American Journal of Plant Sciences
Vol.4 No.2A(2013), Article ID:28450,7 pages DOI:10.4236/ajps.2013.42A048
Cadmium Accumulation in Marsilea minuta Linn. and Its Antioxidative Responses
![]()
1Plant Physiology and Plant Molecular Biology Research Unit, Department of Botany, University of Kalyani, Kalyani, India; 2Department of Biotechnology, Visva Bharati Visvavidyalaya, Santiniketan, India.
Email: #mkadak09@gmail.com
Received December 19th, 2012; revised January 20th, 2013; accepted January 28th, 2013
Keywords: Oxidative Stress; Heavy Metals; Aquatic Fern
ABSTRACT
The present study was carried out to investigate the extent of cadmium (Cd) accumulation with its possible impact on physiological and biochemical basis of heavy metal tolerance in Marsilea minuta Linn. Cd salt (0 µM, 50 µM and 100 µM) was allowed to absorb by the plants for prolong days in hydroponic culture and a significant deterioration of the plant biomass was recorded. However, roots absorbed more metals than the leaves. Plants recorded a significant rise of superoxide (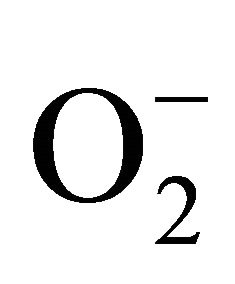 ) and hydrogen peroxide (H2O2). A noticeable amount of protein oxidation and lipid peroxidation were in proportionate to Cd accumulation. Anthocyanin and flavonoid content were decreased as compared to control condition. Superoxide dismutase (SOD), guaiacol peroxidase (GPX) and glutathione reductase (GR) contributed their antioxidative functions according to the Cd doses. The expression of GR was also evident from its activity staining in gel. So, it may suggest that antioxidative enzymes are up regulated and likely to be responsible for tolerance to Cd induced oxidative stress in Marsilea minuta Linn.
) and hydrogen peroxide (H2O2). A noticeable amount of protein oxidation and lipid peroxidation were in proportionate to Cd accumulation. Anthocyanin and flavonoid content were decreased as compared to control condition. Superoxide dismutase (SOD), guaiacol peroxidase (GPX) and glutathione reductase (GR) contributed their antioxidative functions according to the Cd doses. The expression of GR was also evident from its activity staining in gel. So, it may suggest that antioxidative enzymes are up regulated and likely to be responsible for tolerance to Cd induced oxidative stress in Marsilea minuta Linn.
1. Introduction
Cd, one of the heavy metals that is undoubtedly toxic to plants with different interfaces of physiological activities. In general, a decrease in growth and development and its resultant of subdued biomass production is a common feature in plants under Cd contaminated soil [1]. Cd is non redox metal and very frequently affects plant growth by influencing the normal mineral status in a number of ways [2]. Imbalance of nutrient due to impaired membrane potential happens to be the most important. The major source of Cd contamination in ecosystem encompasses the industrial effluents, both particulate and gaseous in nature and shows high solubility in water. Thereby, water bodies in the surrounding areas of industrial belts are characterized with metal pollution with reference to Cd toxicity [3]. Cd is a strong phytotoxic element and thus ensures partial to complete growth inhibition of plants and eventually resulting in death of some plant species [4]. The major attributing factors those expedite and retardation of plants growth are impaired photosynthesis and translocation of solutes, unusual meiotic behaviour, abnormal chloroplast structure and attenuation of chlorophyll, rapid loss of photochemical reactions and enhanced fluorescence etc, the major phytopathological aspect under Cd toxicity is thus established predominantly for the induced chlorosis. Chlorosis under Cd toxicity is also proven to be an Imbalance of Fe:Zn and its concomitant involvement in chlorophyll and other heame containing moieties in plants [5]. On the other extreme of Cd toxicity, it behaves as a pro-oxidant evoking an establishment of elevated cellular redox more towards oxidized state. An exuberation of some free radicals and other reactive oxygen species (ROS) can inevitably ensure the oxidative damages of the macromolecules predominantly of those of lipids, proteins and nucleic acids [6]. In plants the most striking impact of Cd toxicity though preliminary is the leaf chlorosis. This is mostly accomplished by both degradation of chlorophyll or inhibition of chlorophyll biosynthesis through any of its rate limiting states [7]. Now, cholorophyll degradation may be forwarded by damaging of membrane due to lipoxigenase activity or/and following some reactions accumulating aldehyde product of lipid-protein complex [8]. As for example alfaalfa (Medicago sativa L.) plants under Cd stress correlated lipoprotein and concentration dependant chlorophyll-protein complex. However, a number of plant species are reported with varying capacity of Cd accumulation. The most identified Cd hyper-accumulator, to date are included in many angiospermic genera like Agrostis tenuis, Silene vulgaris etc. [9]. However, non-angiospermic plants are also reported in various divisions in plant kingdom to be accumulating as Cd hyper-accumulator, though varying in capacity. Fern species are also reported among the pteridophytes as a specific or selective bioaccumulators of metals, particularly, heavy metals [10]. Recently, Chinese Brake Fern (Pteris vittata Linn.) and other species have primarily been reported to be arsenic, lead, nickel and selenium accumulators [11]. Cd like other heavy metals, is also paid attention for its accumulation in this fern species. Now, Marsilea (Marsilea minuta Linn.), an aquatic fern species is also shown their hyper-accumulation of heavy metals from environment, particularly in contaminated water bodies [12]. The resistance to Cd toxicity in plants is forwarded by either sequestering the metals into the vacuoles and accomplish the antioxidation reactions against Cd induced reactive oxygen species (ROS). Fern species by nature are over producer of a number of secondary metabolites and happens to be more efficient in antioxidation pathways against heavy metals [13]. A number of reducing moieties are over-accumulated in tissues as a part of non-enzymatic antioxidative pathway. These include a lower molecular weight antioxidants like glutathione as well as some antioxidizing enzymes including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR) etc [14]. Admitted well the less reports on the Marsilea species for metal phytotoxicity, it would be worth to describe the relationship between Cd toxicity and scenario of oxidative stress induced by the metal.
