American Journal of Plant Sciences
Vol.07 No.14(2016), Article ID:70977,13 pages
10.4236/ajps.2016.714169
Root Morphology, Plant Growth, Nitrate Accumulation and Nitrogen Metabolism of Temperate Lettuce Grown in the Tropics with Elevated Root-Zone CO2 at Different Root-Zone Temperatures
Jie He*, Lin Qin, Sing Kong Lee
Natural Sciences and Science Education Academic Group, National Institute of Education, Nanyang Technological University, Singapore

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 28, 2016; Accepted: September 26, 2016; Published: September 29, 2016
ABSTRACT
This paper investigated the effects of root-zone (RZ) CO2 concentration ([CO2]) on root morphology and growth, nitrate (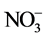 ) uptake and assimilation of lettuce plants at different root-zone temperatures (RZT). Elevated RZ [CO2] stimulated root development, root and shoot growth compared to ambient RZ [CO2]. The greatest increase in root growth was observed in plants grown under elevated RZ [CO2] of 50,000 ppm. However, RZ [CO2] of 10,000 ppm was sufficient to achieve the maximal leaf area and shoot productivity. Lettuce plants exhibited faster shoot and root growth at 20˚C-RZT than at ambient (A)-RZT. However, under elevated RZ [CO2], the magnitude of increased growth was greater at A-RZT than at 20˚C-RZT. Compared to RZ [CO2] of 360 ppm, elevated RZ [CO2] of 10,000 ppm increased
) uptake and assimilation of lettuce plants at different root-zone temperatures (RZT). Elevated RZ [CO2] stimulated root development, root and shoot growth compared to ambient RZ [CO2]. The greatest increase in root growth was observed in plants grown under elevated RZ [CO2] of 50,000 ppm. However, RZ [CO2] of 10,000 ppm was sufficient to achieve the maximal leaf area and shoot productivity. Lettuce plants exhibited faster shoot and root growth at 20˚C-RZT than at ambient (A)-RZT. However, under elevated RZ [CO2], the magnitude of increased growth was greater at A-RZT than at 20˚C-RZT. Compared to RZ [CO2] of 360 ppm, elevated RZ [CO2] of 10,000 ppm increased 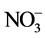 accumulation and nitrate reductase activity (NRA) in both leaves and roots.
accumulation and nitrate reductase activity (NRA) in both leaves and roots.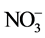 concentrations of leaf and root were higher at 20˚C-RZT than at A-RZT in all plants. NRA was higher in root than in leaf especially under A-RZT. The total reduced nitrogen (TRN) concentration was significantly higher in plants grown under elevated RZ [CO2] of 10,000 ppm than under ambient RZ [CO2] of 360 ppm with greater concentration in 20˚C-RZT plants than in A-RZT plants. These results imply that elevated RZ [CO2] significantly affected root morphology, root and shoot growth and N metabolism of temperate lettuce with greater impacts at A-RZT than at 20˚C-RZT. These findings have practical significance to vegetable production by growing the vegetable crops at cool-RZT with elevated RZ [CO2] to enhance its productivity.
concentrations of leaf and root were higher at 20˚C-RZT than at A-RZT in all plants. NRA was higher in root than in leaf especially under A-RZT. The total reduced nitrogen (TRN) concentration was significantly higher in plants grown under elevated RZ [CO2] of 10,000 ppm than under ambient RZ [CO2] of 360 ppm with greater concentration in 20˚C-RZT plants than in A-RZT plants. These results imply that elevated RZ [CO2] significantly affected root morphology, root and shoot growth and N metabolism of temperate lettuce with greater impacts at A-RZT than at 20˚C-RZT. These findings have practical significance to vegetable production by growing the vegetable crops at cool-RZT with elevated RZ [CO2] to enhance its productivity.
Keywords:
Lettuce, Nitrate Assimilation, Nitrate Uptake, Root Morphology, Root-Zone CO2, Root-Zone Temperature

1. Introduction
Aerial parts of plant physiology such as photosynthesis are closely associated with morphology and physiological activities of roots, especially under stress conditions [1] - [3] . For example, high temperature limits growth and productivity of temperate crops grown in the tropics, which are mainly due to its poor root development [1] . However, temperate lettuce can now be grown in tropical Singapore at any time of the year by cooling their roots while their shoot are subjected to hot ambient temperature [2] [4] [5] . Except for cooling the RZ, elevated RZ [CO2] also enhanced its productivity [6] - [10] . Effects of elevated RZ [CO2] on photosynthetic gas exchange and water use efficiency have received increased attention recently [11] - [17] . We have previously reported the effects of elevated RZ [CO2] on photosynthesis of aeroponically grown lettuce plants at different RZTs [6] - [10] .
