Advances in Chemical Engineering and Science
Vol. 2 No. 2 (2012) , Article ID: 18876 , 5 pages DOI:10.4236/aces.2012.22022
Production of Phosphate-Rich Biofertiliser Using Vermicompost and Anaerobic Digestor Sludge—A Case Study*
Department of Chemical Engineering, National Institute of Technology, Durgapur, India
Email: cmn_recd@yahoo.co.in
Received January 17, 2012; revised February 20, 2012; accepted March 15, 2012
Keywords: Phosphatic biofertiliser; phosphate solubilising bacteria; Phosphate Rich Organic Manure; Vermicompost; Anaerobic digestor sludge; Biogas
ABSTRACT
This paper presents the technology and cost effectiveness of production of phosphate rich biofertiliser (called PROM) by bioconversion of phosphate rock ore into soluble phosphates (that are directly assimilable by plants) in presence of an organic manure such as Vermicompost or anaerobic digestor sludge (discharged from biogas manufacturing units) and using a microbial culture of Bacillus megatherium var phosphaticum. PROM has been found to be an excellent, less expensive, substitute to synthetic phosphatic fertilisers such as SSP, MAP and DAP. This is based on real-life field trials. It is also possible to integrate the production of PROM, with biogas generation and the layout of such a more profitable, integrated scheme is also presented in this paper.
1. Introduction
Phosphorus is one among the most important nutrients for plant growth along with nitrogen and potash. The most important ore of phosphorus is rock phosphate (also called phosphate rock) which is a complex phosphate of calcium. Manufacture of synthetic phosphatic fertilisers (such as triple superphosphate, ammonium phosphate, etc.) demands production of elemental phosphorus or phosphoric acid from phosphate rock, which, by itself, is an energy—intensive process. Biochemical conversion of phosphate rock into soluble phosphates that are directly assimilated by plants becomes attractive in this respect.
Biofertiliser which is thus produced is named as PROM (Phosphate Rich Organic Manure) since it is synthesised by blending finely ground phosphate rock with an organic manure. The most recommended sources of organic manure in this connection (and those which are employed in the present study) are Vermicompost (the residue left behind after composting plant wastes /animal wastes using earth worms) and the sludge discarded from units that manufacture biogas by the anaerobic digestion of animal wastes (cow dung, night soil, poultry litter, piggery waste) or plant wastes (garden debris, agriculture residues, water hyacinth) or municipal/cellulosic wastes. Anaerobic digestor sludge (ADS) has the additional advantage that it is a byproduct and does not have separate manufacturing cost. The specific advantages of PROM are:
1) The process of production of PROM is highly costeffective as it is a low energy process that does not demand high temperature or high pressure (operates at ordinary temperature and pressure), needs no chemical catalyst and does not consume any valuable chemicals;
2) The entire cost of synthesis of phosphoric acid or elemental phosphorus from phosphate rock is eliminated;
3) The raw rock phosphate ore, since is biochemically converted to soluble phosphates, can be fed directly to plants;
4) PROM is equally (if not, more) effective in agriculture as compared to synthetic fertilisers such as single superphosphate (SSP), diammonium phosphate (DAP) or monoammonium phosphate (MAP) as evident from successful field studies performed (data presented subsequently in this paper).
PROM can thus act as a viable substitute to otherwise expensive synthetic phosphatic fertilisers. Though PROM has completed many successful field trials [1,2], commercial production and utilisation of PROM is not still upto the mark. This paper discusses the process of production of PROM in detail (which includes the proposed plant layout), highlights its superior characteristics and illustrates the need for its large scale utilisation.
2. Production of PROM (Materials and Methods)
As stated earlier, PROM is produced by the biochemical conversion of phosphate rock into soluble phosphates.
