Advances in Computed Tomography
Vol. 2 No. 2 (2013) , Article ID: 33019 , 5 pages DOI:10.4236/act.2013.22008
Accuracy of Rapid Prototyping Biomodels Plotted by Three Dimensional Printing Technique: Ex Vivo Study
1Research Center São Leopoldo Mandic, Campinas, Brazil
2School of Dentistry, Federal University of Bahia, Salvador, Brazil
3SENAI-CIMATEC, Salvador, Brazil
4School of Dentistry of Bauru, University of São Paulo, Bauru, Brazil
5School of Dentistry, State University of Feira de Santana, Feira de Santana, Brazil
Email: lucio.safira@ig.com.br, bastosluana@ymail.com, valter.beal@fieb.org.br, razevedo@ufba.br, drfrancischone@yahoo.com.br, izrubira2000@yahoo.com.br, viviane.sarmento@gmail.com
Copyright © 2013 Lucio Costa Safira et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received November 29, 2012; revised March 2, 2013; accepted March 10, 2013
Keywords: Rapid Prototyping; Models; Computed Tomography; Mandible
ABSTRACT
The rapid prototyping biomodels manufacturing is a recent technology with great importance in oral and maxillofacial surgery. It provides a better surgical planning, decrease of anesthesia time and great functional and esthetic results. The aim of this experimental study was to evaluate the accuracy of rapid prototyping biomodels built by three-dimensional printing (3DP) technique, since this is one of the least expensive methods available. Linear measurements of standardized bone defects and anatomic distances were compared using a digital caliper of high precision in nine dry mandibles (gold standard) and their respective biomodels. The Bland-Altman test was used for statistical analysis (5% level of significance). The results showed strong concordance between the dry mandibles and their respective biomodels, with discrepancies smaller than 2 mm in most cases (97.4%). We can conclude that the biomodels built by 3DP technique can be used for surgical planning in Dentistry.
1. Introduction
The increasing demand for excellence in diagnosis and treatment of the changes in bucomaxillofacial complex has become a great challenge for dental surgeons. In this way, the incorporation of modern technologies in Radiology and planning advanced therapies, such as reparative and reconstructive surgery, has assumed a prominent position in the field of biotechnology.
One of those innovations is the rapid prototyping (RP), which uses images from computed tomography (CT) and creates three-dimensional virtual models from the CAD (Computer Aided Design) system [1]. The file is sent to a RP station, which uses the CAM (Computer Aided Manufacturing) system and physically builds the designed object, which in the health field is called biomodel [2].
According to some authors [3,4] the achievement of biomodels allows a more precise diagnosis and consequently a better planning in reconstructive, orthognatic, osteogenic distraction and temporomandibular joint surgeries. The advantages include less surgical and sedation time, as well as a better esthetic and functional outcome, due to the possibility of previous measurement and modeling of replacement pieces in custom prototypes [5-7].
The great complexity that involves all the stages of the biomodels production indicates that they must be followed with extreme caution and precision in order to obtain models compatible with the human anatomy [2,3]. Some authors [8,9] believe that the dimensions, shape and the detailing of these prototypes may be altered by errors originated in any of the stages of the RP process, either on the CT exam, image processing through biomedical softwares or in the construction and post processing of these biomodels.
However, the quality, the anatomical and dimensional fidelity of the biomodels achieved through different RP techniques, as well as its adequate use in surgical planning, are still objects of inquiry [10].
One of these techniques is the three-dimensional printing (3DP) technique, which works like a printer dispersing the binder agent over the powder gypsum constructing the biomodels layer by layer in a incremental method [11].
Some advantages of this technology are: the possibility to generate colorful prototypes (allowing better analysis of vascular structures, for instance), a smaller time of production of the biomodel and a more accessible cost [11]. Therefore, this technology appears to make the biomedical prototypes more accessible in specific surgical procedures due to its potential of reducing costs for the public health services in developing countries [3].