Therefore, in the present study, Marsilea minuta Linn., abundantly grown in lowland or water bodies was taken for consideration. Critical analysis of those contaminants included the abundance of some heavy metals like lead (Pb), mercury (Hg), copper (Cu) and arsenic (As). Thus, the present investigation describes the ability and extent of Marsilea species: 1) to accumulate Cd so as to assess its phytoremediation capacity and 2) to unravel the mechanism for antioxidative responses to Cd, especially at high doses. Precisely the present investigation described the changes of ROS in the Cd accumulated tissue which is well marked physiologically by elevated lipid peroxiadtion and protein carbonylation, upregulation of SOD, GPX and GR, decrease in anthocyanin and flavonoid content in marselia plants.
2. Materials and Methods
2.1. Plant Growth and Treatment
Marsilea minuta Linn., was collected from water pond containing waste water at the industrial contaminated zone of Nadia district, W.B., India. The collected plants were grown for 7 days under Hoagland’s solution. Thereafter, plants were supplemented with varying concentrations (0 µM, 50 µM and 100 µM) of cadmium chloride dissolved in the same solution in different sets. Plants were maintained to grow at field saturation capacity for 15 days in growth chamber at 37˚C ± 1˚C, 85% relative humidity and 14 h light (irradiance 70 - 80 µM/m2/s) with changing solution once a week. On completion of incubation, plants were harvested, separated into roots and rhizomes including leaf biomass and oven dried for estimation of Cd.
2.2. Cd Analysis
The plant samples were dried completely in 550˚C for 8 h to make complete ash in a muffle furnace and dissolved in tri acid mixture of nitric acid, hydrochloric acid and perchloric acid in of 1:1:1. The digested product was used for Cd analysis with atomic absorption spectrophotometer [15].
2.3. Biochemical Analysis
All the biochemical parameters were detected from shoot portions (rhizomes and leaves) of the plants under various treatments according to standard methods.
2.3.1. Assay of Lipid Peroxidation
For lipid peroxidation, fresh samples were completely homogenized in liquid N2 and extracted with 20% trichloroacetic acid containing 0.5% thiobarbituric acid. Malondialdehyde (MDA) content was determined by the procedure of [16].
2.3.2. Assay of Protein Oxidation
For protein oxidation assay, the carbonylated derivatives of protein was measured by dinitrophenylhydrazone in a suspension of 6% sodium dodecyl sulphate [17].
2.3.3. Detection of Superoxide (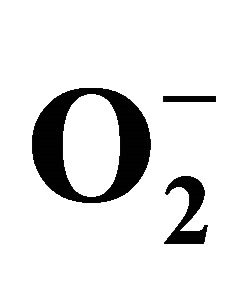 )
)
For measurement of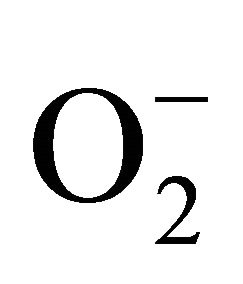 , an aliquot of 0.5 ml supernatant was reacted in 3ml of reaction mixture containing 50 mM Tris-HCl (pH 6.5), 0.2 mM nitroblue tetrazolium, 0.2 mM NADH and 250 mM sucrose for 12 h. The absorbance of the blue colour was read at 530 nm [18].
, an aliquot of 0.5 ml supernatant was reacted in 3ml of reaction mixture containing 50 mM Tris-HCl (pH 6.5), 0.2 mM nitroblue tetrazolium, 0.2 mM NADH and 250 mM sucrose for 12 h. The absorbance of the blue colour was read at 530 nm [18].
