International Journal of Nonferrous Metallurgy
Vol.2 No.1(2013), Article ID:27173,9 pages DOI:10.4236/ijnm.2013.21001
The Uptake of Copper(II) Ions by Chelating Schiff Base Derived from 4-Aminoantipyrine and 2-Methoxybenzaldehyde
School of Chemical Sciences, Universiti Sains Malaysia, Penang, Malaysia
Email: *oocw@usm.my
Received November 28, 2012; revised December 27, 2012; accepted January 10, 2013
Keywords: Schiff Base; Adsorption; Copper(II) Ions; Isotherm; Kinetics
ABSTRACT
The Schiff base, 4-[(2-methoxybenzylidene)amino]-1,5-dimethyl-2-phenyl-1H-pyrizol-3(2H)-one (SB), was used for the first time to adsorb copper(II) ions in aqueous solution. Various parameters such as initial pH, agitation period and different initial concentration of copper(II) ions which influenced the adsorption capacity were investigated. The equilibrium adsorption data for copper(II) ions were fitted to Langmuir, Freundlich and Dubinin-Radushkevish isotherm models. The maximum monolayer adsorption capacity of SB as obtained from Langmuir isotherm was 5.64 mg/g. Kinetic data correlated well with the pseudo second-order kinetic model indicating that chemical adsorption was the rate limiting step.
1. Introduction
Heavy metal ions are the most harmful of the elemental pollutants and are of particular concern because of their toxicities to humans. The human body cannot metabolize heavy metals and it displays bioaccumulation properties causing various diseases and disorders. Metal ions in the environment can accumulate and are biomagnified along the food chain. Therefore, their toxic effects are more pronounced in human. There are many possible sources of these heavy metal ions. These include wastes from metal plating operations, mining operations, tanneries, fertilizer and textiles industries [1,2]. Copper is one of the most useful metals due to its low toxicity, corrosion resistant, workability and electrical conductivity. Copper has antibacterial properties and has a biological role in sustaining life. However, accumulation of copper in human body can cause adverse effect on human health such as stomach and intestinal cancer, liver and also kidney damage [3]. The presences of copper are mainly found in effluents from electroplating and brass manufacturing industries and also in the Cu-based agrichemicals run off from agricultural lands [4]. Many conventional techniques such as ion exchange, reverse osmosis, membrane filtration, precipitation-neutralization etc. have been used to remove heavy metal ions from aqueous solution [5,6]. However, these methods involved high operation cost, generated secondary toxic sludge and were incompetent for the removal of heavy metal ions at low concentration [7]. Alternatively, adsorption onto solid substrate materials is considered as the most suitable process for the removal of heavy metal ions from solution of high and low concentrations.
Over the years, many studies have been conducted on the removal of heavy metal ions from aqueous solution by using adsorbents such as activated carbon, chitosan beads, silica, ion exchange resins and also chelating agents. Chelating agents have been widely employed as adsorbent because it has moderate coordination sites like nitrogen which shows great affinity towards metal ions [8]. One of the important chelating agents receiving an increased interest recently due to the presences of defined chelating groups is Schiff bases. Schiff bases are obtained through the condensation of aldehydes with amines and contain multidentate coordination sites such as Oand N-donor atoms which have high bonding affinity towards many heavy metal ions [6,8,9].
In this study, the efficiency of 4-[(2-methoxybenzylidene)amino]-1,5-dimethyl-2-phenyl-1H-pyrizol-3(2H)-one (written as SB) synthesized from 4-aminoantipyrine and 2-methoxybenzaldehyde was evaluated for the first time in the removal of copper(II) ions from aqueous sotion. Crucial parameters that affected the removal of copper(II) ions, such as initial pH, contact time and difrent initial copper(II) concentration were investigated.
2. Materials and Methods
2.1. Materials
The starting materials such as 4-aminoantipyrine and 2-methoxybenzaldehyde used in this research were purased from Fluka. All the reagents used were of analyticl-reagent grade and were used without further purificaon. Distilled water was used throughout this research.
2.2. Synthesis of 4-[(2-Mthoxybenzylidene)aino]-1,5-dmethyl-2-penyl-1H-prizol-3(2H)-one (SB)
The Schiff base, 4-[(2-methoxybenzylidene)amino]-1,5- dimethyl-2-phenyl-1H-pyrizol-3(2H)-one (SB) was prepared by adding 4-aminoantipyrine (10 mmol, 2.03 g) into 2-methoxybenzaldehyde (10 mmol, 1.36 g) solution that was dissolved in 20 mL of ethanol in the presence of one drop of glacial acetic acid. The mixture was refluxed for 2 hours. The resulting mixture was cooled to room temperature and the resulting precipitate was filtered, washed repeatedly with distilled water and recrystallized from ethanol. The Schiff base (SB) was isolated as a yellow crystalline solid. The structure of SB was shown in Figure 1.