In the aeroponic growing system, plant roots have very little interaction with microorganisms. Thus, RZ [CO2] of aeroponically grown plants is much lower than those plants grown in soil [10] . However, plant roots were able to transport CO2 such as 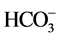 (dissolved inorganic carbon, DIC) to the aerial parts of plants for photosynthesis regardless of growth medium [11] - [17] . Theoretically, light and CO2 levels are major environmental factors that limit plant growth in the aeroponic systems as water deficit is reduced due to the continuous nutrient and water being supplied to the plant roots [1] . It is well known that the amounts of water and nutrient uptake are determined by both the availability of nutrient solution and the morphology and physiology of the root systems [1] [2] . The changes in root morphology of aeroponically grown plants are closely associated with variations in water and mineral nutrient uptake [1] [2] [18] . Well-developed root systems under cooling RZ environment enhanced water and mineral nutrient uptake, especially the
(dissolved inorganic carbon, DIC) to the aerial parts of plants for photosynthesis regardless of growth medium [11] - [17] . Theoretically, light and CO2 levels are major environmental factors that limit plant growth in the aeroponic systems as water deficit is reduced due to the continuous nutrient and water being supplied to the plant roots [1] . It is well known that the amounts of water and nutrient uptake are determined by both the availability of nutrient solution and the morphology and physiology of the root systems [1] [2] . The changes in root morphology of aeroponically grown plants are closely associated with variations in water and mineral nutrient uptake [1] [2] [18] . Well-developed root systems under cooling RZ environment enhanced water and mineral nutrient uptake, especially the  uptake compared to those roots exposed to high temperatures [1] [2] [18] [19] . The
uptake compared to those roots exposed to high temperatures [1] [2] [18] [19] . The 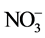 uptake could be associated with exchange for DIC such as
uptake could be associated with exchange for DIC such as . The elevated DIC stimulated respiratory electron transport and increased the incorporation of
. The elevated DIC stimulated respiratory electron transport and increased the incorporation of  into amino acids [11] [20] . In the study of crisp head-type lettuce (L. sativa L. cv. “Wintergreen”) grown aeroponically under different RZ [CO2] at two different air temperatures [8] , we previously found that
into amino acids [11] [20] . In the study of crisp head-type lettuce (L. sativa L. cv. “Wintergreen”) grown aeroponically under different RZ [CO2] at two different air temperatures [8] , we previously found that 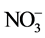 and the total reduced N (TRN) concentrations of shoots were higher in all plants under elevated RZ [CO2] than under ambient RZ [CO2] at 28˚C/22˚C (day/night temperature) and 36˚C/30˚C. However, there was very little information on the impacts of elevated RZ [CO2] on root morphology and the key enzyme such as nitrate reductase (NR) under different RZTs. This paper aimed to study the effects of RZ [CO2] with manipulation of RZT on root morphology and growth. The impacts of RZ [CO2] under different RZTs on
and the total reduced N (TRN) concentrations of shoots were higher in all plants under elevated RZ [CO2] than under ambient RZ [CO2] at 28˚C/22˚C (day/night temperature) and 36˚C/30˚C. However, there was very little information on the impacts of elevated RZ [CO2] on root morphology and the key enzyme such as nitrate reductase (NR) under different RZTs. This paper aimed to study the effects of RZ [CO2] with manipulation of RZT on root morphology and growth. The impacts of RZ [CO2] under different RZTs on 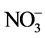 uptake and its assimilation such as NR activity (NRA) were also investigated.
uptake and its assimilation such as NR activity (NRA) were also investigated.
2. Materials and Methods
2.1. Plant Material and Cultural Methods
After germination, seedlings of lettuce (Lactuca sativa L. cv. “Wintergreen”) were transplanted into polyurethane cubes and transferred to the greenhouse for establishment. One week later, they were transplanted to the aeroponic system [1] . Netherlands Standard Composition of nutrient solution was used with pH maintained at ~6.0 and an electrical conductivity of 2.2 mS. The aerial parts of plants were expose to ambient temperature fluctuating from 26˚C - 38˚C. Plant roots were subjected to either 20˚C-RZT or fluctuating ambient (A)-RZT. The maximum photosynthetic photon flux density inside the greenhouse was about 1000 mmol∙m?2∙s?1. Relative humidity was between 65% - 95%.