2.3.4. Detection of H2O2
For H2O2 measurement, the tissue extract was incubated in reaction mixture (50 mM sodium acetate buffer pH 6.5); 1 mM 4-aminoantipyrine, 1 mM 2,4-dichlorophenol indophenol, 50 mM manganese chloride and 0.2 mM NADH) for 30 min [19].
2.3.5. Determination of Anthocyanin and Flavonoid Content
For anthocyanin, the leaf sample was extracted with 1% (v/v) methanolic HCl at 4˚C and the absorbance was read at 525 nm [20]. The residue dissolved in a reaction mixture of 60% ethanol and 5% sodium nitrate. Flavonoid content was quantified according reacting with 1% aluminium chloride followed by addition of 1 M NaOH. The absorbance was read at 510 nm and quantified as suggested earlier [21].
2.3.6. In-Vitro Assay of SOD, GPX and GR
For activity of SOD (EC 1.15.1.1), fresh sample was thoroughly crushed with liquid nitrogen and extracted with buffer containing 50 mM potassium phosphate (pH 7.8), 0.5 mM EDTA, 7.2 mM β-mercaptoethanol, 0.2% bovine serum albumin, 0.1% ascorbic acid under cold condition and was centrifuged at 12,000 × g for 15 min. The assay mixture containing 50 mM phosphate buffer (pH 7.8), 10 mM methionine, 75 mM NBT, 2 mM riboflavin, 100 mM EDTA and 50 µg equivalent of enzyme protein was incubated under fluorescent light for 10 min. Activity of enzyme was assayed according to earlier citation [22]. For GPX (EC 1.11.1.7) activity, fresh sample was homogenized in an ice-cold buffer of 50 mM sodium phosphate (pH 7.0), 0.5 mM dithiothreitol (DTT), 0.1% BSA, 5 mM polyvinyl-pyropropilidone and was centrifuged at 10,000 × g for 15 min at 4˚C. In an assay mixture of 50 mM sodium phosphate (pH 7.0), 9.0 mM guaiacol, 2.0 mM H2O2 was added and the absorbance was read at 420 nm [23]. The activity of GR (EC 1.8.1.7) was measured in an assay mixture containing 0.1 M phosphate buffer (pH 7.8), 1 mM EDTA, 1 mM GSSG, 0.2 mM NADPH and absorbance was read at 340 nm [24]. GR activity in gel staining was detected by loading 100 µg of protein in a 10% non-denaturing polyacrylamide gel and run at 6 V per lane, 4˚C, ~2 h 30 min. The intensity of the polypeptide bands were resolved in a reaction mixture containing 0.5 mM NADH, 3.4 mM GSSG, 0.5 mM EDTA, 25 mM Tris-Cl, (pH 7.6); 0.5 mM dichlorophenol indophenol, 0.25 mM 3-(4,5-dimethyl-2- thiazolyl-2,5-diphenyl-H-tetrazolium bromide) for 10 min in dark condition. The gel was washed with deionized water repeatedly and resolutions of the band were detected in a gel documentation system [25].
2.4. Statistical Analysis
All the observations were recorded with three replications (n = 3) and data were presented as mean ± SE. The statistical analysis was performed by one-way ANOVA analysis, taking P ≤ 0.05 as significant.
3. Result and Discussion
After 15 days of exposure to varying Cd concentrations, there had been recorded hardly any visual or morphological toxic symptoms of the plants. Rather, the change in biomass accumulation was not statistically significant (P ≤ 0.05) as the plants were incubated in those Cd concentrations through the due course (Data not shown). Interestingly, plants recorded discrimination in accumulation of Cd acquisition in leaves and rhizomes along with roots. Since the plants are less differentiated into rhizomes and roots, in the present study both the organs were considered as shoot for metal accumulation. Thus, the maximum ranges of Cd accumulation recorded 0.82 mg/kg to 43.1 mg/kg in roots and leaves respectively (Table 1). The translocation efficiency so computed as ratio of Cd concentration in the leaves to roots and it ranged from 0.742 to 7.198 (Table 1).
Similar findings with the Chinese Brake fern showed maximum accumulation of heavy metals as reported by some authors [26]. This supposedly exhibits an excellent trait for Marsilea plant to withstand heavy metals even non altering its growth significantly.