FTIR (KBr pellet): n 3051, 2933, 2833, 1642, 1609, 1593, 1508 cm−1. Calc. for C19H19N3O2: C, 71.01; H, 5.96; N, 13.08. Found: C, 71.20; H, 5.77; N, 12.80.
2.3. Characterization of the SB
The types of functional groups existing on SB were confirmed by a PerkinElmer Fourier transform infrared (FTIR) System 2000 Model spectrophotometer. Meanwhile, the surface morphology of SB before and after adsorption of copper(II) ions was observed using a Leica Cambridge S360 scanning electron microscope (SEM) coupled with energy dispersive X-ray spectroscopy (EDX).
2.4. Batch Adsorption Experiments
Stock solution of 1000 mg/L of copper(II) ions was prepared using the analytical-reagent grade copper(II) nitrate salt. The stock solution was diluted accordingly to prepare different concentration of copper(II) ions. The

Figure 1. The structure of 4-[(2-methoxybenzylidene)amino]-1,5-dimethyl-2-phenyl-1H-pyrizol-3(2H)-one (SB).
batch adsorption experiments were conducted in 250 mL Erlenmeyer flasks with 50 mL of standard solution and equilibrated using a shaker. The amount of SB used throughout this research was 0.1 g. Each experiment was carried out in duplicates. All filtrates were analysed using atomic absorption spectrometer (AAnalyst 200 AA, PerkinElmer, USA) at a wavelength of 324.75 nm for copper(II) ions.
The effect of pH on the adsorption of copper(II) ions was studied in a pH range of 1.5 - 7.0. The pH of the 10 mg/L of copper(II) ions was adjusted to different pH using appropriate concentration of HCl and NaOH solutions and equilibrated for 3 h. The effect of agitation time was also studied at different time interval ranging from 5 - 240 min at the optimum pH. The isotherm study was conducted under the optimum condition by varying the initial concentration of copper(II) ions from 5 to 30 mg/L. The amount of adsorption at equilibrium was calculated using the following equation:
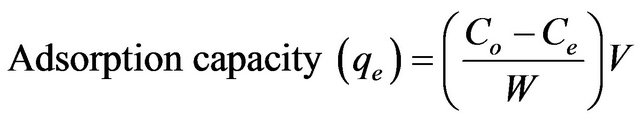 (1)
(1)
where Co is the initial concentration of copper(II) ions (mg/L), Ce is the final concentration of copper(II) ions (mg/L), V is the volume of copper(II) ions solution (L) and W is the weight of SB (g) used.
3. Results and Discussion
3.1. FTIR of the SB
The FTIR spectrum of SB was shown in Figure 2. The peaks at 1642 and 1508 cm−1 were attributed to the C=O group (conjugated) and the C=N bonds, respectively. The present of imine group, C=N confirmed that a Schiff base was formed from the reaction between 4-aminoantipyrine and 2-methoxybenzaldehyde. The peaks found at 1609 and 1593 cm−1 were due to the C=C bonds in the benzene rings of SB. The peaks at 2933 and 2833 cm−1 were attributed to the C-H stretching of the methoxy group. Meanwhile the =C-H stretching was evident at 3051 cm−1. The medium broad peak at 3431 cm−1 could be due to the present of water molecule on the SB because of its hygroscopic nature.
3.2. Adsorption Studies
3.2.1. Effect of Initial pH
The pH of metal solution is the most important parameter governing metal adsorption. This is due to the fact that pH of the metal solution affects the solubility of metal ions and the surface charge of the adsorbent. In most cases, the metal uptake increased at higher pH when the surface charge of the adsorbent is opposite to that of the metal cation. At this point, the attraction force between the adsorbent and the metal cation will increase. Figure 3
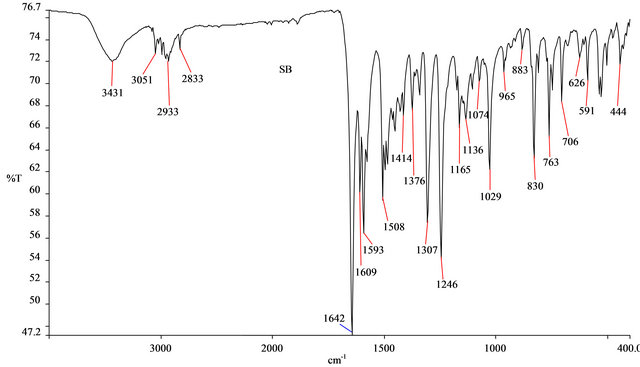
Figure 2. FTIR spectrum of SB.
