Advances in Enzyme Research
Vol.05 No.02(2017), Article ID:76707,11 pages
10.4236/aer.2017.52002
Organogermanium (Ge-132) Suppresses Activities of Stress Enzymes Responsible for Active Oxygen Species in Monkey Liver Preparation
Takafumi Tezuka1*, Atsunori Higashino1, Mitsuo Akiba2, Takashi Nakamura2
1Graduate School of Information Science, Nagoya University, Nagoya, Japan
2Asai Germanium Research Institute Co., Ltd., Hakodate, Japan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 13, 2017; Accepted: May 30, 2017; Published: June 2, 2017
ABSTRACT
Assays of stress enzymes related to active oxygen species were performed by using an in vitro preparation from the liver of a monkey (Japanese Macaque). Ge-132, an organic germanium compound, viz. poly-trans-[(2-carboxyethyl) germasesquioxane] [(GeCH2CH2COOH)2O3]n, suppressed the activities of NADH-dependent oxidase and NADPH-dependent oxidase [NAD(P)H-OD] and xanthine oxidase (XOD) as superoxide-forming enzymes, while promoting the activities of superoxide dismutase (SOD) as a superoxide-scavenging enzyme and catalase (CAT) as an enzyme responsible for degradation of hydrogen peroxide (H2O2). The evidence suggests that the levels of active oxygen species such as  and H2O2 would be reduced by Ge-132. The possible connection between Ge-132 and activities of stress enzymes is discussed on the basis of these results.
and H2O2 would be reduced by Ge-132. The possible connection between Ge-132 and activities of stress enzymes is discussed on the basis of these results.
Keywords:
Active Oxygen Species, Stress Enzymes [CAT, NAD(P)H-OD, SOD, XOD], Ge-132 [(GeCH2CH2COOH)2O3]n, Monkey Liver

1. Introduction
Active oxygen species are produced as a consequence of aerobic respiration and substrate oxidation. In the cells of organisms subjected to various stresses, production of active oxygen species such as , 1O2, ・OH and H2O2 is enhanced [1] [2] [3] . These active oxygen species act directly on enzymes and damage cells. SOD scavenges
, 1O2, ・OH and H2O2 is enhanced [1] [2] [3] . These active oxygen species act directly on enzymes and damage cells. SOD scavenges  in cells and suppresses the formation of 1O2, and ・OH radicals [4] [5] .
in cells and suppresses the formation of 1O2, and ・OH radicals [4] [5] .
Poly-trans-[(2-carboxyethyl)germasesquioxane] which is one of organogermanium compounds (Ge-132) showed the superoxide-scavenging activity of plasma in patients with immunological disorders [6] .
In 1967, poly-trans-[(2-carboxyethyl)germasesquioxane] was synthesized as the first original water-soluble organogermanium compound by Oikawa and Kakimoto [7] (cf. Asai [8] ) and its molecular formula, designated as [(GeCH2CH2COOH)2O3]n, viz. abbreviated Ge-132 was determined by X-ray crystallography [9] . Ge-132 is a water-soluble compound and its safety has been confirmed [10] . The structure of Ge-132 and its hydrolyzed monomer, 3-(trihy- droxygermyl)propanoic acid (THGP), are displayed in Figure 1 [11] . Ge-132 has specific ring structure membered by twelve elements of Ge and O. Moreover, it can hydrolyze to THGPs in aqueous solution. When Ge-132 is absorbed from intestine by post oral intake Ge-132 hydrolyzed to THGP via duodenum, and it should act as monomeric THGP in body. However, in this study, we described it as Ge-132 without distinction. Experiments regarding physiological functions of Ge-132 have been carried out using various organisms (microorganisms, plants and animals) and analgesic [12] , antitumor [13] , antivirus [14] , organ protection [15] antirheumatoid [6] , anti-cataract [16] , immunostimulator [17] antioxidative [18] effects have been documented, along with promoted growth of self-incom- patible pollen tubes [19] and “suppression” of osteoporosis [20] . Moreover, the oxidation rate of low-density lipoprotein (LDL) was reduced by treatment with Ge-132 [21] while secretion and anti-oxidative activity of bile in rodents were promoted [18] . These positive effects of Ge-132 on physiologically bad condition may encourage seriously ill patients and sickly animal keepers. The condition
Figure 1. Structure of poly-trans-[(2-carboxyl)germasesquioxane] (Ge-132) and its hydrolysis product 3-(trihydroxygermyl)propanoic acid (THGPA). (after Yamaguchi et al., 201511).
seems to be caused mainly by active oxygen species. Therefore, our purpose in the present study is to investigate relationship between Ge-132 and stress enzyme activities, that is, Ge-132 induces to lower the level of active oxygen species. Results obtained in the present study are expected to bring good news to various patients.
