestic fuels, the detection of possible leakages of Liquefied Petroleum (LP) gas from production plants, from cylinders during their storage, transport and usage is of utmost importance. This article discusses a study of the response of undoped and chlorine doped electrodeposited n-type Cuprous Oxide (Cu2O) films to of LP gas. Undoped n-type Cu2O films were fabricated in an electrolyte bath containing a solution of sodium acetate and cupric acetate whereas n-type chlorine doped Cu2O thin films were prepared by adding a 0.02 M cuprous chloride (CuCl2) into an electrolyte solution containing lactic acid, cupric sulfate and sodium hydroxide. The n-type conductivity of the deposited films was determined using spectral response measurements. The structural and morphological properties of the fabricated films were monitored using X-ray diffraction (XRD) and Scanning Electron Microscopy (
Keywords:
Liquefied Petroleum Gas, Electrodeposition, Cu2O Thin Films, Chlorine Doped, Undoped, Gas Response

1. Introduction
Environmentally hazardous gases are being released continuously to the atmosphere due to industrialization, increased human activities and the natural processes that take place as a result of drastic changes in the environment. Therefore, monitoring of environment has been of extreme importance for the safety and well-being of human and animal life and nature in general. Consequently, gas sensing has become an important area of research that leads to the development of highly responsive gas sensing devices capable of detecting minute amounts of gases of different types. One such gas that requires monitoring in the context of developing countries is the highly inflammable Liquefied Petroleum (LP) gas which is being used very widely as a domestic fuel. At most times leakage of LP gas from production plants or from cylinders occur during their storage, transport and usage. These leakages at most times can be found from the odor of the gas, however, by then a significant amount of gases may have leaked out to the surroundings. Gas sensor devices enable the early detection of such leakages thus preventing accidents and wastage while helping maintain a safer and cleaner environment.
With the pioneering work reported in 1962 by Seiyema et al. [1] , much technological effort has been made in the field of gas sensing aiming towards improvement of the gas response, selectivity, stability and feasibility for practical use. A principal mechanism employed in gas sensing is the monitoring of the electrical resistance of the sensor material upon its exposure to a particular gas of low concentration. Among the many sensor materials available, use of polycrystalline oxide semiconductor thin films as sensing materials is rapidly expanding. Extensive studies have been made on oxide semiconductors such as SnO2, ZnO and WO3, for their gas sensing applications for various types of gases [2] [3] . Recently, there have been a few studies on the use of p-type Cu2O thin films as a potential gas sensing material, Alahapitiya et al. [4] have used thermally oxidized p-type Cu2O for methane sensing. Shishyano et al. [5] have used electrodeposited p-type Cu2O for NO2 gas sensing for LP gas sensing. Dhawale et al. [6] have explored the possibility of using electron beam irradiated chemically de- posited TiO2 thin films. Shukla et al. [7] have reported thesynthesis of tin oxide thick films and their appli- cations in LP gas sening at room temperature.
It has been known that the electrodeposition can be used to fabricate Cu2O thin films with both n-type and p-type conductivities [8] . Thus the ability to control the conductivity type, the surface morphology etc. coupled with the ease and associated low cost of fabrication make electrodeposited Cu2O thin films a suitable candidate for gas sensing applications. The inherent high resistivity associated with the elctrodeposited Cu2O thin films has been considered a drawback that can be addressed using suitable doping methods for related device applica- tions. For example, chlorine doping during the elctrodeposition has lowered the resistivity of Cu2O thin films significantly [9] . This paper reports the use of electrodeposited chlorine doped n-type Cu2O thin films for monitoring LP gas while making a comparison with the previously reported LP gas sensing behavior of undoped Cu2O thin films [10] . The temperature dependent variation of the gas sensing sensitivity is discussed including the reaction mechanisms that cause the observed behaviour.
