International Journal of Nonferrous Metallurgy
Vol.05 No.04(2016), Article ID:71140,12 pages
10.4236/ijnm.2016.54005
Adsorption of Copper from an Ammonia-Thiosulfate Media Using DOWEX 550A Ion Exchange Resin
Cristian Vargas*, Patricio Navarro
Departamento de Ingeniería Metalúrgica, Universidad de Santiago de Chile, Santiago, Chile

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 25, 2016; Accepted: October 8, 2016; Published: October 11, 2016
ABSTRACT
The study of copper adsorption onto ion exchange resins of anionic type is part of the gold recovery from ammonia-thiosulfate solutions, where copper is the main impurity of the system because it acts as a catalyst of gold dissolution reaction. A study is made of the adsorption and desorption of copper in the form of the 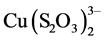 complex in an ammonia-thiosulfate media on an ion exchange resin, DOWEX 550A, classified as a strong base, which in its inner structure has a quaternary amine functional group. In the studied pH range copper adsorption increased with increasing pH, while the presence of thiosulfate decreased it, the same as the ammonia content, due to the greater presence of cuprotetramine,
complex in an ammonia-thiosulfate media on an ion exchange resin, DOWEX 550A, classified as a strong base, which in its inner structure has a quaternary amine functional group. In the studied pH range copper adsorption increased with increasing pH, while the presence of thiosulfate decreased it, the same as the ammonia content, due to the greater presence of cuprotetramine,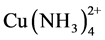 . Elution of the copper complexes from the resin was more efficient with sulfite than with perchlorate.
. Elution of the copper complexes from the resin was more efficient with sulfite than with perchlorate.
Keywords:
Adsorption, Desorption, Copper, Ion Exchange Resin, Ammonia-Thiosulfate

1. Introduction
The study of copper adsorption on anion exchange resins has to do with the recovery of gold as 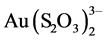 present in the solutions produced by leaching gold ores in an ammonia-thiosulfate media, where copper is the main impurity in the system. The ammonia-thiosulfate system with copper offers high gold leaching rates compared to the cyanide system, because the reaction is catalyzed by cupric ions [1] - [23] . The main reaction in the gold leaching in a thiosulfate-ammonia media is the following:
present in the solutions produced by leaching gold ores in an ammonia-thiosulfate media, where copper is the main impurity in the system. The ammonia-thiosulfate system with copper offers high gold leaching rates compared to the cyanide system, because the reaction is catalyzed by cupric ions [1] - [23] . The main reaction in the gold leaching in a thiosulfate-ammonia media is the following:
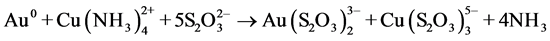 (1)
(1)
With the oxidation reactions of Cu+ to Cu2+ through the following equilibrium:
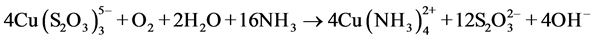 (2)
(2)
The solutions resulting from gold leaching contain gold-thiosulfate, copper-ammonia and copper-thiosulfate complexes, plus tetrathionate in different proportions as a function of the concentrations of copper, ammonia, thiosulfate, and pH.
Recovery of the gold dissolved in the rich leaching solution can take place by activated carbon, solvent extraction, ion exchange resins, or cementation with zinc powder [24] - [31] .
The application of the ion exchange resins technique is very appropriate for solutions with low content of the element of interest, because it is a process that allows concentrating solutions very selectively. The ion ex-change is a reversible chemical reaction that takes place when an ion of a solution or a dissolved complex is exchanged for another ion (or ions) of equal value that is joined to the stationary solid particle.
When anion exchange resins are used, the adsorbed complexes will be both the aurothiosulfate anions, as well as the cuprothiosulfates, plus the trithionate, tetrathionate and other higher polythionates produced by the oxidation of the thiosulfate [32] . For that reason, it becomes necessary to know the behavior of the copper complexes separately in these kinds of resins in a thiosulfate-ammonia media, because copper will always be present in the gold leaching solutions.
The adsorption of copper on ion exchange resins in thiosulfate ammonia medium has not been studied extensively. Dreisinger and Zhang determined that with strong base anion exchange resins there is a high gold load on the resin, with a high adsorption rate due to the alkaline character of the solutions coming from the leaching [33] [34] . Most information is aimed at finding the complexes formed in the copper(I/II)-thi- osulfate-ammonia system by means of speciation diagrams [35] .