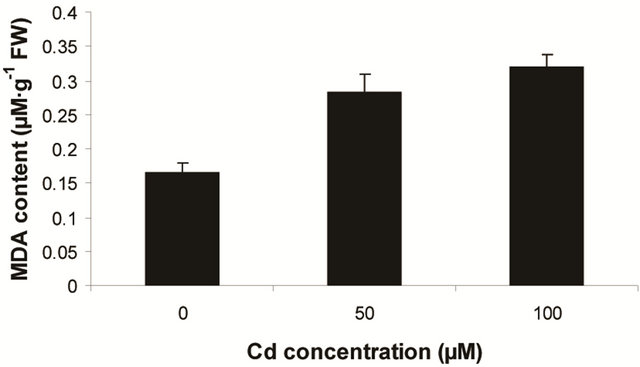
Other fern species like Salvinia, Azolla and Pteris are also reported with the varying capacity of heavy metal accumulation [1]. In the present experiment, Marsilea plants are more prone to allocate the Cd in their leaf tissues and whether and how this agreement is physiologically circumvented still to be ascertained. Likewise, Marsilea plants were sensitive to ROS induced lipid peroxidation. This was detected as an increasing trend of MDA content as a function of Cd concentration (Figure 1(a)). The changes of MDA content though recorded as proportional to dose dependent of Cd but was significant only with highest concentration of metal (i.e 100 µM). Thus, the maximum MDA content recorded to be 1.93 fold over control. This suggests that Marsilea plants had been sensitive to oxidative stress only at excess of Cd contamination. Along with lipid peroxidation, plants are
Table 1. Absorption of cadmium (mg∙kg−1 dry weight) in the leaves and rhizome, translocation efficiency in Marsilea minuta Linn. under varying treatments of cadmium chloride (0 - 100 µM).

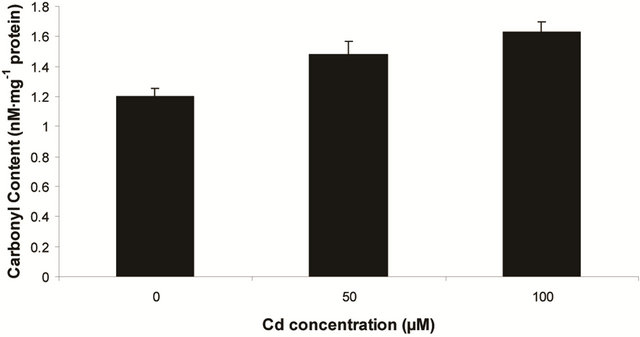
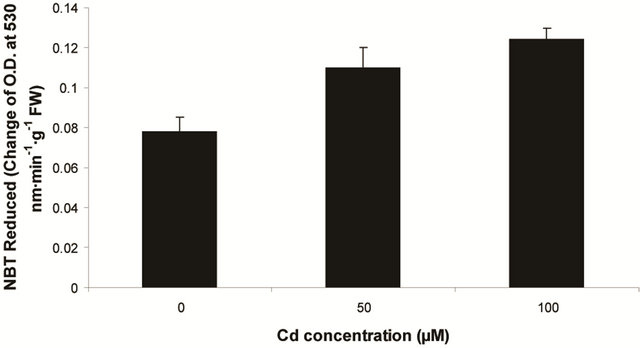
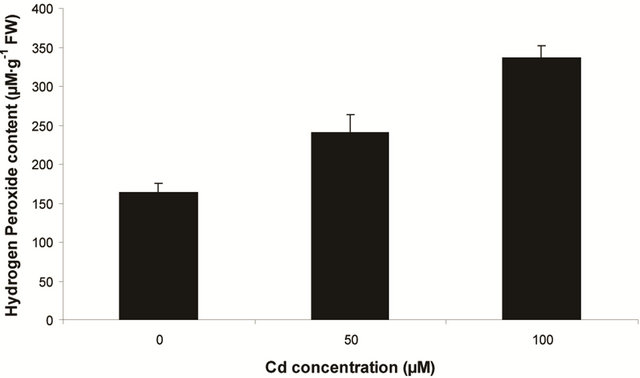
sensitive to oxidation of protein as shown by protein carbamoylation. Thus, a significant protein oxidation was recorded and it ranges from 1.48 unit to 1.63 unit (Figure 1(b)). As a consequence of lipid peroxidation and protein oxidation, plants should have accumulated a significant amount of ROS in the tissue level. As expected in the present experiment, plants recorded a significant amount of superoxide (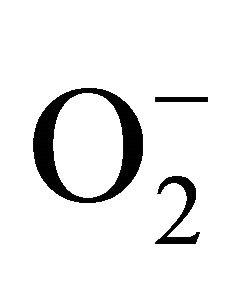 ) and hydrogen peroxide (H2O2) as a function of Cd exposure. It is interesting to note that a linear increase in
) and hydrogen peroxide (H2O2) as a function of Cd exposure. It is interesting to note that a linear increase in 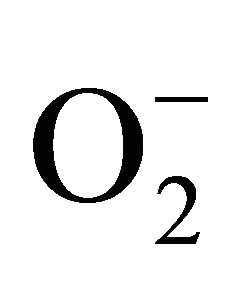 and H2O2 was recorded 0.11unit to 0.124 unit and 240 unit to 337 unit respectively (Figures 1(c) and (d)). As compared to control, plants were much prone to acquisition of H2O2 than
and H2O2 was recorded 0.11unit to 0.124 unit and 240 unit to 337 unit respectively (Figures 1(c) and (d)). As compared to control, plants were much prone to acquisition of H2O2 than 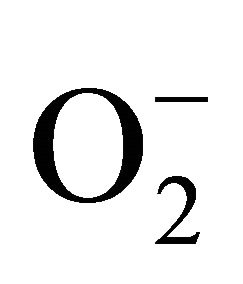 content in its tissue. It was 2.05 fold and 1.58 fold increase over control for H2O2 and
content in its tissue. It was 2.05 fold and 1.58 fold increase over control for H2O2 and 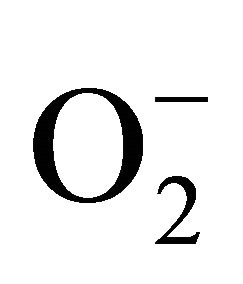 respectively at 100 µM Cd.
respectively at 100 µM Cd.