Feedstock: Pulverised refined phosphate rock (free from silica) of uniform size of around 75 microns blended with well—ground ADS (Anaerobic Digestor sludge) or vermicompost in the ratio 1:2. Average particle size of the blend = less than 1 mm. The vermicompost used is that obtained by composting plant wastes and agricultural residues. The compost is dried in a tray dryer, finely powdered in a hammer mill and then blended with the pulverised rock phosphate ore. For sample-II, ADS is collected from the biogas generator that employs anaerobic digestion of animal wastes (cow dung, night soil, poultry litter). The sample of discharged slurry is dewatered, dried in a tray dryer, finely ground in a ball mill and then mixed with the well-ground ore of phosphate rock in the ratio specified above. Both the ore and the organic manure (vermicompost, ADS) are screened through a set of Indian standard screens in a sieve shaker to ascertain the uniformity of size.
Substrate: An aqueous suspension of above blend in water. The water: solids ratio maintained is 7:3. Small amount of salt petre and gypsum are also added to make up nutrient deficiency and promote bacterial growth.
Bioreactor: Agitated stirred tank (slurry reactor).
Operating temperature (optimum): 30˚C - 35˚C Operating temperature (maximum): 60˚C pH (optimum): 7.0 Operating pressure: 1 atm Microbial culture: Bacillus megatherium var phosphaticum (phosphorus solubilising bacteria)
Size of inoculum: 3% - 5% The process is conducted in two stages. During the first stage, the substrate slurry is added continuously to the bioreactor and is constantly agitated. The suspension is allowed to ferment for about 7 - 10 days (thermophillic stage). Since the consistency of suspension is maintained at (7/3), the pH of the medium remains at around 7.0. The operating temperature is maintained more or less constant and it seldom exceeds 60˚C. At the end of the thermophillic stage, the bioreactor is seeded with an inoculum of phosphate solubilising bacteria (mentioned above) and the agitation is continued. Though the process is aerobic, since the reactor is kept open, atmospheric air/ oxygen diffuses into the substrate and transfer and dissolution of oxygen is further facilitated by agitation of the slurry. No air compressors are required to be employed. At the end of this aerobic stage, it is desirable to employ an additional stage during which the bioreactor is seeded with an inoculum of nitrogen fixing microbes such as Azotobacter and permit an additional residence time of about 5 - 10 days. This stage is, however, optional. We have employed all the three stages in our study.
Downstream processing: The product solution is filtered to separate the solid biofertiliser which is then dried, ground to the desired size, labeled and packaged. The biofertiliser (PROM) so obtained has been found to have the following composition:
Phosphorus content: 16.5% (as soluble P2O5)
C:N ratio: 19:1.
It is fit for direct use in the agricultural field. The composition of PROM has been analysed by the standard procedure with the help of spectrophotometer.
As is the case with most biochemical processes, bioconversion of phosphate rock is also a slow process and thus demands a large residence time for the bioreactor. For a given capacity, the reactor volume required could be significantly large. It is, therefore, recommended to use two to three bioreactors with parallel feeding of substrate slurry.
3. PROM Production Integrated with Biogas Generation [Proposed Plant Layout]
It is most desirable to integrate the production of PROM with manufacture and enrichment of biogas from animal/plant/municipal wastes [3]. On one side, the digestor sludge (ADS) can be blended with phosphate rock to produce PROM and on the other side, a rich fuel such as methane is obtained for use in industries/automobiles. The proposed integrated process shall consist of the following units:
1) Grinding Unit (GU): The refined phosphate rock (after removing silica) is ground to very fine powder (particle size = less than 1mm) in a hammer mill/ball mill that operates in closed circuit with a classifying screen or hydraulic/pneumatic classifier (or elutriator). The product is pulverised phosphate rock of uniform size.
2) Biogas Production Unit (BPU): This consists of the anaerobic digestor (bioreactor) into which the feedstock (animal waste/plant waste/municipal waste) that has been premixed with water in the ratio 1:1 (by mass) is fed continuously. Since the process is anaerobic, the reactor is provided with an air-tight cover. Raw cow dung may be used as the inoculum. The biogas produced (that shall consist of around 60% methane (by volume), 40% carbon dioxide with traces of hydrogen sulphide) is sent to a storage tank. The sludge that is continuously discharged from the reactor is sent to a sedimentation tank, the thick underflow sludge is dried and ground to less than 1 mm size.