The speciation diagrams for the system of interest allow a better understanding of the phenomena that take place, and that is how Senanayake and Muir showed the effect of pH on the distribution of the involved species. For the copper(II)-thiosulfate-ammonia system they found that the predominant species at pH lower than 7.5 is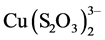 , with 0.1 M concentrations of thiosulfate and ammonia; on the contrary, at higher ammonia concentrations and higher pH, but less than 12, the predominant species is
, with 0.1 M concentrations of thiosulfate and ammonia; on the contrary, at higher ammonia concentrations and higher pH, but less than 12, the predominant species is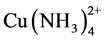 .
.
The objective of this work was to study experimentally by means of discontinuous tests the effect of different variables such as pH, thiosulfate, ammonia and copper concentrations in the adsorption of copper complexes in the  form on the DOWEX 550A ion exchange resin. The effect of the sulfite and perchlorate ions on the desorption of gold from the resin was also studied.
form on the DOWEX 550A ion exchange resin. The effect of the sulfite and perchlorate ions on the desorption of gold from the resin was also studied.
2. Experimental
2.1. Materials
The ion exchange resin used was DOWEX 550A (Dow Chemical Company), classified as a type 1 strong base. In its internal structure it has a quaternary amine functional group, and its outer appearance is as gel-type uniform particles with an average size of 590 ± 50 μm.
For the preparation of the copper solution and the eluting solutions double distilled and deionized water was used and analytical grade reagents, such as copper(II) sulfate pentahydrate [CuSO4∙5H2O], sodium thiosulfate [Na2S2O3∙5H2O], ammonium hydroxide [NH4OH], sodium hydroxide [NaOH], sodium perchlorate [NaClO4∙2H2O], and sodium sulfite [Na2SO3]. All reagents were purchased from Sigma-Aldrich.
2.2. Experimental Techniques
The adsorption tests were carried out in 500-mL reactors with mechanical stirring, keeping constant the following experimental parameters: contact time: 3 h, temperature: 298 K, aqueous solution volume: 400 mL, solution/resin ratio: 833.3 mL/g.
In this stage the percentage of copper adsorbed and the resin’s copper load (g Cu/kg resin) as a function of time were measured for the different experimental conditions. The studied variables and their experimentation ranges are presented in Table 1.
The experimental method used in each experiment was the same, varying only the specific conditions for each case. The following stages were used:
・ The aqueous solution was added to the reactor, and the temperature and the pH were adjusted.
・ The resin was added to the solution and the system was stirred mechanically in order to work with a perfect mixture.
・ Samples of 3 mL were removed occasionally from the aqueous phase for the chemical analysis.
・ The pH and the temperature of the aqueous solution were measured continuously.
・ After 3 h the stirring was stopped, the resin was filtered from the aqueous solution, and it was washed for its later chemical analysis by atomic absorption, and the copper load on the resin was determined from the copper balance present in the solutions.
For the elution tests, the resin was loaded and the desorption experiments were performed in 500-mL reactors with mechanical stirring, keeping the following experimental conditions elutant: Cu load on the resin: 17.4 g Cu/kg resin, pH: 11, temperature: 298 K, contact time: 3 h, solution/resin ratio: 200 mL/g, volume of aqueous solution: 300 mL.
Table 1. Variables and experimental ranges in the adsorption stage.
In this stage the copper concentration in the rich solution was determined, and the percentage of desorbed copper as a function of time for the different experimental conditions was determined. The studied variables and their experimental ranges are shown in Table 2.
In this case the experimental method was the following:
・ The copper bearing resin was loaded in agreement with the experimental conditions studied in the previous stage.
・ The aqueous eluting solution was added and the temperature and the pH were adjusted to the required values.
・ The loaded resin was added and the system was stirred mechanically.
・ Samples of 3 mL were removed occasionally from the aqueous phase for the chemical analysis.
・ The pH and the temperature of the aqueous solution were measured continuously.
・ After 3 h the stirring was stopped, the resin was filtered from the aqueous solution, and it was washed.
・ The same as in the previous stage, the analyses were performed by atomic absorption.
3. Results and Discussion
3.1. Adsorption Stage
3.1.1. Effect of pH
Figure 1 shows the results of the effect of pH on the copper adsorption on the Dowex 550A resin. In general, the adsorption did not exceed 10% at best, and the general shape of these curves indicates that initially the copper is adsorbed at a fast rate in all cases until a maximum value is reached, and then there is a small drop in the adsorption that can be attributed to a partial desorption of the copper.