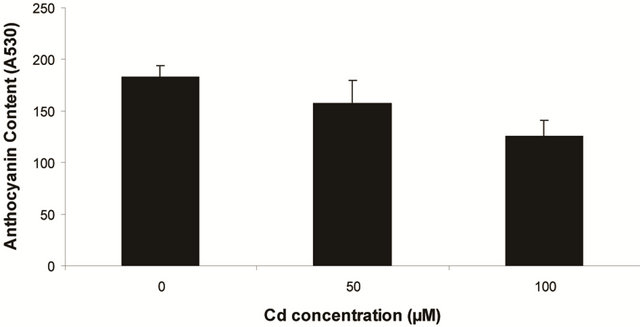
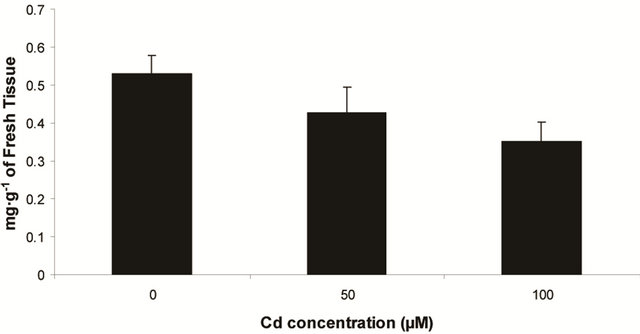
Plants in the present experiment recorded a significant down-regulation of anthocyanin and flavonoid content (Figures 2(a) and (b)). Critically, it was estimated that anthocyanin content in the leaves was decreased by 10.6% over control whereas it was 33.77% of flavonoids. Noticeably, the decrease in anthocyanin content was significant only at 100 µM, but for flavonoids at each concentration. In the present experiment, anthocyanin and flavonoid are two predominant phenolic glycosides those were elevated according to concentration of Cd. Anthocyanin is the compound which can mitigate the oxidative stress by quenching excess energy of ROS directly, or reacting with ROS in the irreversible ways to be self oxidized [27].
Superoxide dismutase (SOD) is the first line of defense, furnished by the plants to enzymatic dismutation of 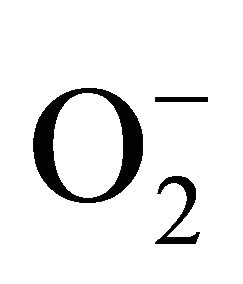 into H2O2 [12]. In the present experiment, the activity of SOD was assayed with formazon generation following NBT reduction. Interestingly, there recorded a linear increase of enzyme activity as a function of Cd concentration as ranged from 1.05 unit to 1.38 unit (Figure 3(a)). It is noteworthy that the SOD activity was maximum at 100 µM Cd and it was 1.89 fold as compared to control. Activity of GPX in the present study recorded a significant up regulation ranging from 1184 unit to 2608 unit under metal stress. The maximum increase in activities was significant (P ≤ 0.05) and it was 2.2 fold (Figure 3(b)). Under the condition of oxidative stress, a frequent decrease of GSH might be indicative for more production of H2O2 than
into H2O2 [12]. In the present experiment, the activity of SOD was assayed with formazon generation following NBT reduction. Interestingly, there recorded a linear increase of enzyme activity as a function of Cd concentration as ranged from 1.05 unit to 1.38 unit (Figure 3(a)). It is noteworthy that the SOD activity was maximum at 100 µM Cd and it was 1.89 fold as compared to control. Activity of GPX in the present study recorded a significant up regulation ranging from 1184 unit to 2608 unit under metal stress. The maximum increase in activities was significant (P ≤ 0.05) and it was 2.2 fold (Figure 3(b)). Under the condition of oxidative stress, a frequent decrease of GSH might be indicative for more production of H2O2 than 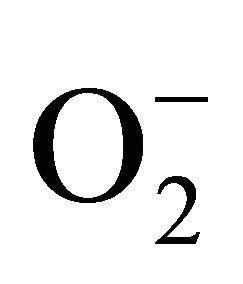 [15]. In the Pteris species, GR have been reported to reduce GSSG into GSH and that was also following the same pattern for
[15]. In the Pteris species, GR have been reported to reduce GSSG into GSH and that was also following the same pattern for
(a) Lipid peroxidation in terms of malondialdehyde (MDA) content (b) Protein oxidation
(c) Generation of 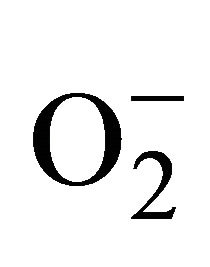 (d) H2O2 Content
(d) H2O2 Content
Figure 1. Lipid peroxidation in terms of malondialdehyde (MDA) content (a); Protein oxidation (b); O2 generation (c) and H2O2 content (d) in leaves of Marsilea plant grown in culture supplemented with cadmium doses 0, 50 and 100 µM. Increase significantly at P ≤ 0.05.