2.2. RZ [CO2] Treatments
Different RZ [CO2] (ambient, 360 ppm and elevated concentrations of 2000, 10,000, 50,000 ppm) were imposed on plants at each of the two RZTs (20˚C-RZT and A-RZT) after the plants were transplanted for three weeks. The ambient [CO2] inside the greenhouse was around 360 to 400 ppm (±5 ppm) while photosynthetic rate was highest between 10.30 to 1100 h. Thus, ambient [CO2] was defined as 360 ppm. Different elevated RZ [CO2] were supplied to different aeroponic troughs, respectively from compressed air cylinders at ~0.5 L∙min−1 using pre-mixed CO2-air mixtures (SOXAL, Singapore Oxygen Air Liquide Pte Ltd.) [9] .
2.3. Analysis of Root Morphology
The root morphology was analysed with WIN MAC RHIZO V3.9 programme (Instruments Regent, Canada) equipped with WIN MAC RHIZO scanner (Québec, Canada) two weeks after treatments. The roots of each plant was detached from the shoot and then placed in a tray of water. The water served to spread out the roots and keep them moist. The roots were first scanned before the total length, root tips, surface area and average root diameter was determined by the programme [19] .
2.4. Measurements of Leaf Area, Shoot and Root Fresh Weight (FW) and Dry Weight (DW)
After elevated [CO2] had been supplied to the RZ for one day, similar sizes of the second leaves from the top were labelled immediately from 5 different plants for each treatment. Leaf areas were drawn on the papers between 0800 h to 0900h for every two to three days for 12 days. The leaves drawn on paper were cut and areas were measured using the Area Measurement System (Delta T-Devices Ltd., England). Every 5 days after treatments, whole plants were removed from the aeroponics system and divided into shoot and roots. FWs were recorded immediately and they were then dried to constant mass in an oven at 80˚C before weighing the DWs on an analytical balance.
2.5. Determination of 
Dried plant tissue of 0.03 g was ground using a pestle and mortar with deionised water and then incubated at 37˚C for 2 hours. Sample turbidity was removed by filtration through a 0.45 µm pore diameter membrane filter prior to analysis. The 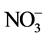 was determined using a Flow Injection Analyser (Model QuikChem 8000, Lachat Instruments Inc., Milwaukee, USA) [19] . The principle of this method was to catalytically reduce
was determined using a Flow Injection Analyser (Model QuikChem 8000, Lachat Instruments Inc., Milwaukee, USA) [19] . The principle of this method was to catalytically reduce 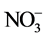 to
to  and measure the amount of
and measure the amount of 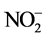 present by a calorimetric reaction.
present by a calorimetric reaction.  is quantitatively reduced to
is quantitatively reduced to 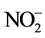 by passage of the sample through a copperized cadmium column. The
by passage of the sample through a copperized cadmium column. The 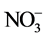 is then determined by diazotizing with sulfanilamide followed by coupling with N-(1-naphthyl) ethylenediamine dihydrochloride. The resulting water soluble dye has a magenta color which is read at 520 nm.
is then determined by diazotizing with sulfanilamide followed by coupling with N-(1-naphthyl) ethylenediamine dihydrochloride. The resulting water soluble dye has a magenta color which is read at 520 nm.
2.6. Determination of Nitrate Reductase Activity (NRA)
Leaf or root samples were rapidly frozen in liquid nitrogen after weighed and stored at −80˚C until use. The frozen sample (0.5 to 1 g) was powdered in liquid nitrogen and ground with 4 ml of extraction buffer, in a mortar with the presence of 0.2 g/g FW insoluble PVP. The extraction buffer contained 0.25 M Tris-HCl (pH 8.5), 3 mM dithiothreitol (DTT), 10 µM flavin adenine dinucleotide (FAD), 1 µM sodium molybdate, 1 mM ethylenediamine-tetra-acetic acid (EDTA) [21] . The extracts were centrifuged at 15,000 g for 10 min at 4˚C. NRA was measured immediately in the supernatant. In vitro NADH:NRA assay was derived from Kaiser and Huber [22] with modification. The reaction medium contained 50 mM Hepes-KOH (pH 7.5), 1 mM DTT, 10 µM FAD, 10 mM KNO3, 0.2 mM NADH, NR extraction, and 15 mM EDTA. The reaction was started by adding of 300 µl NR extraction. Incubation was performed at 25˚C for 20 min, and the reaction was then terminated by adding sulfanilamide (1%(w/v) in 3 N HCl) and the naphthylethylene-diamine dihydrochloride (0.02% w/v). After 30 min at room temperature, the optical density of all the samples was read at 540 nm. The blank was identical to the samples, but the NR extracts were boiled for 5 min before adding into the reaction mixture. NRA was expressed as nmol 
2.7. Determination of TRN Concentration
The concentration of TRN was determined by Kjeldahl digestion of dried samples in concentrated sulphuric acid [23] . The dry sample was placed into a digestion tube with a Kjeldahl tablet and 5 ml of concentrated sulphuric acid. The mixture was then digested in a digestor until the mixture turned clear. After the digestion was completed, the mixture was allowed to cool for 30 min before it was used to determine N concentration by a Kjeltec auto 1030 analyser. This result was later used to calculate the N concentration (mg/g DW) present in the sample.