The amount of adsorbed copper indicates that the process improves at pH 11 in relation to pH 9. The greater adsorption at pH 11 can be attributed to the greater stability of the thiosulfate at that pH, generating less tetrathionate (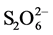 ) which acts as a competitor of the
) which acts as a competitor of the 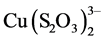 complex in the adsorption process. The reason for the drops of these curves (due to partial desorption) can be attributed to a critical concentration of tetrathionate that causes this situation, and this agrees with the fact that at pH 9 partial desorption takes place in shorter solution-resin contact times. The chemical equilibrium that accounts for this situation can be represented by the following equation.
complex in the adsorption process. The reason for the drops of these curves (due to partial desorption) can be attributed to a critical concentration of tetrathionate that causes this situation, and this agrees with the fact that at pH 9 partial desorption takes place in shorter solution-resin contact times. The chemical equilibrium that accounts for this situation can be represented by the following equation.
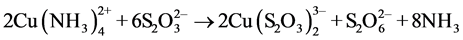 (3)
(3)
Table 2. Variables and experimental ranges in the elution stage.
Figure 1. Effect of pH on copper adsorption. Conditions: 200 ppm Cu; 0.1 M ammonia; 0.1 M thiosulfate.
3.1.2. Effect of Thiosulfate Concentration
Figure 2 shows the results of the effect of thiosulfate on the copper adsorption and load, and it is seen that as the concentration of thiosulfate increases the percentage of adsorbed copper decreases.
When the concentration of thiosulfate is between 0.3 and 0.5 M, 2% to 3% of Cu is adsorbed, achieving a copper load on the resin of 3 to 4 g Cu/kg resin. But working with a 0.1 M thiosulfate concentration the adsorption rises to 8%, with a load of approximately 17 g Cu/kg resin. This situation is explained because the thiosulfate would act as a competitor of copper for the resin’s active sites. According to reaction (3), a lower concentration of thiosulfate generates less tetrathionate, which, as already mentioned, competes with the copper complex in the adsorption process.
Figure 3 shows the adsorption of copper at different thiosulfate concentrations and different contact times. It is seen that for 0.5 M thiosulfate there is no variation in the percentage of adsorbed copper for the different solution-resin contact times, but for a 0.1 M initial thiosulfate concentration the amount of adsorbed copper is a function of time. This behavior can be attributed to the coadsorption of the anionic thiosulfate complexes on the resin. The stoichiometry that represents the adsorption of thiosulfate can be described by the following equation:
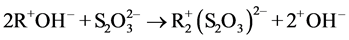 (4)
(4)
3.1.3. Effect of Ammonia Concentration
Figure 4 presents the results of copper load on the Dowex 550A resin as the ammonia concentration is varied. It is seen that as the concentration of dissolved ammonia increases, the copper adsorption and load on the resin decrease. This may be due to the fact that as the ammonia concentration increases, the stability of the 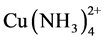 complex in relation to the formation of the
complex in relation to the formation of the 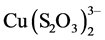 complex increases.
complex increases.
Speciation diagrams were made of the Cu-NH3-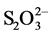 system under the experimental temperature and electrochemical potential conditions. Figure 5 shows that as the amount of ammonia in the solution increases, the stability of the copper-thiosulfate complexes decreases, the presence of the copper-ammonia complex becoming more important, so that at an NH3 concentration of 500 mM the copper-thiosulfate complexes disappear.
system under the experimental temperature and electrochemical potential conditions. Figure 5 shows that as the amount of ammonia in the solution increases, the stability of the copper-thiosulfate complexes decreases, the presence of the copper-ammonia complex becoming more important, so that at an NH3 concentration of 500 mM the copper-thiosulfate complexes disappear.
At higher ammonia concentrations the following situations occur:
・ The formation of cuprotetramine, 
・ There will be a greater amount of free thiosulfate, which may cause a greater production of tetrathionate, causing a decrease of the adsorption of the 
Figure 2. Effect of thiosulfate on copper adsorption. Conditions: 200 ppm Cu; 0.1 M ammonia; pH 11.
Figure 3. Variation of copper adsorption with thiosulfate concentration. Conditions: 200 ppm Cu; 0.1 M ammonia; pH 11.
Figure 4. Effect of ammonia on copper load. Conditions: 200 ppm Cu; 0.1 M thiosulfate; pH 11.