3) Biogas Purification Unit/Sulfur Recovery Unit (SRU): Hydrogen sulfide, if present in biogas, could cause acute corrosion problems when the gas is used as an engine fuel. H2S can be recovered from the biogas as elemental sulfur through a biotechnological route. The basic principle of this process is same as that proposed by Rajvaidya and coworkers [4] for the removal of hydrogen sulphide from air or coke oven gas. The process is sketched in figure 1. The gas is first bubbled through aqueous ferric sulfate solution in reactor-1 (chemical reactor), when H2S present in the gas gets reduced to elemental sulfur:
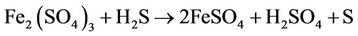 (1)
(1)
The precipitated sulfur (in colloidal form) is filtered off and the residual ferrous sulfate solution is fed to an aerobic bioreactor that is seeded with an inoculum of recombinant thiobacillus ferrooxidant microbes. These microbes catalyse conversion of ferrous sulfate to ferric:
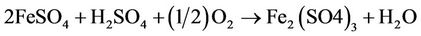 (2)
(2)
The ferric sulfate solution, so regenerated, is recycled back to reactor-1. The process is self-supporting, no principal chemical is consumed, demands no high operating temperature or high operating pressure or chemical catalyst and almost complete removal of hydrogen sulfide is possible regardless of its concentration in the feed gas. The elemental sulfur so recovered can be blended with PROM either as such or in the form of calcium sulfate (after making it react with milk of lime) in predetermined proportion. This has been found to be specifically desirable when PROM is to be fed to citrus plants which need a particular percentage of sulfur in soil for their efficient growth.
Biogas Enrichment Unit (BEU): When biogas produced is to be employed as a boiler/furnace fuel or as an engine fuel (in automobiles such as cars, buses, truckstractors), it is desirable to remove its carbon dioxide content so as to obtain almost pure methane. This can be accomplished by scrubbing the gas with aqueous monoethanolamine (MEA) solution in a packed absorption tower that operates in the countercurrent mode. MEA not only absorbs carbon dioxide but also reacts with it and consequently, the rate of absorption is quite high. The process operates at ordinary temperature and pressure. The rich MEA solution discharged from the bottom of the absorption tower is sent to a stream stripper (desorption unit) where all the CO2 that remains dissolved in the solution is stripped off by steam and the regenerated absorbent (lean MEA solution) is recycled back to the top of the absorption tower. The process is thus quite costeffective since the absorbent is continuously regenerated and recycled for reuse (see figure 2). A well-tested software package has been prepared by the author [5] for the optimum design of such a biogas enrichment unit. It is a multiparameter package that takes into account all the influencing system/operating parameters and also performs a cost optimisation of the process.
Once enriched, biogas becomes almost pure methane and it can be used as a substitute to natural gas in all industrial applications (to note that methane—content of natural gas is more than 93%). Examples are
1) As an industrial fuel in boilers and furnaces (calorific value of methane = 55,000 kJ/kg).
2) As an automobile fuel, in the same way as LNG (Liquefied Natural Gas) and CNG (Compressed Natural Gas) are being employed.
3) As a feedstock for the manufacture of several organic/inorganic chemicals. Methane on steam cracking produces synthesis gas (a mixture of CO and H2) which is the starting material for the synthesis of a variety of industrial chemicals such as methanol, hydrogen gas, ammonia and related fertilisers.