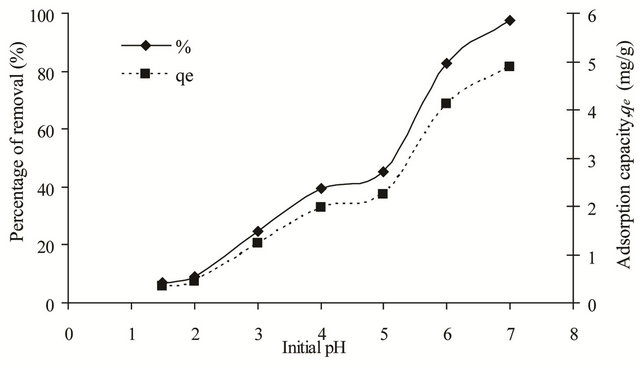
Figure 3. Effect of initial pH on removal of copper(II) ions by SB.
shows the effect of pH on the removal of copper(II) ions by SB. At low pH, the uptake of copper(II) ions was low because the functional groups (O-CH3 and C=N) on the adsorbent surface were protonated giving rise to positively charged adsorption sites. The electrostatic repulsion between the positively charged SB and the copper(II) ions together with the competition from the proton (H+) for the active adsorption sites will contribute to the low adsorption percentage. Furthermore, dissolution of SB was observed at acidic pH of 2 to 4. However, as the pH of the solution increased the adsorption percentage increased as well, whereby at pH 6, 82.66% of copper(II) ions was being adsorbed. At higher pH, the functional groups of the adsorbent were deprotonated creating the negatively charged surface that will attract the copper(II) ions. Less amount of protons in the solution also reduced the competition between copper(II) ions and the protons for the active adsorption sites. In this study, pH 6 was chosen as the optimum adsorption pH. Although at pH 7, the percentage of removal was much higher, it was not considered as the optimum pH because at higher pH, copper(II) ions will form the insoluble Cu(OH)2 precipitate.
3.2.2. Effect of Contact Time
The uptake rate of copper(II) ions onto SB was investigated as it represented the amount of time required before the adsorption process becomes constant and equilibrium was reached. As shown in Figure 4, for the first 90 min, the uptake rate was high and it decreased gradually until it reached equilibrium at 180 min with an adsorption percentage of 83.18%. The high uptake rate observed at initial stage could be due to the adsorption of copper(II) ions onto the external surface of the adsorbent. Meanwhile, the slower uptake rate observed after 90 min is due to the pore diffusion and the quick exhaustion of the available adsorption sites. The adsorption data were then analyzed with pseudo firstand pseudo second-order kinetic models.
3.2.3. Adsorption Kinetics
The adsorption kinetics of metal ions is important for designing the adsorption system and is required for determining the optimum operating conditions for a fullscale batch process [10]. Many adsorption kinetic models
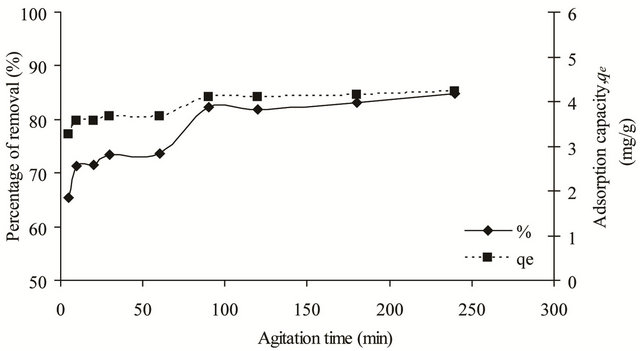
Figure 4. Effect of agitation time on the removal of copper(II) ions by SB.
