International Journal of Nonferrous Metallurgy
Vol.1 No.2(2012), Article ID:21393,4 pages DOI:10.4236/ijnm.2012.12002
Microbial Recovery of Manganese Using Staphylococcus epidermidis
1Centre of Biotechnology, Siksha ‘O’ Anusandhan University, Bhubaneswar, India
2Institute of Minerals and Materials Technology, Bhubaneswar, India
Email: *alok1503@gmail.com
Received May 1, 2012; revised June 7, 2012; accepted June 30, 2012
Keywords: Manganese; Staphylococcus epidermidis; Bioleaching
ABSTRACT
Manganese minerals are widely distributed throughout the globe. The most important industrial uses of Mn are in the manufacture of steel, non-ferrous alloys, carbon-zinc batteries and some chemical reagents. Microbial recovery of manganese from low grade manganese ores using bioleaching was investigated in this paper. A bacterial strain, Staphylococcus epidermidis (MTCC-435) was collected from microbial type culture collection, IMTECH Chandigarh and used for the experiment. The experimental results for bioleaching with S. epidermidis showed that under pH 5.5, particle size −150 μm, pulp density 10%, temperature 35˚C and agitation 200 rpm, about 80% of Mn was recovered within 20 days of incubation.
1. Introduction
The necessity for manganese ore has increased due to the escalating shortage of natural resources and increase in the manufacture of Steel, dry cell batteries and some portion of manganese oxide [1]. The 96% of global production of manganese today is from barely 7 countries viz. CIS, RSA, Brazil, Gabon, Australia, China and India in decreasing order of tonnages raised annually. The global resource base is close to 12 billion tonnes including Indian reserve of about 240 million tonnes. Indian manganese ores are preferred by many as they are generally hard, lumpy and amenable to easy reduction. The high grade ores with manganese concentrations in excess of 35% are preferred industrially, and the low grades with manganese concentration less than 35% are presently ignored because extraction methods applied to low grade manganese ore are not economically viable [2].
The development of bioleaching technology focuses on achieving effective recovery of precious metals by improving the efficiency of bioleaching microorganisms [3,4]. This relates to manganese solubilising activities of manganese-oxidizing microbes and the speciation of intermediate compounds formed during bioleaching processes [5]. The effectiveness of bioleaching is also highly dependent on the physical, chemical and biological factors in the system. The maximum yield of metal leaching may be achieved when these parameters are considered and optimized collectively [6]. In the present research work, bioleaching technology was applied for manganese recovery from low grade manganese ore.
2. Materials and Methods
2.1. Collection of Ore
The low grade manganese ore collected from Ferromanganese mines, Keonjhar district in Odisha, India. The large pieces were crushed in a jaw-crusher separately. Then, all pieces were ground to relatively smaller particles using ball mill machine. The physical and chemical properties of samples were characterized. The presence of other heavy metals was estimated by Atomic Absorption Spectroscopy methods (A. Analyst 200. Perkin Elmer). The Mn content of the ore was 20%.
2.2. Microorganism and Culture Condition
A bacterial strain, Staphylococcus epidermidis (MTCC- 435) was procured from microbial type culture collection, IMTECH Chandigarh. The strain was subculture on nutrient agar medium plates containing 50 mg of MnO2/ml to the medium. The growth of the bacterial colony was observed after 24 h of incubation at 30˚C. Isolated colonies picked up with sterilized wire loop and streaked on nutrient agar medium plate containing 100 mg of MnO2/ml. Leaching study was carried out under shaking condition inside an incubator shaker. At five day interval, pH of each flask was noted under aseptic condition & manganese estimation was done by titration method.