Few studies have evaluated the accuracy of these biomodels and guide the professional the correct indication, especially when millimeter precision is required, as in Implantology, in which they are used in planning of zygomatic and extraoral implants and in surgical guides manufacture [12-14].
Thus, in the present study we evaluate the fidelity of the RP biomodels built by 3DP technique.
2. Materials and Methods
After the project being approved by the local ethics committee of School of Dentistry of Federal University of Bahia (Salvador, Bahia, Brazil), nine human dry mandibles were selected from the collection of the Department of Anatomy. All mandibles were intact, regardless of being edentulous or not. Teeth with restorations or metallic dental prosthesis were excluded.
2.1. Production of the Bone Defects
Standardized circumferential bone defects were produced in both sides of the dry mandibles with trephine drills (11.8 mm and 7.8 mm external diameter) at the parasymphyseal and angle regions using a handpiece attached to an electric bench motor (1200 rpm). Four defects were created in each piece.
Thirty-six bone defects were produced in total: sixteen using the drill of greater diameter (in four mandibles), and twenty using the drill of smaller diameter (in the other five mandibles).
2.2. Computed Tomography Examination
After, the dry mandibles were submitted to the Helical Computed Tomography examination (Somaton Emotion, Siemens AG®, Muenchen, Germany) in a private radiology clinic. They were positioned on the device bench over a polystyrene support and fixed with adhesive tape, in order to simulate the real position of an in vivo examination. Thus, it was possible to eliminate the undesired inclinations of the gantry when acquiring the images.
Axial slices of 1.1 mm thickness were taken with a 1 mm increase and 1.5 pitches. The energetic factors used were 120 kV and 120 mAs, with bone filter. The acquisition time varied from 22 to 25 seconds, and the entire length of the mandible was included in the examination. After data acquisition, the images were saved on CDROM in DICOM format.
2.3. Biomodels Acquisition
The images were taken to a RP laboratory where they were converted into STL files, through the 3D Doctor® (Able Software Corp.®, Lexington, Massachusetts, USA) software.
In the prototyping machine program (ZPrint, Z Corporation®, Burlington, Massachusetts, USA) each STL model was arranged on the virtual space that represented the machine’s plotting area. The 3DP system, which is composed of a plaster and starch based powder and a binder liquid presented in a container, was used. The method of construction of the biomodels is incremental (layers of 0.1 mm thickness). Over each layer of powder that is deposited, a layer of binder was applied at the points where the virtual model corresponded to the structure to be built, working like a printer that disperses ink jets. Then, the platform is slightly lowered, a new layer of powder is added over the previous layer and the process repeated until the piece is done. The loose powder remains on the platform to support the prototype.
After plotting, the biomodels were carefully removed from the machine. The powder excess was removed with an appropriate brush and vacuum cleaner. In this stage, the operators adopted biosafety care procedures in order to prevent aspiration of dust. Then, the models were covered with a liquid cyanoacrylate layer in order to waterproof them, increase the strength and durability of these parts.
2.4. Measurements
Linear measures were made on the bone defects and anatomic measures according to pre-determined points. The first were obtained from the diameter of the internal face of the defects, and then considered internal measures. The anatomic measures were obtained on the surface of the pieces, thus considered external.
All the measurements were obtained using a digital caliper rule (727 series, Starrett®, Itu, São Paulo, Brazil), by the same examiner, twice, within an interval of seven days.
2.5. Statistical Analysis
The averages of the two measurements taken in the dry mandibles and biomodels were used for statistical analysis. The comparison between the mandibles and biomodels measurements was performed by Bland-Altman test (5% level of significance).
3. Results
In each one of the nine mandibles and their respective biomodels, thirteen measurements were done (five towards the horizontal direction and eight towards the vertical) (Figure 1). From the vertical measurements, four were derived from the bone defects (internal measures) and the other four were anatomical (external measure). From the horizontal measurements, four measures were derived from the bone defects and one was anatomical. Hence, 117 measures were obtained from the mandibles and other 117 from the biomodels. As each assessment was performed twice by the same examiner, this resulted in 468 measurements.