have been applied to understand the adsorption kinetics and the rate-limiting step. Among all, pseudo firstand pseudo second-order models are the most commonly used to study the adsorption kinetics of heavy metal ions [11]. The pseudo first-order kinetic model is given in its linear form as:
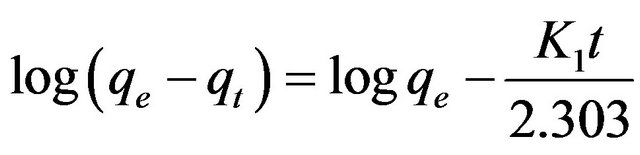 (2)
(2)
where qe is the amount of copper(II) ions adsorbed at equilibrium (mg/g), qt is amount of copper(II) ions adsorbed at time t (mg/g) and K1 is the pseudo first-order kinetic constant (1/min). Linear plot of log(qe-qt) against t for the adsorption of copper(II) ions onto SB was shown in Figure 5. In many cases, the pseudo first-order kinetic model does not fit well to the whole range of contact time. It would only be applicable over the initial stage of the adsorption process [12]
The pseudo second-order kinetic model is based on the assumption that the rate-limiting step in the adsorption of heavy metal ions is chemisorption involving the valence force through the sharing or exchange of electrons between the adsorbate and the adsorbent [2,13-15]. This kinetic model is represented as:
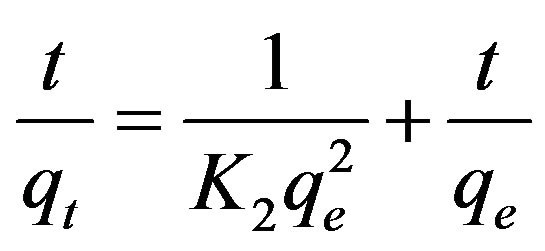 (3)
(3)
where qe and qt are the amount of copper(II) ions adsorbed at equilibrium and time t (mg/g), respectively and K2 is the pseudo second-order kinetic constant (g/mg∙min). Figure 5 showed the linear plots of t/qt against t.
The pseudo second-order kinetic model gave higher correlation (R2 = 0.9991) than the pseudo first-order kinetic model (R2 = 0.8953). Furthermore, the calculated equilibrium adsorption capacity based on pseudo secondorder (4.27 mg/g) was in agreement with the experimenttal data (4.16 mg/g). Hence, the pseudo second-order is more appropriate to represent the adsorption data of copper(II) ions onto SB. The poor fit observed for pseudo first-order kinetic model could be due to a time lag caused by a boundary layer or the external resistance


Figure 5. Pseudo firstand pseudo second-order plots for the removal of copper(II) ions with SB.
controlling at the beginning of the adsorption process [16].
3.2.4. Isotherm Studies
The equilibrium adsorption isotherm is essential to be studied as it describes the interactive behavior between the solutes and the adsorbent. Different initial concentration of copper(II) ions ranging from 5 - 30 mg/L was used to study the effectiveness of SB to remove trace amount of copper(II) ions. As shown in Figure 6, the adsorption capacity increased with an increase in the initial concentration of copper(II) ions at optimum adsorption pH and agitation time. The adsorption capacity reached equilibrium once the active adsorption sites were saturated at the initial concentration of 25 mg/L of copper(II) ions.
The adsorption equilibrium data were fitted with different isotherm models mainly Langmuir, Freundlich and Dubinin-Radushkevich (D-R) isotherm in order to evaluate the adsorption phenomenon. In this study, non-linear method was used to evaluate the fitness of the isotherm models to the experimental data. The non-linear method is a better way to obtain the isotherm parameters compared to the linear least-square method [17]. The plots based on Langmuir, Freundlich and D-R isotherms were shown in Figures 7 and 8, respectively, while the isotherm constants were tabulated in Table 1.
3.2.4.1. Langmuir Isotherm Langmuir isotherm relates the coverage of molecules on a solid surface to the concentration of a medium above
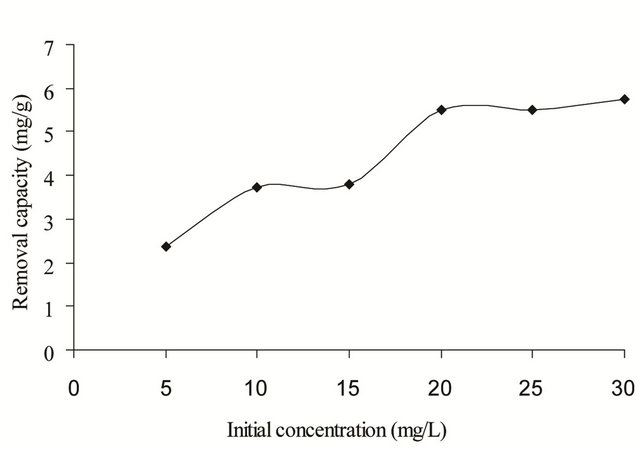
Figure 6. Effect of initial concentration of copper(II) ions on the adsorption capacity of SB.

Figure 7. Langmuir and Freundlich isotherms for the adsorption of copper(II) ions onto SB.