2.8. Statistical Analysis
One-way ANOVA was used to test for significant differences among different RZ [CO2] treatments. Tukey’s multiple comparison tests were used to discriminate the means (MINITAB, Inc., Release 15, 2007).
3. Results
3.1. Root Morphology
Root morphology analysis was carried out 2 weeks after different RZ [CO2] treatments. It was shown that plants grown under elevated RZ [CO2] had longer total root length (Figure 1(A)), greater number of root tips (Figure 1(B)) and larger root surface area (Figure 1(C)) than under ambient RZ [CO2] at both RZTs with greater values obtained from 20˚C-RZT plants than from A-RZT plants. The higher the RZ [CO2] supplied to the plants, the greater the increase in root length, root tip number and root surface area. Elevated RZ [CO2] did not result in any significant changes in average root diameter at either 20˚C-RZT or A-RZT (Figure 1(D)). However, root diameter of A-RZT plants were significantly thicker than those of 20˚C-RZT plants.
3.2. Leaf Growth, Shoot and Root Productivity
Expansion of leaf (leaf area) was monitored one day after elevated RZ [CO2] had been supplied to the RZ. Leaf expansion of all elevated RZ [CO2] plants were faster than that
Figure 1. Total root length (A), total number of root tips (B), total surface area (C) and average root diameter (D) of lettuce plants grown under different elevated RZ [CO2] at 20˚C-RZT and A-RZT for 3 weeks. Each mean is 5 measurements of 5 different leaves. Vertical bars represent the standard errors. Means with different letters above the bars are statistically different (P < 0.05) as determined by Tukey’s multiple comparison test.
of ambient RZ [CO2]. There were no significant differences in leaf areas when the roots were exposed to elevated RZ [CO2] of 10,000 and 50,000 ppm and they were much greater than those plants exposed to elevated RZ [CO2] of 2000 ppm (Figure 2). Responses of leaf expansion to different RZ [CO2] were similar between 20˚C-RZT (Figure 2(A)) and A-RZT (Figure (2B)) plants. Although leaf growth was much faster under 20˚C-RZT than under A-RZT the magnitude of increased leaf area under elevated RZ [CO2] was greater in A-RZT plants than in A-RZT plants.
Both shoot and root productivities were determined every 5 days after elevated RZ [CO2] treatments for 20 days. Greater accumulation of biomass in lettuce plants at elevated RZ [CO2] than at ambient RZ [CO2] at both RZTs. By the end of three weeks, RZ [CO2] of 10,000 ppm was adequate for maximal shoot production since no further increases in both shoot FW (Figure 3(A) and Figure 3(D)) and DW (data not shown) were obtained when plants were subjected to RZ [CO2] of 50,000 ppm at both RZTs with greater increases in plants grown under A-RZT than under 20˚C-RZT. However, a greater increase in root FW (Figure 3(B) and Figure 3(E)) and DW (data not shown) in plants grown under RZ [CO2] of 50,000 ppm than of 10,000 ppm. For shoot/root ratio FW (Figure 3(C) and Figure 3(F)) and shoot/root ratio DW (data not shown), there were no changes in plants grown under RZ [CO2] of 50,000 ppm at both RZTs. However, they were much lower than plants grown under other RZ [CO2].
3.3. 


Figure 2. Changes in leaf area of lettuce plans grown under different elevated RZ [CO2] at 20˚C-RZT (A) and A-RZT (B). Each mean is 5 measurements of 5 different leaves. Vertical bars represent the standard errors. Means with different letters on day 12 are statistically different (P < 0.05) as determined by Tukey’s multiple comparison test.
Figure 3. Changes in shoot FW ((A), (D)), root FW ((B), (E)) and shoot/root ratio FW ((C), (F)) of lettuce plans grown under different elevated RZ [CO2] at 20˚C-RZT and A-RZT. Each mean is 5 measurements of 5 different leaves. Vertical bars represent the standard errors. Means with different letters on day 20 are statistically different (P < 0.05) as determined by Tukey’s multiple comparison test.
Figure 4. 