A vertical anatomic measure from mandible 8 (which showed manufacturing defect on the symphysis region) was excluded of the analysis. So, the statistical analysis evaluated a total of 464 measures.
Observing Tables 1 and 2, it was possible to state that there has been strong concordance between the measures obtained from the dry mandibles and their respective biomodels in all analysis. The r Pearson coefficients were close to 1, and the confidence intervals of the BlandAltman test were narrow. The average difference was 0.18 mm, with a mean distortion of 3.78%. It was also stressed that most of times that discrepancy was less that 1mm (64.7%). A discrepancy between 1 and 2 mm was seen in 32.8% of the sample. And only three measures (2.6%) had differences superior to 2 mm and all were anatomic measures.
It was observed that, in most assessments, the dimensions measured in dry mandibles were higher than those of the biomodels, except in the case of the anatomic measurements, either vertical or horizontal.
4. Discussion
The majority of the studies analyzing the precision of biomodels in the craniofacial region used the stereolithography technology, which is based on the photopolymerization of a liquid resin. This technique has been already evaluated presenting a mean dimensional alteration of 0.62 mm (0.65%) [15] with differences that ranged
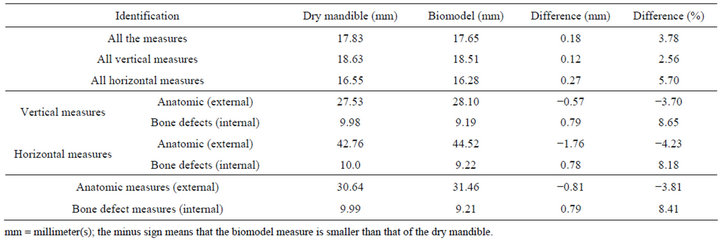
Table 1. Mean values of the linear, vertical and horizontal, anatomic or bone defects measures obtained from the dry mandibles and their respective RP biomodels.
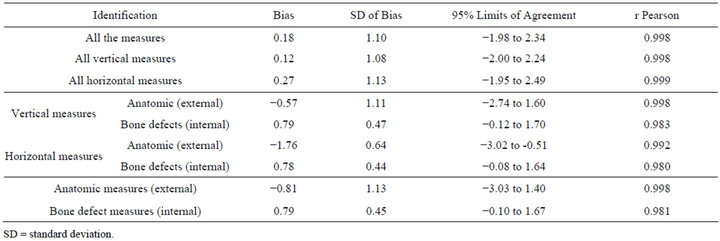
Table 2. Bland-Altman test between measures obtained from dry mandibles and RP biomodels.
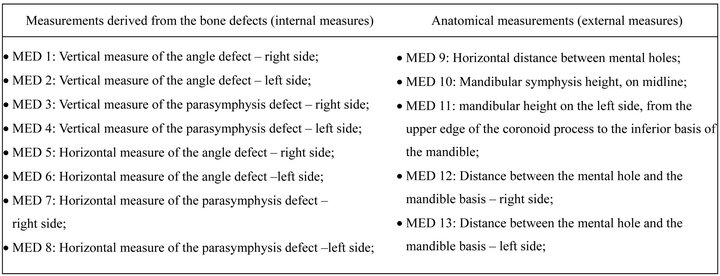
Figure 1. Description of the measurements made in the mandible and its biomodel.
from 0.3 mm to 0.8 mm between the mandible and the stereolithographic model [16].
Efforts directed towards the 3DP system are more recent and some authors reported values inferior to 2.0 mm (or 2.1%) [11] and mean discrepancy of 1.07 mm (or 2.67%) [10]. In this study, the mean discrepancy was of 3.78%. An error variation of 2% is acceptable and the biomodels can be used in diagnostic and in the communication with the patient and surgical planning involving traumas and severe deformities [17].
In the current study, although there was strong concordance, the horizontal measures were more discordant than the vertical. There were no published studies using this type of comparison (by direction).
Later, the measures divided into anatomic measures (external) and bone defects measures (internal) were compared and the greatest differences were found in the group of internal measures, which is consistent to other studies [10,11,14].