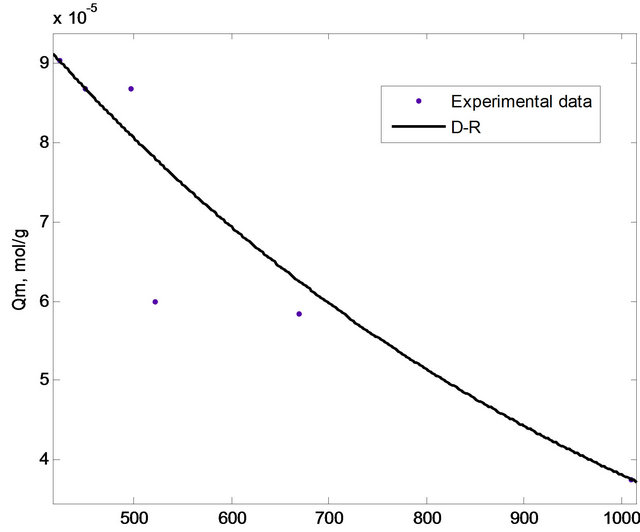
Figure 8. D-R isotherm for the adsorption of copper(II) ions onto SB.
the solid surface at a fixed concentration [11]. It describes monolayer adsorption and is based on the assumption that all the adsorption sites are energetically
Table 1. Isotherm and kinetic constants of different models for the adsorption of copper(II) ions onto SB.

identical and adsorption takes place on a structurally homogenous adsorbent [2]. This model can be represented as:
 (4)
(4)
where Ce is the equilibrium concentration (mg/L), qe is the amount of copper(II) ions adsorbed at equilibrium (mg/g), Vm is the monolayer adsorption capacity (mg/g) and KL is the Langmuir equilibrium constant (L/mg). Based on Figure 7, the steep initial slope observed for the Langmuir plot reflected high KL value (3.127 L/ mg) indicating that the adsorbent has high affinity for copper(II) ions [18,19] with a monolayer adsorption capacity of 5.64 mg/g.
3.2.4.2. Freundlich Isotherm Freundlich isotherm is an empirical equation used for the adsorption on heterogeneous surfaces or the surface adsorption sites of varied affinities [20]. It describes monomolecular layer coverage of adsorbent by the solutes [21]. Freundlich isotherm is given as:
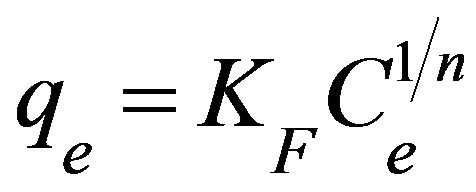 (5)
(5)
where qe is the amount adsorbed at equilibrium (mg/g), Ce is the equilibrium concentration (mg/L), KF is the adsorption capacity (mg/g) and 1/n is the adsorption intensity. Adsorption is said to be favorable when the Freundlich constant 1/n is between 0.1 and 1 and represented a heterogenous surface structure of the adsorbent [22,23]. Meanwhile, smaller value of 1/n indicates stronger interaction between the adsorbent and the heavy metal ions, while 1/n equal to 1 implies linear adsorption leading to identical adsorption energies for all sites [11]. As shown in Table 1, adsorption of copper(II) ions onto SB has a 1/n value of 0.2013 with KF equal to 3.192 mg/g. This showed that SB had a strong interaction with copper(II) ions which makes SB a favourable adsorbent for copper(II) ions.
3.2.4.3. Dubinin-Radushkevich (D-R) Isotherm D-R isotherm, apart from being analogue of Langmuir isotherm, is a more general model than Langmuir isotherm as it does not assume homogenous surface or constant sorption potential [24]. D-R isotherm was derived as the overall adsorption isotherm for the adsorption onto heterogenous solid surface [20]. The D-R isotherm equation is expressed as:
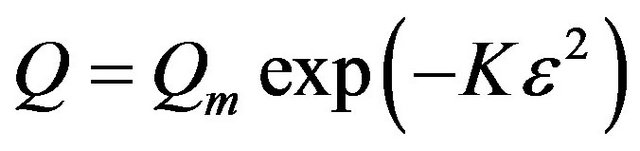 (6)
(6)
where, Q is the amount adsorbed at equilibrium (mol/g), Qm is the maximum adsorption capacity (mol/g), K is the D-R constant (mol/kJ) which is related to the adsorption energy. The Polanyi potential (ε) is given as:
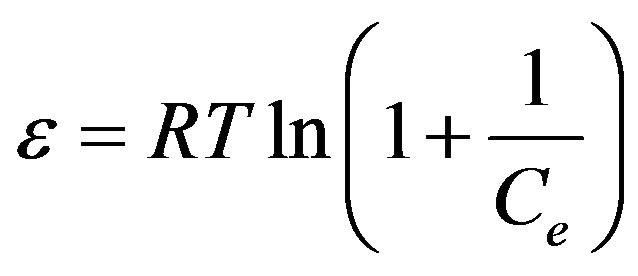 (7)
(7)
where R is the gas constant in kJ/mol K and T is the temperature in Kelvin. The mean energy of adsorption, E is used to estimate the type of adsorption process and can be calculated by the following equation:
 (8)
(8)
The adsorption process can be explained by an ion exchange process if the value of E is between 8 and 16 kJ/mol [2,18]. Meanwhile, E values lower than 8 kJ/mol correspond to physical adsorption (physisorption) [25,26]. The E value for the adsorption of copper(II) ions onto SB was calculated as 1.2299 kJ/mol indicating the involvement of physisorption.