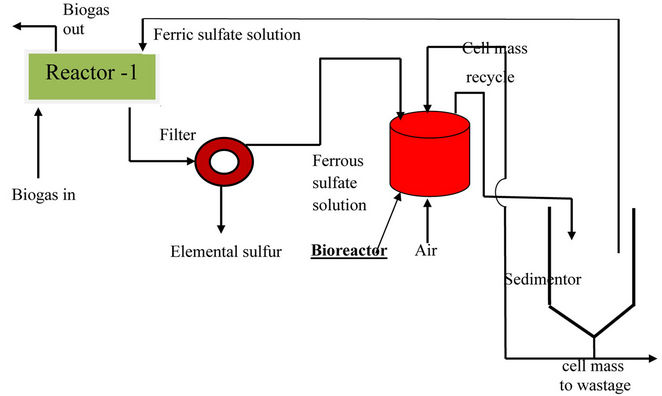
Figure 1. Biochemical recovery of H2S from biogas as elemental sulfur.
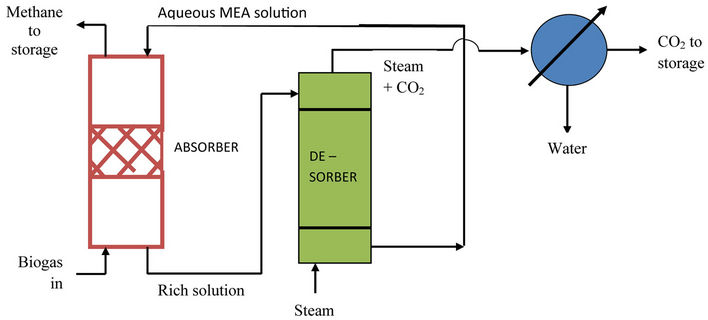
Figure 2. Schematic of biogas enrichment unit.
PROM Production Unit: In this unit, the pulverised phosphate rock from the Grinding Unit (GU) is blended with well-ground ASD produced in BPU and fermented in the aqueous medium to produce PROM as described earlier.
4. PROM in Agriculture
PROM has been found to be easily assimilable by plants and can be fed to plants as such. It is equally effective both in acidic as well as alkaline soils. Elaborate field trials have demonstrated that PROM performs comparably as (often better than) synthetic fertilisers such as SSP, DAP or MAP with respect to the crop yield. A few typical results on the yield of crops such as rice, wheat and barley, grams such as soybeans and groundnut and also on the yield of vegetables such as cabbage and onion are presented below in Table 1. Comparison is being made with the popularly used synthetic phosphatic fertilisers such as SSP (Single Superphosphate) for wheat and soyabeans and DAP (Diammonium Phosphate) for rice, barley, groundnut, cabbages and onions. The figures speak for themselves. The yield of crops, grams and vegetables obtained with PROM is very much comparable with and often superior to that obtained when synthetic phosphatic fertilisers (SSP, DAP) are being employed in soil.
Experiments with flowering plants (Roses, sunflower) and orchard products (mangoes, apples, lemons) have also been equally encouraging. These results ably demonstrate that PROM is a promising substitute to synthetic phosphatic fertilisers.
5. Conclusions
1) Synthesis of phosphatic biofertiliser (PROM) can be successfully achieved starting from raw phosphate rock ore and an organic manure such as Vermicompost or Anaerobic Digestor Sludge (ADS). Microbial cultures such as those of Bacillus megatherium and Azotobacter could also be employed in second and third stages of preparation so as to enhance the quality of biofertiliser.
2) The phosphorus content of PROM is around 16.5% (as soluble P2O5) and is directly assimilable by plants.
3) Production of PROM is much more cost-effective than that of synthetic phosphatic fertilisers such as SSP, MAP, DAP since the entire manufacturing cost of phosphoric acid or elemental phosphorus from phosphate rock is eliminated. The starting material remains the same as raw phosphate rock ore. Though the process is relatively slow, it does not demand any high temperature or high pressure. No chemical catalyst is required and there is no consumption of any valuable chemicals such as sulfuric acid.
4) With respect to plant growth, PROM is equally effective as the synthetic fertilisers presently in market such as SSP and DAP (as evident from field data presented in Table 1).