(a) Anthocyanin (b) Flavanoid
Figure 2. Changes of anthocyanin (a) and flavonoid (b) content in the leaves of Marsilea plant Grown in culture supplemented with cadmium doses 0, 50 and 100 µM. Decrease significantly at P ≤ 0.05.
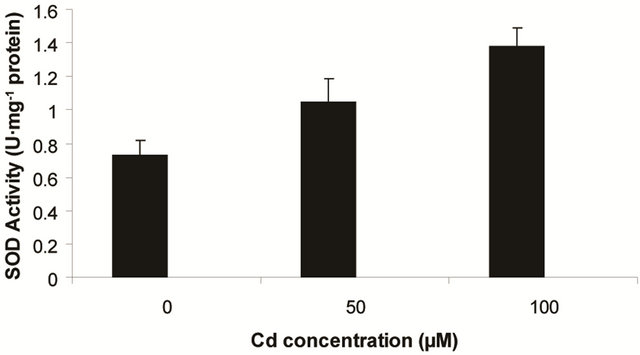
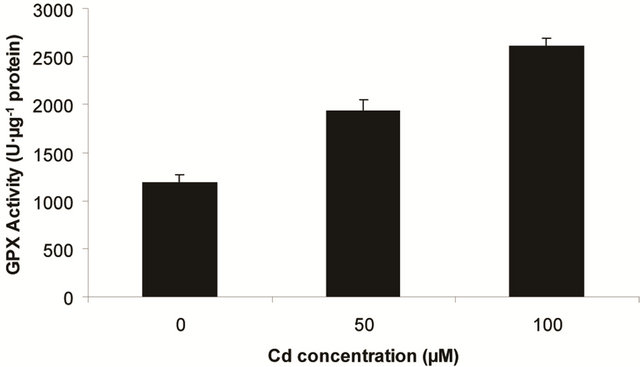
(a) SOD activity (b) GPX activity
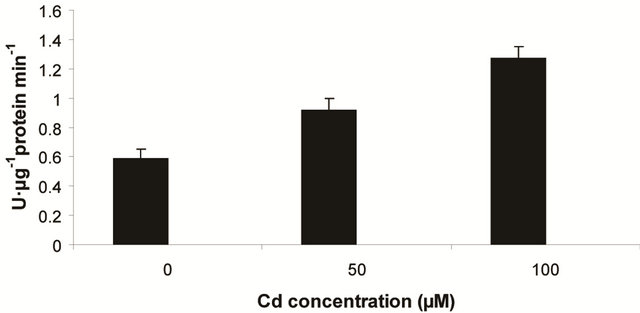
(c) GR activity
Figure 3. Changes in activities of superoxide dismutase (a), guaiacol peroxidase (b) and determination of glutathione reductase activity (c) under varying (0, 50 and 100 µM) of Cd. Increase significantly at P ≤ 0.05.
maintaining the ratio of GSH:GSSG [28]. In the present study, the distinct variation of polypeptides and its ingel activity staining are more convincing with their different nature in roots and leaves. GR activity is interesting to note with a linear trend as a function of Cd concentration. The variation of GR activity was significant at each concentration and it was maximum with 2.15 fold higher as compared to those enzymes (Figure 3(c)).
The variations in the polypeptide bands (32 kDa and 36 kDa) though were not different in number, but in intensities, more in leaves than roots (Figures 4(a) and (b)). The up-regulation of GR activity was maximum in leaves than roots are logistic to replenish the reduced form of
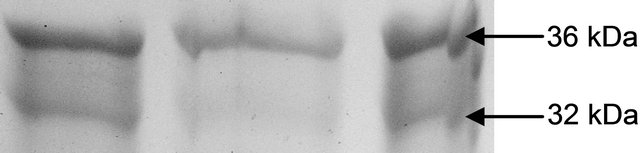
(a) GR isoform in leaf
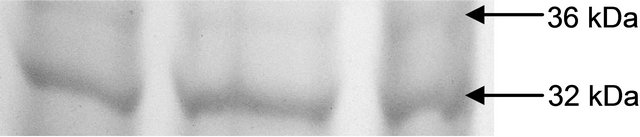
(b) GR isoform in root
Figure 4. Expression of glutathione reductase isoform in leaf (a) and root (b) as detected by activity staining on native PAGE.
glutathione. Roots appear to be less in depleting the GSH for this purpose which was more than in leaves. Thus GR becomes a trait for sustenance of redox in plants under oxidative stress. As recorded in many plants under varying conditions of abiotic stresses inducing oxidative injury.