Lilium longiflorum cv. Hinomoto has a gametophytic self-incompatibility sys- tem. In Hinomoto lilies, a pistil after self-incompatible pollination can thus be considered to be subject to stress. In our previous study [19] , stress enzymes, such as superoxide-forming NAD(P)H-OD, XOD, SOD, CAT and ascorbate peroxidase (APOD), in pistils after self-incompatible pollination showed high activities, as compared with those in the pistils after cross-compatible pollination. Moreover, the growth of pollen tubes in the pistil after self-incompatible pollination was promoted by treatment with germanium compounds [Ge-132; [(GeCH2CH2COOH)2O3]n and GeO2], apparently linked to suppressed levels of activities of superoxide-forming NAD(P)H-OD, XOD, SOD and elevated activities of CAT and APOD in pistils. In other words, Ge-132 seems to stimulate growth of pollen tubes in pistils through suppression of active oxygen species. Since it is conceivable that Ge-132 might also regulate the activities of stress enzymes related to active oxygen species in animal tissues/cells in the same manner as lily pistils.
We performed the present study to examine its effects on the activities of superoxide-forming and superoxide-scavenging enzymes such as NAD(P)H-OD, XOD and SOD, using a preparation from the liver of a Japanese macaque as a substitute for that from lily pistils.
2. Materials and Methods
2.1. Chemicals
An organogermanium [Ge-132; (GeCH2CH2COOH)2O3]n (Figure 1) (Asai Ger- manium Research Institute Co., Ltd., Hakodate, Hokkaido, Japan) was tested in the present study.
2.2. Animal Materials
The liver sample of a six-year-old Japanese macaque (Macaca fuscata) was used in the present study.
Ethics
The liver sample used in the present experiment was part of tissue stocks that were collected for multiple usage on opportunities of animal experiments approved by the Primate Research Institute of Kyoto University, Japan. It was provided by the Institute through the Joint Research System operated by this institute. The present study does not include an animal experiment.
2.3. Preparation of Enzyme Fractions from Liver
All subsequent steps were carried out at 0˚C - 4˚C as below. Portions of liver (2 g) were homogenized in a glass homogenizer in 10 mL of grinding medium composed of 0.1 M morpholinopropane-sulfonic acid (MOPS)-KOH (pH 7.5), 1 mM disodium ethylenediamine-tetraacetic acid (Na2-EDTA), 1 mM dithiothreitol (DTT). The homogenate was centrifuged for 7 min at 15,000 g and the supernatant was sonicated five times at 0˚C, for 10 s each time, with a sonicator (model 5202; Ohtake Works Co. Ltd., Tokyo) at an output of 100 W. The sonicated supernatant was centrifuged for 7 min at 15,000 g. An aliquot of the resulting supernatant was used for assays of the activities of NAD(P)H-OD, XOD and CAT. The remaining supernatant, after removal of the aliquot for assays of the activities of the above-mentioned enzymes, was dialyzed against 10 mM MOPS-KOH (pH 7.5) containing 1 mM Na2-EDTA and 1 mM DTT for 4 h at 4˚C and used for assays of the activity of SOD.
2.4. Assays of Enzymatic Activities
The activities of enzymes were estimated at 25˚C with a spectrometer (model U-3210; Hitachi, Tokyo). In order to make the original (stock) solution of Ge-132, powdery Ge-132 was dissolved in small amount of distilled water and adjusted at pH 7.0 with 1 M NaOH. After that, the original solution of Ge-132 was adjusted to 100 μM with distilled water.
NAD(P)H-OD was assayed by a modified version of the method of Azzi et al. [22] in a reaction mixture (1 mL) that contained 20 mM N-tris(hydroxyme- thyl)methyl-2-aminoethanesulfonic acid (TES)-KOH (pH 7.0), 40 μM acetylated cytochrome c, 40 μM NADPH or NADH, 10 or 0 μg/mL SOD and 30 μL enzyme fraction (0.8 mg protein), and 0, 0.001, 0.01, 0.1 or 1 μM Ge-132. The reaction was carried out at 25˚C, following increase in absorbance (A) at 550 nm (A550).