3.3. Adsorption Mechanism
The adsorption of solute on any sorbent can happen either through physical bonding, ion exchange, complexation, chelation or a combination of any of these interactions [11]. The adsorbent used in the present study is a Schiff base which has the -OCH3 and C=N functional groups as the active sites to interact with copper(II) ions. At the optimum pH, the oxygen and nitrogen atoms on these active sites behave as the electron donors and can form complex with the copper(II) ions as shown in Figure 9. This process can be classified as chemisorption which involves the sharing of electrons between the adsorbate and the adsorbent.
The removal of copper(II) ions by SB, however, was not solely governed by chemisorption. The mean adsorption energy, E calculated from the D-R isotherm suggested that physical adsorption might have taken place as well. This was proven based on the SEM-EDX analysis carried out on SB before and after the adsorption of copper(II) ions. The SEM-EDX micrographs were shown in Figure 10. The SEM image for SB before the adsorption of copper(II) ions (Figure 10(a)) shows an even and smooth surface morphology. Many small particles which clustered on the surface of SB were also found. Based on the EDX analysis, the presences of carbon, hydrogen and nitrogen were detected. The gold peaks (Au) found in the spectra were due to the gold purposely settled to increase electrical conductive of the sample. After the adsorption of copper(II) ions (Figure 10(b)) surface morphology of SB changed drastically. The surface was found to be very uneven and many small coarser particles were formed on the surface. The EDX spectra showed the presences of copper on the surface of SB especially on these smaller particles. These observation proved that copper(II) ions were deposited on the surface of the adsorbent by means of physical adsorption. Physical adsorption occurs through the existences of van der Waals forces between the adsorbent and the adsorbate.
4. Conclusion
Schiff base derived from 4-aminoantipyrine and 2- methoxybenzaldehyde can act as a potential adsorbent to remove copper(II) ions from the aqueous solution. Present study showed that SB has a monolayer adsorption capacity of 5.64 mg/g for copper(II) ions. Chemisorption of copper(II) ions could be occurred through the interaction of copper(II) ions with the methoxy and imine groups on SB. Copper(II) ions also can be removed via physical adsorption. The kinetic studies showed that the removal of copper(II) ions by SB followed the pseudo second-order kinetic reaction.
5. Acknowledgements
The authors would like to express their sincere gratitude to Universiti Sains Malaysia for funding this study
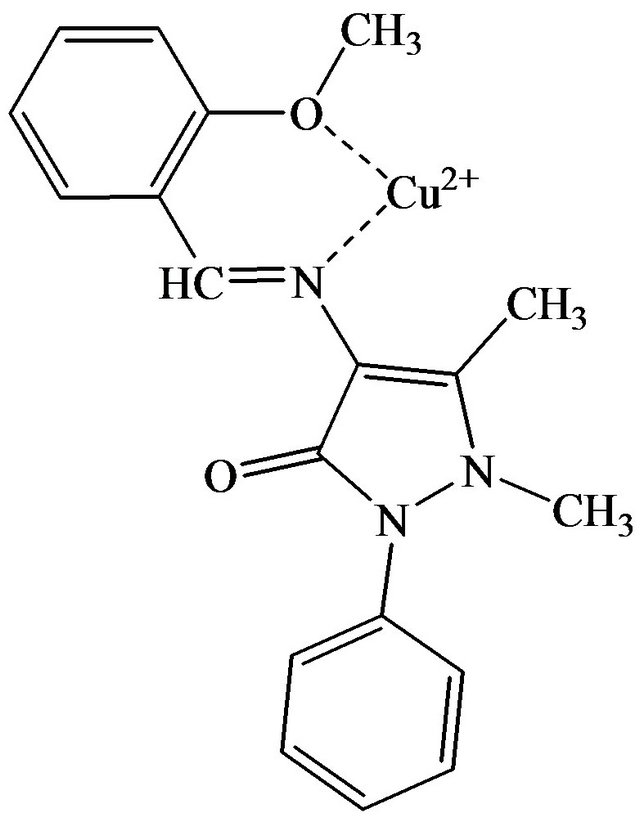
Figure 9. Proposed mechanism for the adsorption of copper(II) ions onto SB.