The concentration of TRN was significantly higher in plants grown under elevated RZ [CO2] of 10,000 ppm than under ambient RZ [CO2] of 360 ppm at both growth stages (Figure 5). At each RZ [CO2], 20˚C-RZT plants had higher concentration of TRN than those of A-RZT plants. Furthermore, the concentrations of TRN in leaves were higher than in roots for all plants.
NRA was higher in root than in leaf for each given RZ [CO2] especially under A-RZT at both growth stages of 6 (Figure 6(A)) and 12 (Figure 6(B)) days after treatments.
Figure 5. TRN concentration of leaves and roots after different RZT and [CO2] treatments for 6 (A) and 12 days (B). Vertical bars represent the standard errors. Means with different letters are statistically different (P < 0.05; n = 5) as determined by Tukey’s multiple comparison test.
Figure 6. NRA of leaves and roots after different RZT and [CO2] treatments for 6 (A) and 12 days (B). Vertical bars represent the standard errors. Means with different letters are statistically different (P < 0.05; n = 4) as determined by Tukey’s multiple comparison test.
For both leaf and root, NRA was higher in plants under RZ [CO2] of 10,000 ppm than under RZ [CO2] of 360 ppm.
4. Discussion
We have previously reported that elevated RZ [CO2] stimulated photosynthesis of aeroponically grown lettuce plants and enhanced water use efficiency and plant growth, with greater increase at higher ambient temperature [8] and hot A-RZT compared to cool ambient temperature and cool-RZT [9] . Elevated levels of atmospheric CO2 promote not only leaf photosynthesis but also photoassimilate partitioning to roots which stimulate root development [24] [25] . This study showed that elevated RZ [CO2] also promoted root development and root growth with longer total root length (Figure 1(A)), greater number of root tips (Figure (1B)), larger root surface area and greater root biomass accumulation (Figure 2(B) and Figure 2(E)) at both 20˚C-RZT and A-RZT. Furthermore, the magnitudes of increased total root length, root tips, root surface area and root biomass were greater in A-RZT plant than in 20˚C-RZT plants.
It was reported that increased plant growth at elevated atmosphericCO2 depends on the source/sink ratio and “sink capacity” of plants [26] . In the present study, elevated RZ [CO2] increased from 10,000 to 50,000 ppm did not result in further increases in leaf area (Figure 2), shoot and root FW (Figure 3) and DW (data not shown) in both 20˚C-RZT and A-RZT. However, root productivity (root FW and DW) increased further under elevated RZ [CO2] of 50,000 ppm with constant shoot/root ratio throughout the entire 3 weeks of treatment compared to plants grown under other RZ [CO2] of 360, 2000 and 10,000 ppm at both RZTs (Figure 2(C) and Figure 2(F)). These results support the fact that plants with larger root systems under elevated RZ [CO2] enhanced the capacity of utilizing photosynthetic products with greater amount of photoassimilate portioning to their roots [8] [9] . We have previously reported that lettuce plants grown at 20˚C-RZT had not only greater root biomass but also longer total length, greater number of tips and larger surface area as compared with A-RZT plants [28] . In the present study, elevated RZ [CO2] also promoted root growth and development at both 20˚C-RZT and A-RZT. Our previous study also showed that plants grown at 20˚C-RZT had smaller average diameter of roots compared to those grown at A-RZT [28] . However, in this study, regardless of RZ [CO2] treatments, there were no differences in average root diameter at either 20˚C-RZT or A-RZT (Figure 1(D)). The temperature of a sink could affect its metabolic rate and thus its capacity to utilize carbohydrate [27] - [29] . Elevated RZ [CO2] could also affect the physiological activities of roots and thus alter the pattern of photoassimilate partitioning. This merits our further studies.
A well-developed large root system generally increased the capacity of plants to exploit water and mineral nutrients especially under other adversely environmental conditions [13] [14] . It has been reported that elevated RZ [CO2] could enhance the growth of tomato (Lycopersicon esculentum) seedlings, especially under stress conditions such as salinity and high temperature. Greater number of fine roots in larger root system (Figure 1) which resulted in a greater amount of 




The elevated DIC stimulated respiratory electron transport and increased the incorporation of 





In our previous study, it was found that lettuce plants grown under elevated RZ [CO2], had lower light saturated stomatal conductance, gs sat but higher light saturated photosynthetic CO2 assimilation rate, Asat. Higher Asat under elevated RZ [CO2] could partially be resulted from the incorporation of DIC that improved the incorporation of N into amino acids in the roots as a consequence of a greater supply of anaplerotic carbon for protein synthesis [8] [9] . Our previous study also showed that higher 
Assimilation of 





Acknowledgements
All experiment work on subtropical and temperate vegetables grown aeroponically was supported by the Academic Research Fund (Grant reference numbers: RP12/01, RI7/05 HJ, Ministry of Education, Singapore and Singapore Millennium Foundation (Project code: SMF-Farming System).