XOD was assayed by a modified version of the method of Hashimoto [23] . The assay was carried out in a reaction mixture (1.5 mL) that contained 100 mM potassium phosphate (K-PO4) (pH 7.5), 0.13 mM xanthine, 0.2 mM K-oxonate and 100 μL enzyme fraction (1.8 mg proteins), and 0, 0.001, 0.01, 0.1 or 1 μM Ge-132. The reaction proceeded at 30˚C for 25 min and then was quashed by addition (50 μL) of 100% trichloroacetic acid (TCA). After centrifugation for 5 min at 15,000 g, enzymatic activities in the resulting supernatant were estimated by measuring A292.
SOD was assayed with a modified version of the method of Asada et al. [24] , in a reaction mixure (1 mL) that contained 50 mM K-PO4 (pH 7.8), 0.1 mM Na2-EDTA, 0.1 mM xanthine, 20 μM cytochrome c, 0.012 U XOD and enzyme fraction (0 or 0.4 mg protein), and 0, 0.001, 0.01, 0.1 or 1 μM Ge-132. The reaction was carried out at 25˚C, following increase in A550. Exceptionally, the activity of SOD was shown by units defined by Asada et al. [24] .
CAT was assayed with a modified version of the method of Beers and Sizer [25] , in a reaction mixture (1 mL) that contained 90 mM K-PO4 (pH 7.9), 0.043% H2O2 and enzyme fraction (0.6 mg protein), and 0, 0.001, 0.01, 0.1 or 1 μM Ge-132. The reaction was carried out at 25˚C, following increase in A240.
The specific activities of enzymes as mentioned above were presented as μmol/mg protein/min [NAD(P)H-OD, XOD and CAT] or units/mg protein (SOD). Protein was quantified by the method of Lowery et al. [26] , with bovine serum albumin as the standard. All experiments were repeated three times with similar results and representative findings are shown.
3. Results
3.1. Effects of Ge-132 on the Activities of NAD(P)H-OD
The activity of NAD(P)H-OD as superoxide-forming enzymes was strongly suppressed by Ge-132 (Figure 2(a)), with a negative exponential curve. This was


Figure 2. Effects of Ge-132 on suppression of superoxide-forming enzyme activities in a monkey liver preparation. (a) NADPH- and NADH-oxidase; (b) xanthine-oxidase. Data represent mean ± s.e.m. (n = 3 measurements). RSD values were calculated according to an equation of [standard deviation ÷ arithmetic mean], and their values of NADPH-oxi- dase, NADH-oxidase and xanthine-oxidase (XOD) were 4.3%, 5.1% and 4.3%, respectively.
more pronounced in the case of NADH-OD, as compared with NADPH-OD. In the assay condition without Ge-132 (0 μM), NADPH-OD showed higher (3.6- fold) activity than NADH-OD. With Ge-132 at 1 μM, NADPH-OD showed high (5.3-fold) activity, as compared with NADH-OD. In other words, the activities of NADPH-OD with 0.001, 0.01, 0.1 or 1 μM Ge-132 were suppressed up to 63.8%, 47.8%, 35.9% or 31.9%, respectively, and those of NADH-OD with 0.001, 0.01, 0.1 or 1 μM Ge-132 were suppressed up to 57.1%, 42.9%, 28.6% or 21.4%, respectively, as compared with respective activities (100%) without Ge-132 (0 μM).
3.2. Effects of Ge-132 on the Activities of XOD
The activity of XOD was also suppressed by Ge-132 (Figure 2(b)), with a negative parabola curve. Namely, the activities with 0.001, 0.01, 0.1 or 1 μM Ge-132 were suppressed up to 90.8%, 89.1%, 80.8% or 45.3%, respectively, as compared with that (100%) without Ge-132 (0 μM).
3.3. Effects of Ge-132 on the Activities of SOD
In contrast, the activity of SOD as a superoxide-scavenging enzyme was promoted by Ge-132 (Figure 3(a)), with an apparent asymptotic curve. The activities with 0.001, 0.01, 0.1 or 1 μM Ge-132 were elevated 174.2%, 171.4%, 176.9% or 177.5%, respectively, as compared with that (100%) without Ge-132 (0 μM).
3.4. Effects of Ge-132 on the Activities of CAT
Activity of CAT as an enzyme responsible for the degradation of hydrogen peroxide was also promoted by Ge-132 (Figure 3(b)), with an asymptotic curve. The activities with 0.001, 0.01, 0.1 or 1 μM Ge-132 were elevated 162.9%, 182.2%, 188.7% or 193.5%, respectively, as compared with that (100%) without Ge-132 (0 μM).