 (a)
(a) (b)
(b)
Figure 10. SEM-EDX spectra of SB (a) before and (b) after the adsorption of copper(II) ions.
through the Research University grant no. 1001/PKIMIA /811134.
REFERENCES
- E. A. Susan, J. O. Trudy, R. M. Bricka and D. D. Adrian, “A Review of Potentially Low-Cost Sorbents for Heavy Metals,” Water Research, Vol. 33, No. 11, 1999, pp. 2469-2479. doi:10.1016/S0043-1354(98)00475-8
- N. Unlu and M. Ersoz, “Adsorption Characteristics of Heavy Metal Ions onto a Low Cost Biopolymeric Sorbents from Aqueous Solutions,” Journal of Hazardous Materials, Vol. 136, No. 2, 2006, pp. 272-280. doi:10.1016/j.jhazmat.2005.12.013
- C. I. Lee, W. F. Yang and C. I. Hsieh, “Removal of Copper(II) by Manganese-Coated Sand in a Liquid FluidizedBed Reactor,” Journal of Hazardous Materials, Vol. 114, No. 1-3, 2004, pp. 45-51. doi:10.1016/j.jhazmat.2004.06.033
- N. Boujelben, J. Bouzid and Z. Elouear, “Adsorption of Nickel and Copper onto Natural Iron Oxide-Coated Sand from Aqueous Solutions: Study in Single and Binary Systems,” Journal of Hazardous Materials, Vol. 163, No. 1, 2009, pp. 376-382. doi:10.1016/j.jhazmat.2008.06.128
- G. Palma, J. Freer and J. Baeza, “Removal of Metal Ions by Modified Pinus radiata Bark and tannins from Water Solutions,” Water Research, Vol. 37, No. 20, 2003, pp. 4974-4980. doi:10.1016/j.watres.2003.08.008
- Q. F. Yin, B. Z. Ju, S. F. Zhang, X. B. Wang and J. Z. Yang, “Preparation and Characteristics of Novel Dialdehyde Aminothiazhole Starch and Its Adsorption Properties for Cu(II) Ions from Aqueous Solution,” Carbohydrate Polymers, Vol. 72, No. 2, 2008, pp. 326-333. doi:10.1016/j.carbpol.2007.08.019
- G. Vazquez, J. Gonzalez-Alvarez, S. Freire, M. LopezLorenzo and G. Antorrena, “Removal of Cadmium and Mercury Ions from Aqueous Solution by Sorption on Treated Pinus pinaster Bark: Kinetics and Isotherms,” Bioresource Technology, Vol. 82, No. 3, 2002, pp. 247- 251. doi:10.1016/S0960-8524(01)00186-9
- M. H. Mashhadizadeh, M. Pesteh, M. Talakesh, I. Sheeikhshoaie, M. M. Ardakani and M. A. Karimi, “Solid Phase Extraction of Copper(II) by Sorption on Octadecyl Silica Membrane Disk Modified with a New Schiff Base and Determination with Atomic Absorption Spectrometry,” Spectrochimica Acta Part B, Vol. 63, No. 8, 2008, pp. 885-888. doi:10.1016/j.sab.2008.03.018
- P. A. Amoyaw, M. Williams and X. R. Bu, “The Fast Removal of Low Concentration of Cadmium(II) from Aqueous Media by Chelating Polymers with Salicylaldehyde Units,” Journal of Hazardous Materials, Vol. 170, No. 1, 2009, pp. 22-26. doi:10.1016/j.jhazmat.2009.05.028
- E. A. Oliveira, S. F. Montanher, A. D. Andrade, J. A. Nobrega and M. C. Rollemberg, “Equilibrium Studies for the Sorption of Chromium and Nickel from Aqueous Solutions Using Raw Rice Barn,” Process Biochemistry, Vol. 40, No. 11, 2005, pp. 3485-3490. doi:10.1016/j.procbio.2005.02.026
- J. Febrianto, A. N. Kosasih, J. Sunarso, Y.-H. Ju, N. Indraswati and S. Ismadji, “Equilibrium and Kinetic Studies in Adsorption of Heavy Metals Using Biosorbent: A Summary of Recent Studies,” Journal of Hazardous Materials, Vol. 162, No. 2-3, 2009, pp. 616-645. doi:10.1016/j.jhazmat.2008.06.042
- Y. S. Ho and G. Mckay, “The Sorption of Lead(II) Ions on Peat,” Water Research, Vol. 33, No. 2, 1999, pp. 578- 584. doi:10.1016/S0043-1354(98)00207-3