2.3. Effect of Process Parameters
Process optimization experiments for temperature, pH, Particle size, pulp density and carbon concentration were carried out. Leaching experiments were done in 250 ml flask containing 100 ml of sterilized minimal salt medium for a period of 20 days. For pH optimization, the five standards used were 4.5, 5.5, 6.5, 7.5 and 8.5. The respective pH was adjusted with 0.5N NaOH and 0.5N HCl. For temperature optimization the five standard temperatures considered were 25˚C, 30˚C, 35˚C, 40˚C and 45˚C. Different size fractions of manganese ore ranging from +4 mm, –4 mm, +1 mm, –1 mm, +150 μm and −150 μm at 2% pulp density were considered. Pulp densities ranging from 2%, 4%, 6%, 8% and 10 % (w/v) of the sieved particle size –150 μm were taken for the experiment. Aseptic conditions were maintained throughout the experiment. Uninoculated media containing manganese ore served as control. Samples were drawn at regular intervals and were analyzed by titration method to determine the percentage of manganese leached in the liquid medium.
2.4. Manganese Bioleaching
The low grade manganese ore was used for these experiments. Effect of temperature and pH variation was studied. Leaching experiments were done in 250 ml flask containing 90 ml of sterilized Bromfield medium (BM) containing ammonium sulphate 0.027 g, dihydroxy potassium phosphate 0.025 g, yeast extract 0.1 g and sucrose 10 g.