Cite this paper
He, J., Qin, L. and Lee, S.K. (2016) Root Morphology, Plant Growth, Nitrate Accumulation and Nitrogen Metabolism of Temperate Lettuce Grown in the Tropics with Elevated Root-Zone CO2 at Different Root-Zone Temperatures. American Journal of Plant Sciences, 7, 1821-1833. http://dx.doi.org/10.4236/ajps.2016.714169
References
- 1. He, J. (2010) Mineral Nutrition of Aeroponically Grown Subtropical and Temperate Crops in the Tropics with Manipulation of Root-Zone Temperature at Different Growth Irradiances. Plant Stress, 4, 14-30.
- 2. He, J. (2009) Impact of Root-Zone Temperature on Photosynthetic Efficiency of Aeroponically Grown Temperate and Subtropical Vegetable Crops in the Tropics. In: Buchner, Th.B. and Ewingen, N.H., Eds., Theory and Applications in Energy, Biotechnology and Nanotechnology, Nova Science Publishers Inc., New York, Chapter 4, 111-143.
- 3. Yang, J.C. (2011) Relationships of Rice Root Morphology and Physiology with the Formation of Grain Yield and Quality and the Nutrient Absorption and Utilization. Scientia Agricultura Sinica, 1, 36-46.
- 4. He, J. and Lee S.K. (1998) Growth and Photosynthetic Characteristics of Lettuce (Lactuca sativa L.) Grown under Fluctuating Hot Ambient Temperatures with the Manipulation of Cool Rootzone Temperature. Journal of Plant Physiology, 152, 387-391.
http://dx.doi.org/10.1016/S0176-1617(98)80252-6 - 5. He, J. and Lee, S.K. (1998) Growth and Photosynthetic Responses of Three Aeroponically Grown Lettuce Cultivars (Lactuca sativa L.) to Different Rootzone Temperatures and Growth Irradiances under Tropical Aerial Condition. The Journal of Horticultural Science and Biotechnology, 73, 173-180.
http://dx.doi.org/10.1080/14620316.1998.11510961 - 6. He, J., Austin, P.T., Nichols, M.A. and Lee, S.K. (2004) Effect of Root-Zone CO2 on Productivity and Photosynthesis in Aeroponically Grown Lettuce Plants. Acta Horticulturae, 648, 39-45.
http://dx.doi.org/10.17660/ActaHortic.2004.648.5 - 7. He, J., Austin, P.T., Nichols, M.A. and Lee, S.K. (2007) Elevated Root-Zone CO2 Protects Lettuce Plants from Midday Depression of Photosynthesis. Environmental and Experimental Botany, 61, 94-110.
http://dx.doi.org/10.1016/j.envexpbot.2007.04.001 - 8. He, J., Austin, P.T., Nichols, M.A. and Lee, S.K. (2010) Effects of Elevated Root-Zone CO2 and Air Temperature on Photosynthetic Gas Exchange, Nitrate Uptake and Total Reduced Nitrogen Content in Aeroponically Grown Lettuce Plants. Journal of Experimental Botany, 61, 3959-3969.
http://dx.doi.org/10.1093/jxb/erq207 - 9. He, J., Qin, L. and Lee, S.K. (2013) Root-Zone CO2 and Root-Zone Temperature Effects on Photosynthesis and Nitrogen Metabolism of Aeroponically Grown Lettuce (Lactuca sativa L.) Plants in the Tropics. Photosynthetica, 52, 330-340.
http://dx.doi.org/10.1007/s11099-013-0030-5 - 10. He, J. (2015) Elevated Root-Zone CO2 on Photosynthesis of Vegetable Crops at Different Air and Root-Zone Temperatures. In: Khan, N., Ed., Photosynthesis: Functional Genomics, Physiological Processes and Environmental Issues, Nova Science Publishers, Inc., New York, Chapter 4, 25-54.