4. Conclusion
It is well understood that Marsilea plant in the present experiment recorded their capability for hyper-accumulation of heavy metals under controlled condition in hydroponic culture. Still, plants have to be proven for their efficacy of hyper-absorption of metals under field condition. Future scopes are there to decipher the characteristic of Marsilea plant on cellular and molecular basis in depth, so that biotechnologically this species could be improvised in phyto-remediation purposes.
REFERENCES
- R. W. Feng, C. Y. Wei, S. X. Tu and X. Sun, “Interactive Effects of Selenium and Arsenic on Their Uptake by Pteris vittata L. under Hydropronic Conditions,” Environmental and Experimental Botany, Vol. 65, No. 2-3, 2009, pp. 363-368. doi:10.1016/j.envexpbot.2008.11.013
- B. Semane, A. Cuypers, K. Smeets, F. Van Belleghem, N. Horemans and H. Sehat, “Cadmium Responses in Arabidopsis thaliana: Glutathione Metabolism and Antioxidative Defense System,” Physiologia Plantarum, Vol. 129, No. 3, 2007, pp. 519-528. doi:10.1111/j.1399-3054.2006.00822.x
- S. S. Sharma and K. J. Dietz, “The Relationship between Metal Toxicity and Cellular Redox Imbalance,” Trends in Plant Science, Vol. 14, No. 1, 2009, pp. 43-50. doi:10.1016/j.tplants.2008.10.007
- M. Pielichowska and M. Wierzbicka, “Uptake and Localization of Cadmium by Biscutella laevigata, a Cadmium Hyperaccumulator,” Acta Biologica Cracoviensia Series Botanica, Vol. 46, No. 4, 2004, pp. 57-63.
- R. A. Root, R. J. Miller and D. E. Koeppe, “Uptake of Cadmium—Its Toxicity, and Effect on the Iron Ratio in Hydroponically Grown Corn,” Journal of Environmental Quality, Vol. 4, No. 4, 1975, pp. 473-476.
- A. Roychoudhury, S. Basu and D. N. Sengupta, “Antioxidants and Stress-Related Metabolites in the Seedlings of Two Indica Rice Varieties Exposed to Cadmium Chloride Toxicity,” Acta Physiologiae Plantarum, Vol. 34, No. 3, 2012, pp. 835-847. doi:10.1007/s11738-011-0881-y
- P. J. Sobrino, V. C. Ortega, C. M. L. Flores, C. Escobar, C. F. F. Del and L. E. Hernández, “Differential Alterations of Antioxidant Defenses as Bioindicators of Mercury and Cadmium Toxicity in Alfalfa,” Chemosphere, Vol. 77, No. 7, 2009, pp. 946-954. doi:10.1016/j.chemosphere.2009.08.007
- C. Fusco, F. Zaina, S. Atanasio, M. Romano, A. Negrini and S. Negrini, “Physical Exercises in the Treatment of Adolescent Idiopathic Scoliosis: An Updated Systematic Review,” Physiotherapy Theory and Practice, Vol. 27, No. 1, 2011, pp. 80-114. doi:10.3109/09593985.2010.533342
- M. Beladi, A. Kashani, D. Habibi, F. Paknajad and M. Golshan, “Uptake and Effects of Lead and Copper on Three Plant Species in Contaminated Soils,” African Journal of Agricultural Research, Vol. 6, No. 15, 2011, pp. 3483-3492.
- R. W. Feng and C. Y. Wei, “Antioxidation Mechanisms on Selenium Accumulation in Pteris vittata L., Potential Selenium Phytoremediation Plant,” Plant Soil and Environment, Vol. 58, No. 3, 2012, pp. 105-110.
- L. Q. Ma, K. M. Komar, C. Tu, W. H. Zhang, Y. Cai and E. D. Kennelley, “A Fern That Hyperaccumulates Arsenic,” Nature, Vol. 409, No. 6820, 2001, p. 579. doi:10.1038/35054664
- R. Singh, D. P. Singh, N. Kumar, S. K. Bhargava and S. C. Barman, “Accumulation and Translocation of Heavy Metals in Soil and Plants from Fly Ash Contaminated Area,” Journal of Environmental Biology, Vol. 31, No. 4, 2010, pp. 421-430.