3. Results and Discussion
3.1. Ore Characteristics
The moisture content of the collected ore was estimated to be 15%. The heavy metal content of the ore was estimate to be Fe (570 g·kg–1), Si (147 g·kg–1), Mn (210 g·kg–1) and Zn (146 g·kg–1). The phase of Mn and Fe in the collected are sample are in their oxide forms.
3.2. Process Parameters Optimization
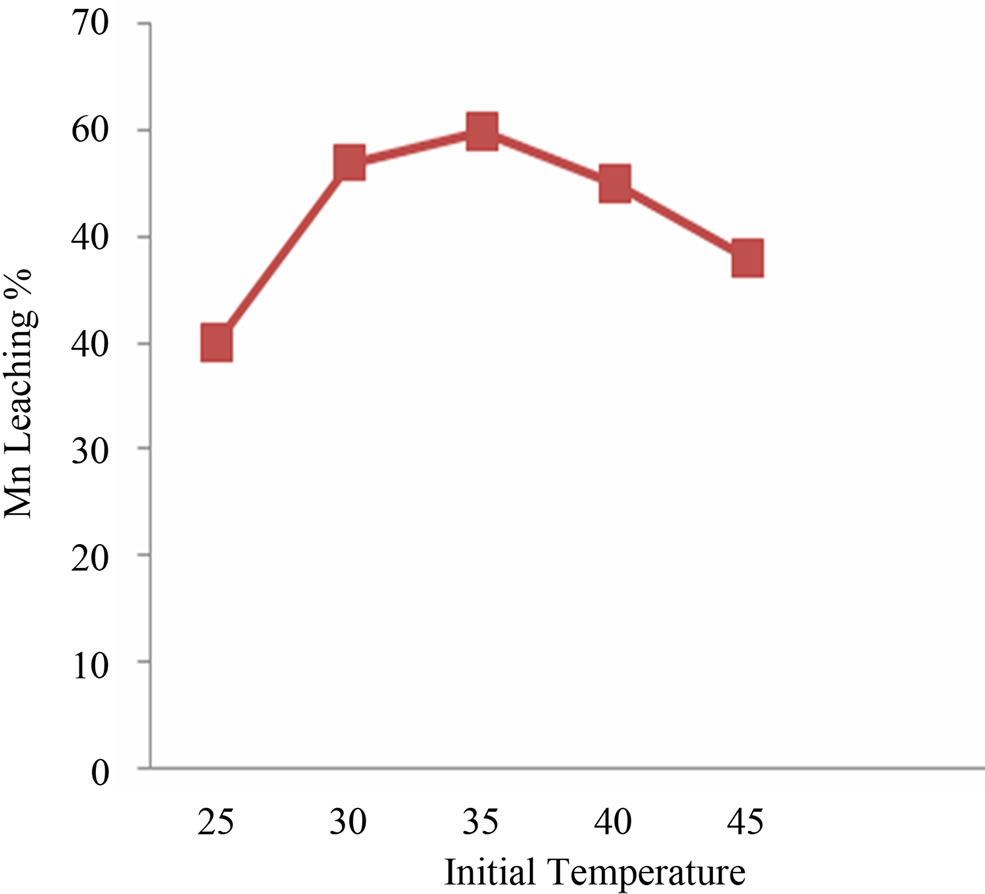
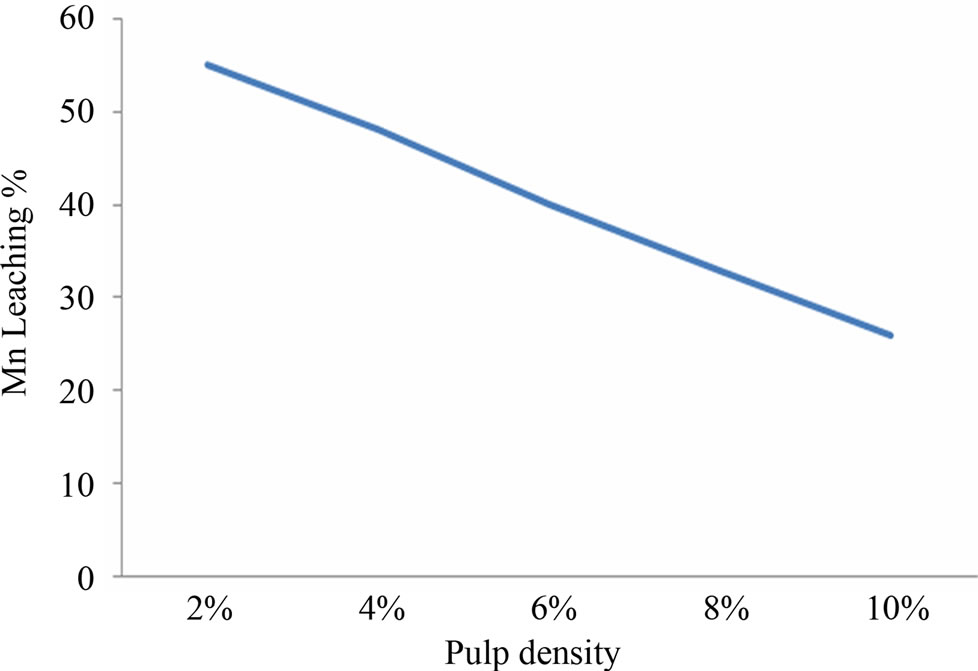
Efficient Mn bioleaching was observed at 5.5 pH and temperature 30 - 35˚C (Figures 1(a) and (b)). Interpretation of the experimental data obtained showed that there was significant rise in the leaching percentage (up to 68%) by the Staphylococcus epidermidis sp. Best manganese leaching solubilisation was observed at –150 μm, it was assumed that manganese extraction yield was maximum (64%) for fine particle size and due to the accessibility of the reaction areas with the particle size to the microbial strain (Figure 2(a)). With different pulp density of manganese ore, the highest dissolved manganese concentration was achieved (44%) with 2% (w/v). The results suggest that with increase in pulp density manganese leaching efficiency decreased which may be due to unavailability of nutritional stress to the microorganism (Figure 2(b)).