- 11. Cramer, M.D., Lewis, O.A.M. and Lips, S.H. (1993) Inorganic Carbon Dioxide Fixation and Metabolism in Maize Roots as Affected by Nitrate and Ammonium Nutrition. Physiologia Plantarum, 89, 632-639.
http://dx.doi.org/10.1111/j.1399-3054.1993.tb05226.x - 12. Cramer, M.D. and Lips, S.H. (1995) Enriched Rhizosphere CO2 Concentrations Can Ameliorate the Influence of Salinity on Hydroponically Grown Tomato Plants. Physiologia Plantarum, 94, 425-432.
http://dx.doi.org/10.1111/j.1399-3054.1995.tb00949.x - 13. Cramer, M.D. and Richards, M.B. (1999) The Effect of Rhizosphere Dissolved Inorganic Carbon on Gas Exchange Characteristics and Growth Rates of Tomato Seedlings. Journal of Experimental Botany, 50, 79-87.
http://dx.doi.org/10.1093/jxb/50.330.79 - 14. Viktor A. and Cramer, M.D. (2003) Variation in Root-Zone CO2 Concentration Modifies isotopic Fractionation of Carnon and Nitrogen in Tomato Seedlings. New Phytologist, 157, 45-54.
http://dx.doi.org/10.1046/j.1469-8137.2003.00650.x - 15. Cramer, M.D., Shane, M.W. and Lambers, H. (2005) Physiological Changes in White Lupin Associated with Variation in Root-Zone CO2 Concentration and Cluster-Root P Mobilization. Plant, Cell & Environment, 28, 1203-1217.
http://dx.doi.org/10.1111/j.1365-3040.2005.01358.x - 16. Ford, C.R., Wurzburger, N., Hendrick, R.L. and Teskey, R.O. (2007) Soil DIC Uptake and Fixation in Pinus taeda Seedlings and Its C Contribution to Plant Tissues and Ectomycorrhizal Fungi. Tree Physiology, 27, 375-383.
http://dx.doi.org/10.1093/treephys/27.3.375 - 17. Berveiller, D. and Damesin, C. (2008) Carbon Assimilation by Tree Stems: Potential Involvement of Phosphoenolpyruvate Carboxylase. Trees, 22, 149-157.
http://dx.doi.org/10.1007/s00468-007-0193-4 - 18. Qin L., He, J., Lee, S.K. and Dodd, I.C. (2007) An Assessment of Ethylene Mediation of Lettuce (Lactuca sativa) Root Growth at High Temperatures. Journal of Experimental Botany, 58, 3017-3024.
http://dx.doi.org/10.1093/jxb/erm156 - 19. Tan, L.P., He, J. and Lee, S.K. (2002) Effects of Root-Zone Temperature on the Root Development and Nutrient Uptake of Lactuca sativa L. cv “Panama” Grown in an Aeroponic System in the Tropics. Journal of Plant Nutrition, 25, 297-314.
http://dx.doi.org/10.1081/PLN-100108837 - 20. Minchin, P.E.H. and Thorpe, M.R. (1996) What Determines Carbon Partitioning between Competing Sinks? Journal of Experimental Botany, 47, 1293-1296.
http://dx.doi.org/10.1093/jxb/47.Special_Issue.1293 - 21. Kuo, T.-M., Warner, R.L. and Kleinhofs, A. (1982) In Vitro Stability of Nitrate Reductase from Barley Leaves. Phytochemisry, 21, 531-533.
http://dx.doi.org/10.1016/0031-9422(82)83134-8 - 22. Kaiser, W.M. and Huber, S.C. (1997) Correlation between Apparent Activation State of Nitrate Reductase (NR), NR Hysteresis and Degradation of NR Protein. Journal of Experimental Botany, 48, 1367-1374.
http://dx.doi.org/10.1093/jxb/48.7.1367 - 23. Allen, S.E. (1989) Analysis of Vegetation and Other Organic Materials. In: Allen, S.E., Ed., Chemical Analysis of Ecological Materials, Blackwell Scientific Publication, Oxford, 46-61.
- 24. Norby, R.J., Gunderson, C.A., Wullschleger, S.D., O’Neill, E.G. and McCracken, M.K. (1992) Productivity and Compensatory Responses of Yellow-Poplar Trees in Elevated CO2. Nature, 357, 322-324.
http://dx.doi.org/10.1038/357322a0 - 25. Norby, R.J. (1994) Issues and Perspectives for Investigating Root Responses to Elevated Atmospheric Carbon Dioxide. Plant Soil, 165, 9-20.
http://dx.doi.org/10.1007/BF00009958 - 26. Makino, A. and Mae, T. (2004) Photosynthesis and Plant Growth at Elevated Levels of CO2. Plant and Cell Physiology, 40, 999-1006.
http://dx.doi.org/10.1093/oxfordjournals.pcp.a029493 - 27. Ainsworth, E.A. and Rogers, A. (2007) The Response of Photosynthesis and Stomatal Conductance to Rising [CO2]: Mechanisms and Environmental Interactions. Plant, Cell & Environment, 30, 258-270.