4. Discussion
In our previous study, the growth of pollen tubes in the pistils of Hinomoto lilies after self-incompatible pollination was promoted by treatment with Ge-132 [19] . Since the Hinomoto lily has a self-incompatibility system and high activities of stress enzymes in pistils after self-incompatible pollination as compared with the case after cross-compatible pollination, promotion by Ge-132 of the growth of pollen tubes after self-incompatible pollination could have been due to its regulation of stress enzymes such as NAD(P)H-OD, XOD, SOD, CAT and APOD. In short, reduced levels of active oxygen species such as 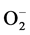 and H2O2 might have been directly involved in Ge-132 promotion of pollen tubes growth.
and H2O2 might have been directly involved in Ge-132 promotion of pollen tubes growth.
In a preliminary experiment using monkey liver cells, the activity of NAD(P) H-OD was actually suppressed by Ge-132 (data not shown), apparently due to non-competitive inhibition, involving substrates [NAD(P)H].
In the present study, activities of free radical-forming and free radical-sca- venging enzymes in the liver of Japanese macaque were regulated by Ge-132.


Figure 3. Effects of Ge-132 on promotion of superoxide-scavenging enzyme activities in a monkey liver preparation. (a) superoxide dismutase; (b) catalase. Data represent mean ± s.e.m. (n = 3 measurements). RSD values were calculated according to an equation of [standard deviation/arithmetic mean], and their valurs of superoxide dismutase (SOD) and catalase (CAT) were 3.2% and 2.1%, respectively.
Namely, Ge-132 suppressed the activities of the  -forming NAD(P)H-OD and XOD, and promoted those of the
-forming NAD(P)H-OD and XOD, and promoted those of the 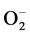 scavenging SOD and the H2O2-de- grading CAT as shown in Figure 2 and Figure 3. These phenomena may generally lead to decrease in the levels of active oxygen species like
scavenging SOD and the H2O2-de- grading CAT as shown in Figure 2 and Figure 3. These phenomena may generally lead to decrease in the levels of active oxygen species like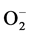 , H2O2, etc. in the cells of biological organisms.
, H2O2, etc. in the cells of biological organisms.
Regarding the results of assays of NAD(P)H-OD (Figure 2(a)) and XOD (Figure 2(b)), the formation of superoxide anion (O2-) in the liver is considered to be limited in the cell membrane fraction, as compared with in the cytosol fraction in the cells. NAD(P)H-OD are membrane-specific enzymes, viz. mem- brane intrinsic and extrinsic enzymes, and XOD is a cytosol-specific enzyme.
It has been reported that activities of NAD(P)H-OD in the lily are suppressed by cAMP [27] , while other stress enzymes such as XOD, SOD, CAT and APOD are not affected. On the basis of this evidence, Ge-132 may generally play an important role in the induction (formation) of cAMP in the cells of organisms. Analysis of the relationships between the induction of cAMP and Ge-132 is necessary.
In this study, the assays of stress enzymes as mentioned above were carried out using a supernatant fraction after centrifugation (15,000 g) of liver homogenate. Therefore, the activities of SOD in the liver cells may quite slightly involve with the effects of SOD contained in erythrocytes in ven-blood, veni-blood and arterial blood in the liver.
The activities of stress enzyme are probably regulated by Ge-132 without distinction of plants (lily pistils) or animals (monkey liver).
5. Conclusions
Ge-132 may play important roles in maintaining a low level of active oxygen species in order to ease various stresses. In other words, treatment with Ge-132 in a living body might be expected to reduce active oxygen species by regulation of the activities of stress enzymes. This might explain promotion of recovery from diseases in persons and animals [8] . A schematic representation of a putative model for the relationship between Ge-132 and activities of stress enzymes in the monkey liver is shown in Figure 4.
Figure 4. Schematic representation of a putative model for the relationship between Ge-132 and the activities of stress enzymes, such as NAD(P)H-oxidase [NAD(H)-OD], xanthine oxidase [XOD], superoxide dismutase [SOD], catalase [CAT] and peroxidase [POD], in the monkey liver. *POD converts H2O2 to H2O according to a reaction formula such as H2O2 + **AH2 → 2H2O + **A. Black arrowhead: suppressed by Ge-132, White arrowhead: promoted by Ge-132, Dotted arrowhead: unacted upon by Ge-132.