Figure 5. Speciation of the Cu-NH3-
3.1.4. Effect of Copper Concentration
Figure 6 shows the effect of the concentration of dissolved copper on the copper load on the resin. Under the experimental conditions such as pH, and NH3 and 

As the dissolved copper concentration increases, the copper load on the resin also increases. For the three copper concentrations studied there was a slight desorption after 90 to 120 minutes of solution-resin contact.
3.2. Desorption Stage
The elution or desorption tests were performed to replace the 
3.2.1. Elution of Copper with Perchlorate (
Figure 7 shows the kinetics of copper elution from the loaded resin by an aqueous solution with different perchlorate concentrations. Increasing the concentration of perchlorate from 0.1 M to 0.5 M did not cause an important increase in the desorption of the copper complex from the resin after 155 minutes of solution-resin contact. The following stoichiometry represents the desorption reaction of the 

Figure 6. Effect of initial copper concentration and its adsorption in the resin. Conditions: pH 11; 0.1 M ammonia; 0.1 M thiosulfate.
Figure 7. Effect of perchlorate in the copper elution. Conditions: resin loaded with 17.35 g Cu/kg resin; 0.1 M ammonia; pH 11.
As the perchlorate concentration was increased from 0.1 M to 0.5 M, the initial exchange rate increased considerably (reaction 6), so that working with a 0.5 M perchlorate concentration the maximum copper elution of 50% was achieved after 20 minutes of solution-resin contact. But using 0.1 and 0.2 M
3.2.2. Elution of Copper with Sulfite (
Figure 8 shows the effect of sulfite concentration in the eluent solution on the elution of copper from the loaded resin. The results show that the elution of the 



Sulfite solutions were more efficient than those of perchlorate for eluting the 
4. Conclusions
Under different experimental conditions the 
Figure 8. Effect of sulfite in the copper elution. Conditions: resin loaded with 17.35 g Cu/kg resin; 0.1 M ammonia; pH 11.
resin at pH 11 after 90 minutes of contact. Increasing the concentration of thiosulfate in the aqueous solution (0.1 M to 0.5 M) decreases the adsorption of the 
Elution of the copper complexes from the resin with sulfite ions is better and faster than with perchlorate ions. A good elution with sulfite is beneficial because this anion is part of the thiosulfate oxidation system. Elution with sulfite ion is more sensitive to changes in concentration, achieving 100% elution for a 0.5 M and 84.79% elution for 0.2 M, while elution was 53.65% at 0.5 M perchlorate concentration and 51.59% at 0.2 M concentration.
Acknowledgements
Support by the Dirección de Investigaciones Científicas y Tecnológicas of the Universidad de Santiago de Chile (DICYT) through project 051414ND is gratefully acknowledged.
Cite this paper
Vargas, C. and Navarro, P. (2016) Adsorption of Copper from an Ammonia-Thiosulfate Media Using DOWEX 550A Ion Exchange Resin. International Journal of Nonferrous Metallurgy, 5, 33-44. http://dx.doi.org/10.4236/ijnm.2016.54005
References
- 1. Zipperian, D. and Raghavan, S. (1998) Gold and Silver Extraction by Ammoniacal Thiosulfate Leaching from a Rhyolite Ore. Hydrometallurgy, 19, 361-375.
http://dx.doi.org/10.1016/0304-386x(88)90041-2 - 2. Langhans, J.W., Lei, K.P. and Carnahan, T.G. (1992) Copper-Catalyzed Thiosulfate Leaching of Low-Grade Gold Ores. Hydrometallurgy, 29, 191-203.
http://dx.doi.org/10.1016/0304-386x(92)90013-p - 3. Abbruzzese, C., Fornari, P., Massidda, R., Veglio, F. and Ubaldini S. (1995) Thiosulphate Leaching for Gold Hydrometallurgy. Hydrometallurgy, 39, 265-276.
http://dx.doi.org/10.1016/0304-386x(95)00035-f - 4. Breuer, P.L. and Jeffrey, M.I. (2000) Thiosulfate Leaching Kinetics of Gold in the Presence of Copper and Ammonia. Minerals Engineering, 13, 1071-1081.
http://dx.doi.org/10.1016/s0892-6875(00)00091-1 - 5. Aylmore, M.G. and Muir, D.M. (2000) Thiosulfate Leaching of Gold—A Review. Minerals Engineering, 14, 135-174.
http://dx.doi.org/10.1016/s0892-6875(00)00172-2 - 6. Jeffrey, M.I. (2001) Kinetics Aspects of Gold and Silver Leaching in Ammonia-Thiosulfate Solutions. Hydrometallurgy, 60, 7-16.