It may be also noted that the dimensions assessed in the biomodels were superior to those of the dry mandibles in the case of external measures, whereas the inverse occurred for the internal measures. That may be associated to problems at the time of segmentation of images when a thicker periosteum is produced in the biomodel increasing the distances between two external points and decreasing them between two internal points (which is called “dumb-bell effect”) [10].
It was not possible to define the exact moment that the distortions occurred, in the current methodology, but it’s probable that the error must be mainly caused by the RP machine ZPrint (Z Corporation®), since its manufacturer indicates in the user manual that there is a distortion of 1.0 mm greater on the Y axis (vertical direction), inherent to the biomodel construction process.
We can also attribute to the axial slice thickness used in this study the cause for the discrepancies, although two authors [10,15] stated that a variation between 1.0 and 2.0 mm in the slice thickness offers a satisfactory image quality and is in the safety limits to the patient, in terms of radiation dose. Nowadays, modern CT equipments may produce slices thinner, and this certainly improves the RP models quality.
It is important to consider that the error may be also introduced during the post-processing of the virtual 3D models by dedicated softwares [18]. So, all steps must be conducted thoroughly, since the CT acquisition until the plot phase.
In our study, we can observer that the discrepancies were smaller than 2 mm in most cases (97.4%). This difference is irrelevant for the majority of the purposes of the biomodels in bucomaxillofacial surgery. For the dental implant, those differences, however, must be observed carefully.
5. Acknowledgments
The authors would like to thank to the private radiologic clinic (Lithocenter, Salvador, Bahia, Brazil) by enabling the accomplishment of the computed tomography examinations, to SENAI-CIMATEC (Salvador, Bahia, Brazil) by making the rapid prototyping laboratory available, and to FAPESB (Salvador, Bahia, Brazil) by the financial support.
REFERENCES
- J. Winder and R. Bibb, “Medical Rapid Prototyping Technologies: State of the Art and Current Limitations for Application in Oral and Maxillofacial Surgery,” Journal of Oral and Maxillofacial Surgery, Vol. 63, No. 7, 2005, pp. 1006-1015. doi:10.1016/j.joms.2005.03.016
- A. Suggar, R. Bibb, C. Morris and J. Parhouse, “The Development of a Collaborative Medical Modeling Service: Organizational and Technical Considerations,” British Journal of Oral and Maxillofacial Surgery, Vol. 42, No. 5, 2004, pp. 323-330. doi:10.1016/j.bjoms.2004.02.025
- J. V. L. Silva, M. F. Gouvêia, A. Santa Barbara, E. Meurer and C. A. C. Zavaglia, “Rapid Prototyping Applications in the Treatment of Craniomaxillofacial Deformities —Utilization of Bioceramics,” Key Engineering Materials, Vol. 254-256, 2004, pp. 687-690. doi:10.4028/www.scientific.net/KEM.254-256.687
- E. L. S. Rosa, C. F. Oleskovicz and B. N. Aragão, “Rapid Prototyping in Maxillofacial Surgery and Traumatology: Case Report,” Brazilian Dental Journal, Vol. 15, No. 3, 2004, pp. 243-247. doi:10.1590/S0103-64402004000300015
- J. R. Cunnningham, M. J. Madsen and G. Peterson, “Stereolithographic Modeling Technology Applied to Tumor Resection,” Journal of Oral and Maxillofacial Surgery, Vol. 63, No. 6, 2005, pp. 873-879. doi:10.1016/j.joms.2005.02.027