We hypothesize that Ge-132 plays an important role in the stress signaling for stress enzymes in the monkey liver. Thus, Ge-132 might contribute greatly to protection against diseases such as cancer, pulmonary complaints, rheumatism, etc. and to improvement in our health.
Nowadays, Ge-132 is applied extensively in order to improve various symptoms in medical and veterinary fields as shown in many reports [6] [10] [12] - [18] [20] [21] . Moreover, the positive effects of Ge-132 have been well known according to serious accounts as personal communication through many carcinoma patients and others who take Ge-132 capsules every day.
In the future, Ge-132 application may pervade more according to the account designated in Figure 4 in medical and veterinary fields. Furthermore, Ge-132 application may be widely spread in the fields of plants and microorganisms in addition to medical and veterinary fields. In our preliminary experiments, actually, Ge-132 showed effectively physiological action in plants and bacteria. Therefore, Ge-132 may have a great future as a physiologically suitable modulator in various fields.
Acknowledgements
The liver sample was provided by courtesy of the Primate Research Institute of Kyoto University. Authors would like to take this opportunity of thanking the Institute.
Disclosure Statement
The authors report no conflict of interest.
Cite this paper
Tezuka, T., Higashino, A., Akiba, M. and Nakamura, T. (2017) Organogermanium (Ge-132) Suppresses Activities of Stress Enzymes Responsible for Active Oxygen Species in Mon- key Liver Preparation. Advances in Enzyme Research, 5, 13-23. https://doi.org/10.4236/aer.2017.52002
References
- 1. Doke, N. (1983) Involvement of Superoxide Anion Generation in the Hypersensitive Response of Potato Tuber Tissues to Infection with an Incompatible Race of Phytophythora infelstans and to the Hyphal Wall Components. Physiological Plant Pathology, 23, 345-357.
- 2. ádám, A., Farkas, T., Somlyai, G., et al. (1989) Consequence of Generation during a Bacterially Induced Hypersensitive Reaction in Tobacco: Deterioration of Membrane lipids. Molecular Plant Pathology, 34, 13-26.
- 3. Scandalios, J.G. (1993) Oxygen Stress and Superoxide Dismutases. Plant Physiology, 101, 7-12.
- 4. Fridovich, I. (1974) Superoxide Dismutases. Advances in Enzymology and Related Areas of Molecular Biology, 41, 35-97.
- 5. Fridovich, I. (1975) Superoxide Dismutases. Annual Review of Biochemistry, 44, 147-159.
https://doi.org/10.1146/annurev.bi.44.070175.001051 - 6. Pronai, L. and Arimori, S. (1992) Decreased Plasma Superoxide Scavenging Activity in Immunological Disorder—Carboxyethylgermanium sesquioxide (Ge-132) as a Promoter of Prednisolone. Biotherapy, 4, 1-8.
https://doi.org/10.1007/BF02171703 - 7. Oikawa, H. and Kakimoto, N. (1967) β-Trichlorogermylethyl derivatives. Abstract of the 21st Annual Meeting of the Chemical Society of Japan (Japanese Ed). p. 2964. The Chemical Society of Japan, Tokyo, Japan
- 8. Asai, K. (1980) Miracle Cure—Organic Germanium. Japan Publications, Inc., Tokyo.
- 9. Tsutsui, M., Kakimoto, N., Axtell, D.D., et al. (1976) Crystal Structure of “Carboxyethylgermanium Sesquioxide”. Journal of the American Chemical Society, 98, 8287-8289.
https://doi.org/10.1021/ja00441a081 - 10. Sanai, T., Okuda, S., Onoyama, K., Oochi, N., Takaichi, S., Mizuhira, V. and Fujishima, M. (1991) Chronic Tubulointerstitial Changes Induced by Germanium Dioxide in Comparison with Carboxyethylgermanium Sesquioxide. Kidney International, 40, 882-890.
https://doi.org/10.1038/ki.1991.289 - 11. Yamaguchi, H., Shimada, Y., Takeba, T., Nakamura, T. and Mano, N. (2015) A Novel Extraction Method Based on a Reversible Chemical Conversion for the LC/ MS/MS Analysis of the Stable Organic Germanium Compound Ge-132. Analytical Chemistry, 87, 2042-2047.
https://doi.org/10.1021/ac504466u - 12. Hachisu, M., Takahashi, H., Koeda, T. and Sekizawa, Y. (1983) Analgesic Effect of Novel Organogermanium Compound, Ge-132. Journal of Pharmacobio-Dynamics, 6, 814-820.