http://dx.doi.org/10.1016/s0304-386x(00)00151-1 - 7. Schmitz, P.A., Duyvesteyn, S., Johnson, W.P., Enloe, L. and McMullen, J. (2001) Ammoniacal Thiosulfate and Sodium Cyanide Leaching of Preg-Robbing Goldstrike Ore Carbonaceous Matter. Hydrometallurgy, 60, 25-40.
http://dx.doi.org/10.1016/s0304-386x(00)00154-7 - 8. Navarro, P., Vargas, C., Villarroel, A. and Alguacil, F.J. (2002) On the Use of Ammoniacal/ Ammonium Thiosulphate for Gold Extraction from a Concentrate. Hydrometallurgy, 65, 37-42.
http://dx.doi.org/10.1016/s0304-386x(02)00062-2 - 9. Molleman, E. and Dreisinger, D. (2002) The Treatment of Copper-Gold Ores by Ammonium Thiosulfate Leaching. Hydrometallurgy, 66, 1-21.
http://dx.doi.org/10.1016/s0304-386x(02)00080-4 - 10. Breuer, P.L. and Jeffrey, M.I. (2003) The Reduction of Copper(II) and the Oxidation of Thiosulfate and Oxysulfur Anions in Gold Leaching Solutions. Hydrometallurgy, 70, 163-173.
http://dx.doi.org/10.1016/s0304-386x(03)00078-1 - 11. Chu, C.K., Breuer, P.L. and Jeffrey, M.I. (2003) The Impact of Thiosulfate Oxidation Products on the Oxidation of Gold in Ammonia Thiosulfate Solutions. Minerals Engineering, 16, 265-271.
http://dx.doi.org/10.1016/s0892-6875(02)00369-2 - 12. Wan, R. and LeVier, K. (2003) Solution Chemistry Factors for Gold Thiosulfate Heap Leaching. International Journal of Mineral Processing, 72, 311-322.
http://dx.doi.org/10.1016/s0301-7516(03)00107-8 - 13. Jeffrey, M.I., Breuer, P.L. and Chu, C.K. (2003) The Importance of Controlling Oxygen Addition during the Thiosulfate Leaching of Gold Ores. International Journal of Mineral Processing, 72, 323-330.
http://dx.doi.org/10.1016/s0301-7516(03)00108-x - 14. Muir, D.M. and Aylmore, M.G. (2004) Thiosulphate as an Alternative to Cyanide for Gold Processing—Issues and Impediments. Mineral Processing and Extractive Metallurgy, 113, 2-12.
http://dx.doi.org/10.1179/037195504225004661 - 15. Zhang, X.M., Senanayake, G. and Nicol, M.J. (2004) A Study of the Gold Colloid Dissolution Kinetics in Oxygenated Ammoniacal Thiosulfate Solutions. Hydrometallurgy, 74, 243-257.
http://dx.doi.org/10.1016/j.hydromet.2004.05.007 - 16. Senanayake, G. (2004) Gold Leaching in Non-Cyanide Lixiviant Systems: Critical Issues on Fundamentals and Applications. Minerals Engineering, 17, 785-801.
http://dx.doi.org/10.1016/j.mineng.2004.01.008 - 17. Senanayake, G. (2005) Role of Copper(II), Carbonate and Sulphite in Gold Leaching and Thiosulphate Degradation by Oxygenated Alkaline Non-Ammoniacal Solutions. Minerals Engineering, 18, 409-426.
http://dx.doi.org/10.1016/j.mineng.2004.08.001 - 18. Senanayake, G. (2005) A Surface Adsorption/Reaction Mechanism for Gold Oxidation by Copper(II) in Ammoniacal Thiosulfate Solutions. Journal of Colloid and Interface Science, 286, 253-257.
http://dx.doi.org/10.1016/j.jcis.2004.12.041 - 19. Feng, D. and van Deventer, J.S. (2006) Ammoniacal Thiosulphate Leaching of Gold in the Presence of Pyrite. Hydrometallurgy, 82, 126-132.
http://dx.doi.org/10.1016/j.hydromet.2006.03.006 - 20. Feng, D. and van Deventer, J.S. (2007) Effect of Hematite on Thiosulphate Leaching of Gold. International Journal of Mineral Processing, 82, 138-147.
http://dx.doi.org/10.1016/j.minpro.2006.09.003 - 21. Feng, D. and van Deventer, J.S. (2007) The Role of Oxygen in Thiosulphate Leaching of Gold. Hydrometallurgy, 85, 193-202.