2. Experimental
Both undoped and chlorine doped n-type Cu2O thin films were deposited on Ti substrates, a process that has been well established in the research group [9] . Prior to the deposition, the substrates were cleaned thoroughly with detergent, dilute nitric acid, in an acetone bath, and lastly with distilled water. The undoped Cu2O thin films were potentiostatically electrodeposited in a three-electrode electrochemical cell that contained aqueous solutions of 0.1 M sodium acetate and 0.01 M cupric acetate and hence termed as an acetate bath. Deposition was carried out for duration of 45 minutes at 60˚C underpotentiostatic condition of a −200 mV [9] . However, difficulties were encountered when chlorine doping was attempted using an acetate bath due to precipitation. Doping was therefore accomplished in an electrolytic bath containing aqueous solutions of 3 M lactic acid, 0.45 M cupric sulfate, and 4 M sodium hydroxide. Sodium hydroxide was used to adjust the pH of the electrolyte around 9.5. A solution of CuCl2 was used as the chlorine precursor by varying its concentration. Resistivity measurements of the resulting Cu2O thin films showed that initially the resistance to drop drastically and then gradually beyond a concentration of 0.02 M CuCl2. Thus for gas sensing measurements, Cu2O films chlorine doped using a 0.02 M CuCl2 solution was used. The deposition was carried out for a period of 45 minutes, at a constant temperature of 60˚C, under potentiostatic conditions of −275 mV. The n-type conductivity of the deposited films was verified using the spectral response measurements [9] . The surface morphological and structural characterization of the films was determined using SEM (Philips XL40) and XRD (SHIMADZU SSX-550) analysis.
A film sample was then enclosed in a gas sensing chamber made of stainless steel. Chamber contained two compartments; the top through which the gas was flown and the bottom where the heating element was housed. In order to measure the electrical resistance, contact probes were fixed on to the surface of the film sample which was placed on an asbestos heating platform. Externally, the probes were connected to a multimeter, which in turn was connected to a computer data logger (Figure 1).
All the measurements were made under atmospheric conditions by using a flow through technique. While maintaining a constant gas flow rate of 0.005 ml/s, films were exposed to LP gas for approximately 30 - 40 s. and then the gas flow was stopped. The sensing temperature was varied between 30˚C and 100˚C while monitoring the temperature with a thermocouple (type K) which was in contact with surface area of the substrate which was not covered by the Cu2O film. The temperature was controlled using a thermostat with a temperature controller. The electrical resistivity measurements were made using computer interfaced a Keithley 2100 digital multimeter. The measurements were taken over a period of approximately 120 s after the gas was sent in to the chamber and repeated when the temperatures were brought down to the room temperature. This procedure was repeated at different temperatures in order to determine the temperature that corresponds to the maximum gas sensitivity.
3. Results and Discussion
3.1. Structure and Morphology
3.1.1. X-Ray Diffraction (XRD)
Figure 2 shows the XRD patterns of the undoped and chlorine doped Cu2O thin films. It can be seen that both doped and undoped Cu2O thin films show single phase, polycrystalline characteristics. It can be seen from spectrum B in Figure 2, that the undoped Cu2O films show a preferred orientation resulting in a stronger (220) reflection. However, doping has caused the preferred orientation to change to produce a stronger (200) reflection in the chlorine doped Cu2O film samples.
3.1.2. Scanning Electron Microscopy
Figure 3(a) and Figure 3(b) show the SEM pictures of electrodeposited undoped and chlorine doped Cu2O
Figure 1. Block diagram of gas sensing setup.
Figure 2. X-ray diffraction spectra of (a) chlorine doped Cu2O thin film and (b) undoped Cu2O thin film.

Figure 3. SEM pictures of (a) undoped Cu2O thin film deposited on Ti substrate and (b) chlorine doped Cu2O thin film deposited on Ti substrate with a 0.02 M CuCl2 added to the electrolyte bath.
samples indicating that both films have uniform polycrystalline coverage. However, effect of doping has caused the average polycrystalline grain size to reduce towards nanoscale compared to that of the undoped Cu2O thin films in which the average grain size is in the micro scale. Through SEM measurements, it was found that the average polycrystalline grain size of chlorine doped Cu2O thin films showed a concentration dependence on CuCl2. It was observed that the increased CuCl2 concentration caused the average grain size to reduce gradually providing a larger effective surface area to interact with the gas molecules [11] .