- Z. E. Hawa, H. I. Jaafar and E. K. Mohd, “Phenolics and Flavonoid Compounds, Phenylalanine Ammonia Lyase and Antioxidant Activity Responses to Elevated CO2 in Labisia pumila (Myrisinaceae),” Molecules, Vol. 17, No. 6, 2012, pp. 6331-6347. doi:10.3390/molecules17066331
- P. Satapathy, V. M. M. Achary and B. B. Brahma, “Aluminium-Induced Abiotic Stress Counteracts Fusarium Infection in Cajanas cajan (L.) Millsp,” Journal of Plant Interactions, Vol. 7, No. 2, 2012, pp. 121-128. doi:10.1080/17429145.2011.584133
- N. Ghosh, M. K. Adak, P. D. Ghosh, S. Gupta, D. N. Sengupta and C. Mandal, “Differential Responses of Two Rice Varieties to Salt Stress,” Plant Biotechnology Reports, Vol. 5, No. 1, 2011, pp. 89-103. doi:10.1007/s11816-010-0163-y
- R. L. Heath and L. Packer, “Photoreoxidation in a Isolated Chloroplasts I. Kinetics & Stoichiometry of Fatty Acid Peroxidation,” Archives in Biochemistry and Biophysics, Vol. 125, No. 1, 1968, pp. 189-198. doi:10.1016/0003-9861(68)90654-1
- A. Roychoudhury, S. Basu and D. N. Sengupta, “Antioxidants and Stress Related Metabolits in the Seedlings of Two Indica Rice Varieties Exposed to Cadmium Chloride Toxicity,” Acta Physiologia Plantarum, Vol. 34, No. 3, 2012, pp. 835-847. doi:10.1007/s11738-011-0881-y
- A. Kiba, C. Miyake, K. Toyoda, Y. Ichinose, T. Yamada and T. Shairaishi, “Superoxide Generation in Extracts from Isolated Plant Cell Walls Is Regulated by Fungal Signal Molecules,” Phytopathology, Vol. 87, No. 8, 1997, pp. 846-852. doi:10.1094/PHYTO.1997.87.8.846
- A. Ishida, K. Ookubo and K. Ono, “Formation of Hydrogen Peroxide by NAD(P)H Oxidation with Isolated Cell Wall Associated Peroxidase from Cultured Liverwort Cells. Marchantia polymorpha L.,” Plant Cell Physiology, Vol. 28, No. 4, 1987, pp. 723-726.
- A. Roychoudhury, C. Roy and D. N. Sengupta, “Transgenic Tobacco Plant Overexpressing the Heterologous Lea Gene Rab 16A from Rice during High Salt and Water Deficit Display Enhanced Salinity Stress,” Plant Cell Reports, Vol. 26, No. 10, 2007, pp. 1839-1859. doi:10.1007/s00299-007-0371-2
- S. Basu, A. Roychoudhury, P. P. Saha and D. N. Sengupta, “Comparative Analysis of Some Biochemical Responses of Three Indica Rice Varieties during Polyethylene Glycol-Mediated Water Stress Exhibits Distinct Varietal Differences,” Acta Physiologia Plantarum, Vol. 32, No. 3, 2010, pp. 551-563. doi:10.1007/s11738-009-0432-y
- O. Borsani, P. Diaz, M. F. Agius, V. Valpuesta and J. Monza, “Water Stress Generates an Oxidative Stress through the Induction of a Specific Cu/Zn Superoxide Dismutase in Loyus corniculatus Leaves,” Plant Science, Vol. 161, No. 4, 2001, pp. 757-763. doi:10.1016/S0168-9452(01)00467-8
- A. Drotar, P. Phelps and R. Fall, “Evidence for Glutathione Peroxidase Activities in Cultured Plant Cells,” Plant Science, Vol. 42, No. 1, 1985, pp. 35-40. doi:10.1016/0168-9452(85)90025-1
- I. Cakmak, D. Strbac and H. Marschner, “Activities of Hydrogen Peroxide-Scavenging Enzymes in Germinating Wheat Seeds,” Journal of Experimental Botany, Vol. 44, No. 258, 1993, pp. 127-132. doi:10.1093/jxb/44.1.127
- C. Mandal, N. Ghosh, S. Maiti, K. Das, S. Gupta, N. Dey and M. K. Adak, “Antioxidative Responses of Salvinia (Salvinia natans Linn.) to Aluminium Stress and It’s Modulation by Polyamine,” Physiology and Molecular Biology of Plants, Vol. 19, No. 1, 2013, pp. 91-103. doi:10.1007/s12298-012-0144-4
- X. D. Cao, L. Q. Ma and C. Tu, “Antioxidative Response to Arsenic in the Arsenic Hyperaccumulation Chinese Brake fern (Pteris vittata L.),” Environmental Pollution, Vol. 128, No. 3, 2004, pp. 317-325. doi:10.1016/j.envpol.2003.09.018
- S. Kochhar and V. K. Kochhar, “Expression of Antioxidant Enzymes and Heat Shock Proteins in Relation to Combined Stress Cadmium and Heat in Vigna mungo Seedlings,” Plant Science, Vol. 168, No. 4, 2005, pp. 921- 929. doi:10.1016/j.plantsci.2004.11.013
- M. Srivastava, L. Q. Ma and J. A. Cotruvo, “Uptake and Distribution of Selenium in Different Fern Species,” International Journal of Phytoremediation, Vol. 7, No. 1, 2005, pp. 33-42. doi:10.1080/16226510590915792
NOTES
*K. Das and M. K. Adak have contributed equally.
#Corresponding author.
#