5) PROM production concomitant with biogas generation (from plants/animal solid wastes) is possible and this shall enhance the overall economy of the scheme.
6) Biogas produced in the above integrated scheme, after H2S removal and enrichment (CO2 removal), could be conveniently used as a promising industrial fuel/automobile fuel (and also for the production of syngas).
7) Elemental sulphur recovered from biogas during the above scheme could be blended with PROM either as such or in the form of calcium sulfate. This is particularly advantageous when PROM is being fed to citrus plants that need a specific percentage of sulphur in soil for efficient growth. The process of recovery of sulphur from biogas through biotechnological route (sketched in figure 1) is also highly cost-effective since it operates at ordinary temperature and pressure, consumes no chemicals (ferric sulfate solution is continuously regenerated in the bioreactor and recycled) and permits almost complete removal of H2S.
8) Large scale, commercial production of PROM must be thus encouraged all over the world, since it is a phosphatic biofertiliser that is much less expensive to manu-
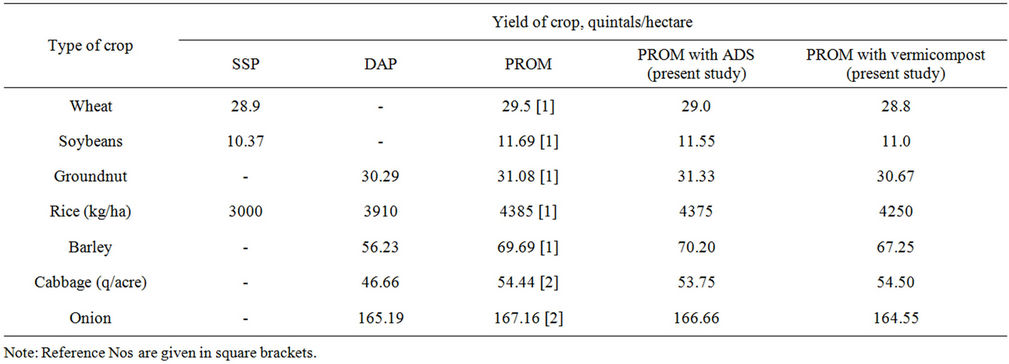
Table 1. Comparison of PROM with synthetic phosphatic fertilisers.
facture than synthetic phosphatic fertilisers.
9) Scope for future work involves studies on kinetics of PROM production and development of reliable kinetic equations. Research projects in this connection have already been initiated by the author.
6. Acknowledgements
The assistances and suggestions received from Dr. D. M. R. Sekhar (Head, Beneficiation Plant, Jordan Phosphate Mines Co. Ltd., Jordan) and members of PROM Society of India and from the engineers/scientists of Synergy Biotech Ltd, Durgapur, India during the different stages of this study are gratefully acknowledged.
REFERENCES
- D. M. R. Sekhar and N. C. Aery, “PROM Manual,” Himanshu Publishers, Udaipur, 2005.
- N. C. Aery, N. S. Rathore, M. K. Katewa and M. R. Masih, “PROM—Volume 1,” Himanshu Publishers, Udaipur, 2006.
- C. M. Narayanan, “An Integrated Process Layout for Manufacture and Utilisation on of PROM,” Proceedings of the All India Seminar on Latest Developments in Phosphatic Fertilisers, New Delhi, November 2006.
- A. S. Rajvaidya, et al., “Precombustion Desulfurisation of Gaseous Fuels,” Proceedings of the All India Seminar on Automotive Fuels, Nagpur, 23-24 August 2002.
- C. M. Narayanan and B. C. Bhattacharya, “Computer Aided Analysis and Optimisation of Biogas Enrichment Process,” Journal of Energy Heat Mass Transfer, Vol. 12, No. 1, 1990, pp. 17-24.
NOTES
*This paper has been presented and discussed in the International Conference on Solid Waste Management and Technology at Philadelphia, USA, during March 27-30, 2011. The paper has been revised and modified based on the discussions during the Conference.