3.1.3. Resistivity Measurements
Figure 4 shows the resistivity measurements taken of the chlorine doped Cu2O samples in which the CuCl2 precursor concentration in the electrolyte was varied during the deposition of the films. It can be seen that initially, the resistivity of the films reduces from MΩ cm range down to kΩ cm range when the CuCl2 precursor concentration in the electrolyte was 0.02 M. Further increase in the concentration causes the resistivity to reduce gradually through the kΩ cm range.
This decrease in the resistivity was quite significant when compared to the resistivity of undoped Cu2O thin
Figure 4. Variation of resistivity of chlorine doped Cu2O thin films with CuCl2 concentration in the electrolyte solution maintained at pH 9.5 and temperature 60˚C.
films fabricated electrochemically using the acetate bath which produced Cu2O thin films in which the resistivity was larger by over 5 orders of magnitude than the chlorine doped Cu2O thin films obtained with the 0.02 M CuCl2 precursor concentration in the electrolyte in the lactate bath.
3.2. Gas Sensing Properties
As mentioned above, the gas sensing properties were measured by monitoring the resistance of the Cu2O thin films upon exposure to LP gas and its stoppage after a certain period. The response to the gases are represented as resistance vs. time curves and the magnitude of sensitivity vs. time curves where the magnitude of sensitivity is termed as
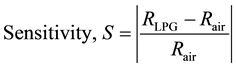 (1)
(1)
Here RLPG is the resistance of the film upon exposure to LP gas and Rair is the resistance of the film when it is under normal atmospheric conditions.
Response at Low Temperatures
Figure 5(a) shows the variation of resistance of undoped Cu2O films as a function of time around room temperature. When the undoped Cu2O films were exposed to the LP gas, films showed a negative response, i.e. the resistance of the film decreased with the exposure time and gradually recovered to its initial value under the normal atmospheric conditions when the gas flow was stopped [10] . In a magnitude of sensitivity vs. time graph, this behavior is depicted by a single response peak as shown in the Figure 5(b).
This behavior can be well understood in terms of the mechanism given by Shukla [7] on LP gas sensing behavior of SnO2 thin films. Under atmospheric conditions, the oxygen adsorbed on the surface of the n-type Cu2O thin film extracts electrons from its conduction band to form  on the film surface causing the film to be at a higher equilibrium resistance initially. Once the LP gas is sent into the chamber, it reacts with the chemisorbed oxygen removing them from the surface giving the electrons back to the conduction band of the Cu2O film as shown by the Equation (2).
on the film surface causing the film to be at a higher equilibrium resistance initially. Once the LP gas is sent into the chamber, it reacts with the chemisorbed oxygen removing them from the surface giving the electrons back to the conduction band of the Cu2O film as shown by the Equation (2).
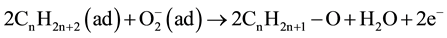 (2)
(2)
This reduces the resistance of the film until the LP gas flow in to the chamber is stopped. The stoppage of the gas flow causes atmospheric oxygen to gradually adsorb on the Cu2O film once again allowing the resistance to recover back to its value under atmospheric conditions.
In contrast, upon exposure to LP gas, the resistance of the chlorine doped Cu2O films increased (RLPG > Rair) initially showing a positive response and then showed a negative response (RLPG < Rair) as in the case of undoped

Figure 5. Variation of (a) resistance and, (b) sensitivity of undoped Cu2O thin films due to the exposures of films to LP gas at a flow rate 0.005 ml/s at different temperatures.
Cu2O films yielding two peaks in the magnitude of sensitivity vs. time graph. Figure 6(a) and Figure 6(b) show the resistance and the magnitude of sensitivity variations at temperatures of 30˚C, 35˚C, 39˚C, 40˚C and 42˚C obtained for chlorine doped Cu2O thin films upon exposure to the LP gas. In contrast to the response given by undoped Cu2O samples in which the variation of resistance gives rise to only a single peak, it can be seen that at temperatures of 30˚C, 35˚C and 39˚C, there are two responses, with the first peak appearing around 10 s and the second peak appearing around 30 s, before the gas is made to stop flowing. The second peak in the sensitivity vs. time graph in the Figure 6(b) is comparable to the peaks seen when undoped Cu2O was used as shown in Figure 5(b).