http://dx.doi.org/10.1016/j.hydromet.2006.09.003 - 22. Heath, J.A., Jeffrey, M.I., Zhang, H.G. and Rumball, J.A. (2008) Anaerobic Thiosulfate Leaching: Development of in Situ Gold Leaching Systems. Minerals Engineering, 21, 424-433.
http://dx.doi.org/10.1016/j.mineng.2007.12.006 - 23. Jeffrey, M.I., Watling, K., Hope, G.A. and Woods, R. (2008) Identification of Surface Species That Inhibit and Passivate Thiosulfate Leaching of Gold. Minerals Engineering, 21, 443-452.
http://dx.doi.org/10.1016/j.mineng.2008.01.006 - 24. Zhao, J., Wu, Z. and Chen, J. (1997) Extraction of Gold from Thiosulfate Solutions with Alkyl Phosphorus Esters. Hydrometallurgy, 46, 363-372.
http://dx.doi.org/10.1016/S0304-386X(97)00031-5 - 25. Vargas, C., Cifuentes, G., Navarro, P. and Orrego, P. (2004) Anodic Behavior of Zinc in Thiosulphate Media. Revista de Metalurgia, 40, 101-108.
http://dx.doi.org/10.3989/revmetalm.2004.v40.i2.249 - 26. Navarro, P., Alvarez, R., Vargas, C. and Alguacil, F.J. (2004) On the Use of Zinc for Gold Cementation from Ammoniacal-Thiosulphate Solutions. Minerals Engineering, 17, 825-831.
http://dx.doi.org/10.1016/j.mineng.2004.02.001 - 27. Kejun, L., Yen, W.T., Shibayama, A., Miyazaki, T. and Fujita, T. (2004) Gold Extraction from Thiosulfate Solution Using Trioctylmethylammonium Chloride. Hydrometallurgy, 73, 41-53.
http://dx.doi.org/10.1016/j.hydromet.2003.07.007 - 28. Vargas, C., Navarro, P., Araya, E., Pavez, F. and Alguacil, F.J. (2006) Recovery of Gold from Solutions with Ammonia and Thiosulfate Using Activated Carbon. Revista de Metalurgia, 42, 222-233.
http://dx.doi.org/10.3989/revmetalm.2006.v42.i3.22 - 29. Navarro, P., Vargas, C., Alonso, M. and Alguacil, F.J. (2006) The Adsorption of Gold on Activated Carbon from Thiosulfate-Ammoniacal Solutions. Gold Bulletin, 39, 93-97.
http://dx.doi.org/10.1007/BF03215535 - 30. Navarro, P., Vargas, C., Alonso, M. and Alguacil, F.J. (2007) Towards a More Environmentally Friendly Process for Gold: Models on Gold Adsorption onto Activated Carbon from Ammoniacal Thiosulfate Solutions. Desalination, 211, 58-63.
http://dx.doi.org/10.1016/j.desal.2006.03.590 - 31. Nicol, M.J. and O’Malley, G. (2002) Recovering Gold from Thiosulfate Leach Pulps via Ion Exchange. JOM, 54, 44-46.
http://dx.doi.org/10.1007/BF02709221 - 32. Navarro, P., Vargas, C., Reveco, V. and Orellana, J. (2006) Recovery of Gold from Ammonia-Thiosulfate Media with Amberlite IRA-410 Ionic Exchange Resin. Revista de Metalurgia, 42, 354-366.
http://dx.doi.org/10.3989/revmetalm.2006.v42.i5.33 - 33. Zhang, H. and Dreisinger, D. (2004) The Recovery of Gold from Ammoniacal Thiosulfate Solutions Containing Copper Using Ion Exchange Resin Columns. Hydrometallurgy, 72, 225-234.
http://dx.doi.org/10.1016/S0304-386X(03)00183-X - 34. Zhang, H. and Dreisinger, D. (2002) The Adsorption of Gold and Copper onto Ion-Exchange Resins from Ammoniacal Thiosulfate Solutions. Hydrometallurgy, 66, 67-76.
http://dx.doi.org/10.1016/S0304-386X(02)00077-4 - 35. Senanayake, G. (2004) Analysis of Reaction Kinetics, Speciation and Mechanism of Gold Leaching and Thiosulfate Oxidation by Ammoniacal Copper(II) Solutions. Hydrometallurgy, 75, 55-75.
http://dx.doi.org/10.1016/j.hydromet.2004.06.004