- F. Gode and E. Pehlivan, “Adsorption of Cr(III) Ions by Turkish Brown Coals,” Fuel Processing Technology, Vol. 86, No. 8, 2005, pp. 875-884. doi:10.1016/j.fuproc.2004.10.006
- D. Singh, K. P. Singh and V. K. Singh, “Trivalent Chromium Removal from Wastewater Using Low Cost Activated Carbon Derived from Agricultural Waste Material and Activated Carbon Fabric Cloth,” Journal of Hazardous Materials, Vol. 135, No. 1-3, 2006, pp. 280-295. doi:10.1016/j.jhazmat.2005.11.075
- V. C. Taty-Costodes, H. Fauduet, C. Porte and A. Delacroix, “Removal of Cd(II) and Pb(II) Ions from Aqueous Solutions by Adsorption onto Sawdust of Pinus sylvestris,” Journal of Hazardous Materials, Vol. B105, 2003, pp. 121-142. doi:10.1016/j.jhazmat.2003.07.009
- G. McKay, Y. S. Ho and J. C. Y. Ng, “Biosorption of Copper from Waste Waters: A Review,” Separation & Purification Reviews, Vol. 28, No. 1, 1999, pp. 87-125. doi:10.1080/03602549909351645
- Y. S. Ho, “Isotherms for the Sorption of Lead onto Peat: Comparison of Linear and Non-Linear Methods,” Polish Journal of Environmental Studies, Vol. 15, No. 1, 2006, pp. 81-86.
- K. Vijayaraghavan, T. V. N. Padmesh, K. Palanivelu and M. Velan, “Biosorption of Nickel (II) Ions onto Sargassum wightii: Application of Two-Parameter and ThreeParameter Isotherm Models,” Journal of Hazardous Materials, Vol. B133, 2006, pp. 304-308. doi:10.1016/j.jhazmat.2005.10.016
- B. Volesky, J. Weber and R. Vieira, “Biosorption of Cd and Cu by Different Types of Sargassum biomass,” Process Metallurgy, Vol. 9, 1999, pp. 473-482. doi:10.1016/S1572-4409(99)80136-9
- A. Dąbrowski, “Adsorption—From Theory to Practice,” Advances in Colloid and Interface Science, Vol. 93, No. 1-3, 2001, pp. 135-224. doi:10.1016/S0001-8686(00)00082-8
- S. Al-Asheh, F. Banat, R. Al-Omari and Z. Duvnjak, “Prediction of Binary Sorption Isothems for the Sorption of Heavy Metals by Pine Bark Using Single Isotherm Data,” Chemosphere, Vol. 41, 2000, pp. 659-665. doi:10.1016/S0045-6535(99)00497-X
- T. Karthikeyan, S. Rajgopal and L. R. Miranda, “Chromium (VI) Adsorption from Aqueous Solution by Hevea Brasilinesis Sawdust Activated Carbon,” Journal of Hazardous Materials, Vol. B124, 2005, pp. 192-199. doi:10.1016/j.jhazmat.2005.05.003
- S. P. Mishra, D. Tiwari, R. S. Dubey and M. Mishra, “Biosorptive Behaviour of Casein for Zn2+, Hg2+ and Cr3+: Effect of Physico-Chemical Treatment,” Bioresource Technology, Vol. 63, 1998, pp. 1-5. doi:10.1016/S0960-8524(97)00110-7
- W. Zheng, X.-M. Li, F. Wang, Q. Yang, P. Deng and G.-M. Zeng, “Adsorption Removal of Cadmium and Copper from Aqueous Solution by Areca—A Food Waste,” Journal of Hazardous Materials, Vol. 157, No. 2-3, 2008, pp. 490-495. doi:10.1016/j.jhazmat.2008.01.029
- M. Mahramanlioglu, I. Kizilcikli and I. O. Bicer, “Adsorption of Fluoride from Aqueous Solution by Acid Treated Spent Bleaching Earth,” Journal of Fluorine Chemistry, Vol. 115, No. 1, 2002, pp. 41-47. doi:10.1016/S0022-1139(02)00003-9
- O. A. Wahab, “Kinetic and Isotherm Studies of Copper (II) Removal from Wastewater Using Various Adsorbents,” Egyptian Journal of Aquatic Research, Vol. 30, No. 1, 2007, pp. 125-143.
NOTES
*Corresponding author.