- M. Robiony, I. Salvo, F. Costa, et al., “Virtual Reality Surgical Planning for Maxillofacial Distraction Osteogenesis: The Role of Reverse Engineering Rapid Prototyping and Cooperative Work,” Journal of Oral and Maxillofacial Surgery, Vol. 65, No. 6, 2007, pp. 1198-1208. doi:10.1016/j.joms.2005.12.080
- J. D. Wagner, B. Baack, G. A. Brown and J. Kelly, “Rapid 3-Dimensional Prototyping for Surgical Repair of Maxillofacial Fractures: A Technical Note,” Journal of Oral and Maxillofacial Surgery, Vol. 62, No. 7, 2004, pp. 898-890. doi:10.1016/j.joms.2003.10.011
- Y. Tang, H. T. Loh, J. Y. H. Fuh, et al., “Accuracy Analysis and Improvement for Direct Laser Sintering,” 2011. https://dspace.mit.edu/bitstream/1721.1/3898/2/IMST001.pdf
- L. J. Stumpel, “Deformation of Stereolithographically Produced Surgical Guides: An Observational Case Series Report,” Clinical Implant Dentistry and Related Research, Vol. 14, No. 3, 2012, pp. 442-453. doi:10.1111/j.1708-8208.2010.00268.x
- P. S. Chang, T. H. Parker, C. W. Patrick Jr. and M. J. Miller, “The Accuracy of Stereolithography in Planning Craniofacial Bone Replacement,” Journal of Craniofacial Surgery, Vol. 14, No. 2, 2003, pp. 164-170. doi:10.1097/00001665-200303000-00006
- D. N. Silva, M. G. de Oliveira, E. Meurer, M. I. Meurer, J. V. L. Silva and A. S. Barbara, “Dimensional Error in Selective Laser Sintering and 3D-Printing of Models for Craniomaxillary Anatomy Reconstruction,” Journal of Cranio-Maxillofacial Surgery, Vol. 36, No. 8, 2008, pp. 443-449. doi:10.1016/j.jcms.2008.04.003
- N. Kitai, Y. Yoshitaka and T. Kenji, “A Stent Fabricated on a Selectively Colored Stereolithographic Model for Placement of Orthodontic Mini-Implants,” The International Journal of Adult Orthodontics & Orthognathic Surgery, Vol. 17, No. 4, 2002, pp. 264-266.
- J. Chow, E. Hui, P. K. Lee and W. Li, “Zygomatic Implants—Protocol for Immediate Occlusal Loading: A Preliminary Report,” Journal of Oral and Maxillofacial Surgery, Vol. 64, No. 5, 2006, pp. 804-811. doi:10.1016/j.joms.2006.01.021
- G. A. Di Giacomo, P. R. Cury, N. S. de Araujo, W. R. Sendyk and C. L. Sendyk, “Clinical Application of Stereolithographic Guides for Implant Placement: Preliminary Results,” Journal of Periodontology, Vol. 76, No. 4, 2005, pp. 503-507. doi:10.1902/jop.2005.76.4.503
- J. Y. Choi, J. H. Choi, N. K. Kim, et al., “Analysis of Errors in Medical Rapid Prototyping Models,” International Journal of Oral and Maxillofacial Surgery, Vol. 31, No. 1, 2002, pp. 23-32. doi:10.1054/ijom.2000.0135
- J. Kragskov, S. Sindet-Pedersen, C. Gyldensted and K. L. Jensen, “A Comparison of Three-Dimensional Computed Tomography Scans and Stereolithographic Models for Evaluation of Craniofacial Anomalies,” Journal of Oral and Maxillofacial Surgery, Vol. 54, No. 4, 1996, pp. 402- 411. doi:10.1016/S0278-2391(96)90109-3
- J. Asaumi, N. Kawai, Y. Honda, H. Shigehara, T. Wakasa and K. Kishi, “Comparison of Three-Dimensional Computed Tomography with Rapid Prototype Models in the Management of Coronoid Hyperplasia,” Dentomaxillofacial Radiology, Vol. 30, 2001, pp. 330-335. doi:10.1038/sj.dmfr.4600646
- E. G. Ferraz, L. C. S. Andrade, A. R. dos Santos, V. R. Torregrossa, M. R. S. Freire and V. A. Sarmento, “Effect of Different Surface Processing Protocols in Three-Dimensional Images for Rapid Prototyping,” Advances in Engineering Software, Vol. 42, No. 6, 2011, pp. 332-335. doi:10.1016/j.advengsoft.2011.02.011