Therefore, the first peak appears to have arisen due to the presence of chloride ions in the doped Cu2O films. Thus for the sake of clarity, the first peak is termed as the chlorine peak whereas the second peak is termed as the Cu2O peak. Furthermore, it can be seen that the chlorine peak reduces its intensity with increasing temperature disappearing completely at 42˚C whereas the intensity of the Cu2O peak continues to increase.
This behavior can be understood in the following manner. Overall reduction of the resistance of the chlorine doped thin films measured under atmospheric conditions is attributed to the increase of electron density in the conduction band of the Cu2O thin film which occurs due to the electrons coming from the donor impurity levels. Thus the doping concentration controls the overall resistivity of the Cu2O film. However, when the films are exposed to the LP gas, due to the formation of electrostatic interactions between induced dipoles of LP gas molecules with the chloride ions in the film, the electron pumping process to the conduction band is retarded causing the resistance to increase initially. When the interaction with chloride ions reaches its saturation, the adsorbed oxygen in the film starts to react with the LP gas molecules as stated above according to the Equation (2) causing the resistance to decrease similar to the undoped Cu2O films until the gas flow is stopped. It can also be seen that compared to the undoped Cu2O films, the initial response to the LP gas arising from the chlorine doped Cu2O films is quicker.
When the chlorine doped thin films are exposed to LP gas at temperatures > 42˚C, the absence of the chlorine peak indicates that the gas is not responsive to chloride ions present in the film sample due to the weakening of the dipole interactions between the LP gas molecules and the chlorides at higher temperature. Thus the contribution to the sensing behavior arises only from the adsorbed 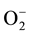 on the surface of the film. Therefore, the chlorine doped Cu2O films show a response similar to the undoped Cu2O thin film samples however, showing contrasting differences in the peak sensitivities measured as a function of temperature. Figure 7(a) and Figure 7(b) show the sensitivity measurements made of the chlorine doped samples for corresponding resistance variation
on the surface of the film. Therefore, the chlorine doped Cu2O films show a response similar to the undoped Cu2O thin film samples however, showing contrasting differences in the peak sensitivities measured as a function of temperature. Figure 7(a) and Figure 7(b) show the sensitivity measurements made of the chlorine doped samples for corresponding resistance variation

Figure 6. Temperature dependent variation of (a) resistance and (b) sensitivity of chlorine doped Cu2O thin films deposited in electrolyte solution with 0.02 M CuCl2 when films were exposed to LP gas at a flow rate of 0.005 ml/s. (Inset: ▲ variation of maximum sensitivity in the chlorine peak, and ■ variation of maximum sensitivity in the Cu2O peak of the chlorine doped Cu2O thin films with the temperature).

Figure 7. Variation of sensitivity of (a) chlorine doped Cu2O thin films at the high temperatures, (b) chlorine doped Cu2O thin films at temperatures around 55˚C and, (c) undoped Cu2O thin films at the different temperatures due to the exposure of films to LP gas. (Highlighted in red are the variations of sensitivity that yields the maximum peak sensitivity.)
when the LP gas was sent changing the film temperature in the range from 50˚C - 95˚C. Measurements showed that the intensity of the Cu2O sensitivity peak of the chlorine doped sample increased with temperature up to about 55˚C and decreased with further increase in temperature. By varying the temperature around 55˚C, it was found that the maximum sensitivity was resulted in at 52˚C. In contrast, the undoped Cu2O films showed the maximum sensitivity around 85˚C as shown by the Figure 7(c).
Above behavior was repeatedly observed when both doped and undoped Cu2O thin film samples were exposed to the LP gas indicating that doping affects the overall gas sensing behavior of the films also.