http://dx.doi.org/10.1111/j.1365-3040.2007.01641.x - 28. He, J., Tan, L.P. and Lee S.K. (2009) Root-Zone Temperature Effects on Photosynthesis, 14C-Photoassimilate Partitioning and Growth of Temperate Lettuce (Lactuca sativa cv. “Panama”) Grown in the Tropics. Photosynthetica, 47, 95-103.
http://dx.doi.org/10.1007/s11099-009-0015-6 - 29. Schurr, U., Heckenberger, U., Herdel, K., Walter, A. and Feil, R. (2000) Leaf Development in Ricinus communis during Drought Stress: Dynamics of Growth Processes, of Cellular Structure and of Sink-Source Transition. Journal of Experimental Botany, 51, 1515-1529.
http://dx.doi.org/10.1093/jexbot/51.350.1515 - 30. Evans, J.R. (1989) Photosynthesis and Nitrogen Relationships in Leaves of C3 Plants. Oecologia, 78, 9-19.
http://dx.doi.org/10.1007/BF00377192 - 31. Ellsworth, D.S., Reich, P.B., Naumburg, E.S., Koch, G.W., Kubiske, M.E. and Smith, S.D. (2004) Photosynthesis, Carboxylation and Leaf Nitrogen Responses of 16 Species to Elevated pCO2 across Four Free-Air CO2 Enrichment Experiments in Forest, Grassland and Desert. Global Change Biology, 10, 2121-2138.
http://dx.doi.org/10.1111/j.1365-2486.2004.00867.x - 32. Pettersson, R. and McDonald, J.S. (1994) Effects of Nitrogen Supply on the Acclimation of Photosynthesis to Elevated CO2. Photosynthesis Research, 39, 389-400.
http://dx.doi.org/10.1007/BF00014593 - 33. Van der Merwe, C.A. and Cramer, M.D. (2000) The Influence of Dissolved Inorganic Carbon in the Root-Zone on Nitrogen Uptake and the Interaction between Carbon and Nitrogen Metabolism. In: Loucao, M.A. and Lips, S.H., Eds., Nitrogen in a Sustainable Ecosystem—From the Cell to the Plant, Backhuys Publishers, Leiden, 145-151.
- 34. Cookson, S.J., Williams, L.E. and Miller, A.J. (2005) Light-Dark Changes in Cytosolic Nitrate Pools Depend on Nitrate Reductase Activity in Arabidopsis Leaf Cells. Plant Physiology, 138, 1097-1105.
http://dx.doi.org/10.1104/pp.105.062349 - 35. Kaiser, W.M. and Brendel-Benisch, E. (1991) Rapid Modulation of Spinach Leaf Nitrate Reductase Activity by Photosynthesis. Modulation in Vivo by CO2 Availability. Plant Physiology, 96, 363-367.
http://dx.doi.org/10.1104/pp.96.2.363 - 36. Cen, Y.P. and Layzell, D.B. (2003) In Vivo Gas Exchange Measurement of the Site and Dynamics of Nitrate Reduction in Soybean. Plant Physiology, 131, 1147-1156.
http://dx.doi.org/10.1104/pp.102.019430 - 37. Scheurwater, I., Koren, M., Lambers, H. and Atkin, O.K. (2002) The Contribution of Roots and Shoots to Whole Plant Nitrate Reduction in Fast- and Slow-Growing Grass Species. Journal of Experimental Botany, 55, 1635-1642.
http://dx.doi.org/10.1093/jxb/erf008 - 38. Fresneau, C., Ghhahghaie, J. and Cornic, G. (2007) Drought Effect on Nitrate Reductase and Sucrose-Phosphate Synthase Activities in Wheat (Triticum durum L.): Role of Leaf Internal CO2. Journal of Experimental Botany, 58, 2983-2992.
http://dx.doi.org/10.1093/jxb/erm150 - 39. Li, Y., Gao, Y., Xu, X., Shen, Q. and Guo, S. (2009) Light-Saturated Photosynthetic Rate in High-Nitrogen Rice (Oryza sativa L.) Leaves Is Related to Chloroplastic CO2 Concentration. Journal of Experimental Botany, 60, 2351-2360.
http://dx.doi.org/10.1093/jxb/erp127 - 40. Peterson, R.B. (1983) Estimation of Photorespiration Based on the Initial Rate of Postillumination CO2 Release: II. Effects of O2, CO2, and Temperature. Plant Physiology, 73, 983-988.
http://dx.doi.org/10.1104/pp.73.4.983 - 41. Peterhansel, C. and Maurino, V.G. (2011) Photorespiration Redesigned. Plant Physiology, 155, 49-55.
http://dx.doi.org/10.1104/pp.110.165019