- 13. Suzuki, F., Brutkiewicz, R.R. and Pollard, R.B. (1985) Importance of T-Cells and Macrophages in the Antitumor Activity of Carboxyethylgermanium Sesquioxide (Ge-132). Anticancer Research, 5, 479-484.
- 14. Aso, H., Suzuki, F., Ebina, T. and Ishida, N. (1989) Antiviral Activity of Carboxyethyl-Germaniumesquioxide (Ge-132) in Mice Infected with Influenza Virus. Journal of Biological Response Modifiers, 8, 180-189.
- 15. Masaki, Y., Kumano, K., Iwamura, M., Endo, T., Sakai, T., Koshiba, K., Nakamura, K., Yokota, K., Sato, K., Uchida, H. and Aso, K. (1989) Protective Effect of an Organic Germanium Compound on Warm Ischemia and Prolonged Kidney Preservation. Transplantation Proceedings, 21, 1250-1251.
- 16. Unaka, N.J., Johnson, M., Tsui, J., Cherian, M. and Abraham, E.C. (1995) Effect of Germanium-132 on Galactose Cataracts and Glycation in Rats. Experimental Eye Research, 61, 155-164.
- 17. Ikemoto, K., Kobayashi, M., Fukumoto, T., Morimatsu, M., Pollard, R.B. and Suzuki, F. (1996) 2-Carboxyethylgermanium Sesquioxide, a Synthetic Organogermanium Compound, as an Inducer of Contrasuppressor T Cells. Experientia, 52, 159-166.
https://doi.org/10.1007/BF01923363 - 18. Nakamura, T., Nagura, T., Akiba, K., Sato, K., Tokuji, Y., Ohnishi, M. and Osada, K. (2010) Promotive Effects of the Dietary Organic Germanium Poly-Trans-[(2-Car-boxyethyl)Germasesquoxane] (Ge132) on the Secretion and Antioxidative Activity of Bile in Rodents. Journal of Health Science, 56, 72-80.
https://doi.org/10.1248/jhs.56.72 - 19. Tezuka, T., Tsuruhara, A., Suzuki, H. and Takahashi, Y.S. (1997) A Connection between the Self-Incompatibility Mechanism and the Stress Response in Lily. Plant and Cell Physiology, 38, 107-112.
https://doi.org/10.1093/oxfordjournals.pcp.a029139 - 20. Matsumoto, H., Jiang, G.-Z., Hashimoto, T., Kuboyama, N., Yamane, J., Nonaka, K. and Fujii, A. (2002) Effect of Organic Germanium Compound (Ge-132) on Experimental Osteoporosis in Rats: The Relationship between Transverse Strength and Bone Mineral Density (BMD) or Bone Mineral Content (BMC). International Journal of Medical Sciences, 1, 10-16.
- 21. Wakabayashi, Y. (2001) Effect of Germanium-132 on Low-Density Lipoprotein Oxidation and Atherosclerosis in Kurosawa and Kusanagi Hypercholesterolemic Rabbits. Bioscience, Biothechnology, and Biochemistry, 65, 1893-1896.
https://doi.org/10.1271/bbb.65.1893 - 22. Azzi, A., Meterucco, P.F. and Richter, C. (1975) The Use of Acetylated Ferricytochromome c for the Detection of Superoxide Radicals Produced in Biological Membranes. Biochemical and Biophysical Research Communications, 65, 590-603.
- 23. Hashimoto, S. (1974) A New Spectrophotometric Assay Method of Xanthine Oxidase in Crude Tissue Homogenate. Analytical Biochemistry, 62, 426-435.
- 24. Asada, K., Urano, M. and Takahashi, M. (1973) Subcellular Location of Superoxide Dismutase in Spinach Leaves and Preparation and Properties of Crystalline Spinach Superoxide Dismutase. The FEBS Journal, 36, 257-266.
https://doi.org/10.1111/j.1432-1033.1973.tb02908.x - 25. Beers Jr., R.F. and Sizer, I.W. (1952) A Spectrophotometric for Measuring the Breakdown of Hydrogen Peroxide by Catalase. Journal of Biological Chemistry, 195, 133-140.
- 26. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent. Journal of Biological Chemistry, 193, 256-275.
- 27. Tsuruhara, A., Suzuki, H. and Tezuka, T. (1999) Inhibition of the Activity of NAD-(P)H-Dependent Oxidase in Pistils of Lilium longiflorum by cAMP. Plant Cell and Physiology, 40, 1093-1098.
https://doi.org/10.1093/oxfordjournals.pcp.a029492