4. Conclusion
In conclusion, it can be clearly seen that the chlorine doping has altered the gas sensing behavior of n-type Cu2O thin films significantly. While chlorine doping causes the resistance of Cu2O films to go down drastically, the presence of chloride ions in the film has resulted in an additional sensitivity peak that exists closer to the room temperature. It can be interpreted that the doping has caused LP gas molecules to interact initially with more active chloride ions providing a faster positive response, i.e. 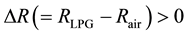 and then with the
and then with the 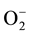 ions on the Cu2O film providing a negative response, i.e. ΔR < 0 as in the case of undoped Cu2O films. It can also be seen that the maximum sensitivity of chlorine doped thin films to the LP gas occurs at a lower temperature compared to the undoped Cu2O films.
ions on the Cu2O film providing a negative response, i.e. ΔR < 0 as in the case of undoped Cu2O films. It can also be seen that the maximum sensitivity of chlorine doped thin films to the LP gas occurs at a lower temperature compared to the undoped Cu2O films.
Acknowledgements
University Grant Commission of Sri Lanka is gratefully acknowledged for financial assistance provided through the research grant UGC/ICD/RG 2011. KNDB is thankful to the Open University of Sri Lanka, for granting leave to carry out this research study.
References
- Seiyama, T., Kato, A., Fujiishi, K. and Nagatani, M. (1962) A New Detector for Gaseous Components Using Semiconductive Thin Films. Analytical Chemistry, 34, 1502-1503. http://dx.doi.org/10.1021/ac60191a001
- Parmar, M. and Rajanna, K. (2011) Copper (II) Oxide Thin Film for Methanol and Ethanol Sensing. International Journal on Smart Sensing and Intelligent Systems, 4, 710-725.
- Yamazoe, N., Sakai, G. and Shimanoe, K. (2003) Oxide Semiconductor Gas Sensors. Catalysis Surveys from Asia, 7, 63-73. http://dx.doi.org/10.1023/A:1023436725457
- Ahalapitiya, H.J., Samarasekara, P. and Kun, G. (2009) Methane Gas Sensors Application of Cuprous Oxide Synthesized by Thermal Oxidation. Physica Status Solidi (a), 206, 332-337. http://dx.doi.org/10.1002/pssa.200824126
- Shishiyanu, T.S. and Lupan, O.I. (2006) Novel NO2 Gas Sensor Based on Cu2O Thin Films. Sensors and Actuators B: Chemical, 113, 468-476. http://dx.doi.org/10.1016/j.snb.2005.03.061
- Dhawle, D.S., et al. (2009) Liqueified Petoleum Gas (LPG) Sensing Perfomance of Electron Beam Irradiated Chemically Deposited Ti2O Thin Film. Sensors and Actuators B: Chemical, 141, 58-64. http://dx.doi.org/10.1016/j.snb.2009.06.025
- Shukla, T. (2012) Synthesis of Tin Oxide Thick Film and Its Investigation as a LPG Sensor at Room Temperature. Journal of Sensor Technology, 2, 102-108. http://dx.doi.org/10.4236/jst.2012.23015
- Siripala, W. and Kumara, K.P. (1989) A Photo Electrochemical Investigation of n- and p-Type Semiconducting Behavior of Copper Oxide Films. Semiconductor Science and Technology, 4, 465. http://dx.doi.org/10.1088/0268-1242/4/6/007
- Jayathilaka, K.M.D.C., Siripala, W. and Jayanetti, J.K.D.S. (2013) Efficiency Improvement of Cuprous Oxide Based Cl Doping. Proceedings of the International Conference (Solar Asia), Kuala Lumpur, 91-96.
- Bandara, K.N.D., Jayathilaka, K.M.D.C., Siripala, W. and Jayanetti, J.K.D.S. (2012) Electrodeposited Nano Crystalline Cu2O Thin Films for Gas Sensing Applications. Proceedings of the 68th Technical Sessions, Sri Lanka Association for the Advancement of Science (SLAAS), 56.
- Han, X.F. (2011) Electrochemical n-Type Doping in Metal Oxides and Its Application in Photovoltaic. Ph.D. Thesis, University of Texas at Arlington, Arlington, 32-33.