 (a)
(a)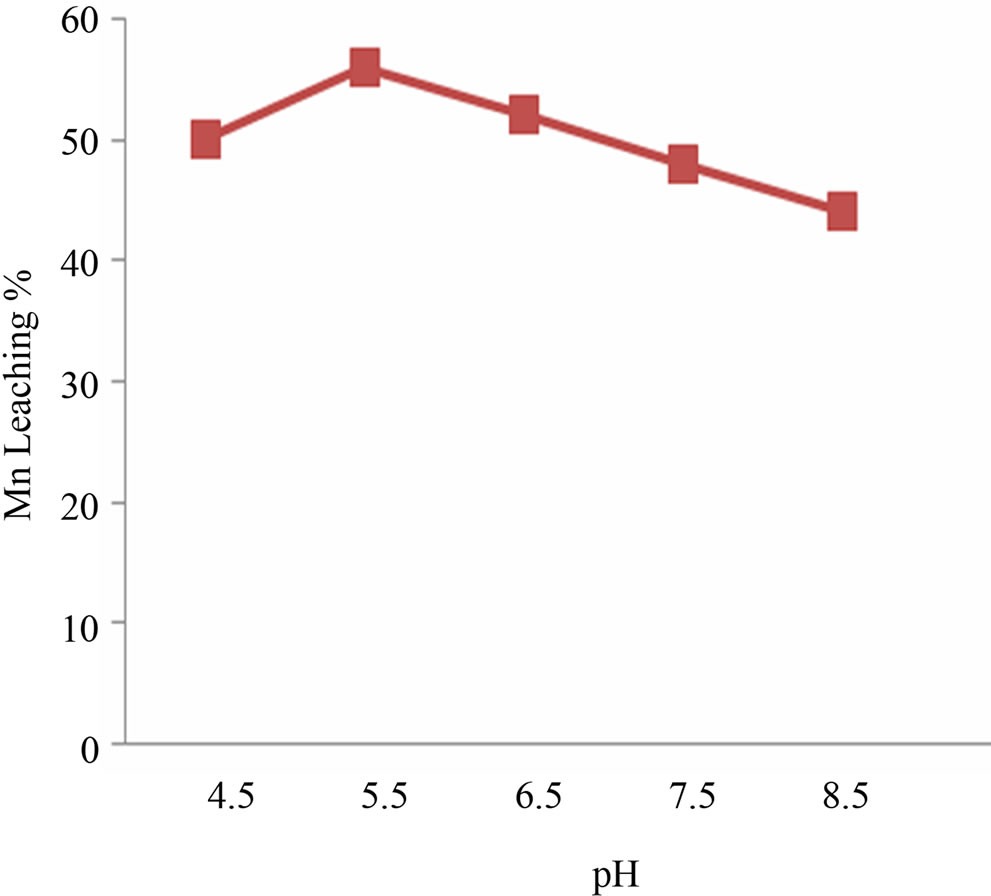 (b)
(b)
Figure 1. (a) and (b) pH and temperature optimization for Mn leaching.
3.3. Mn Bioleaching with Staphylococcus epidermidis (MTCC-435)
Manganese bioleaching efficiency of S. epidermidis was considerably affected by process parameters like initial pH, and temperature. At optimum pH 5.5, particle size –150 μm, pulp density 10%, temperature 35˚C and agitation 200 rpm, about 80 % Mn bioleaching was achieved in 20th day with 10% sucrose as carbon source (Figure 3).
Best Mn bioleaching was evidenced at 5.5 pH. Mn leaching and pH relationship was not surprising because microbial production of organic acid lowers the media pH. Organic acid act as proton source helping metal leaching [7]. For optimum temperature it can be said that bacteria leaches best at its optimum growth temperature which is 30˚C - 37˚C. Higher temperatures (42˚C) significantly reduced bacterial growth and manganese bioleaching due to loss of viability or metabolic activity of cells [8]. Interpretation of the experimental data obtained showed that there was significant rise in the leaching percentage (up to 80%) by the S. epidermidis sp up to 20th day.
4. Conclusion
In this study, the bacterial species S. epidermidis was selected for its potential for manganese bioleaching. S. epidermidis is not usually pathogenic. The maximum recovery efficiency of Mn was 80% by the selected bacterial species at ph 5.5, particle size −150 μm, pulp density 10%, temperature 35˚C and agitation 200 rpm with sucrose as carbon source after 20 day of incubation. The results suggest potential application of this species for environmental clean up from industrial and mining waste and act as an alternative to pyrometallurgical and
 (a)
(a)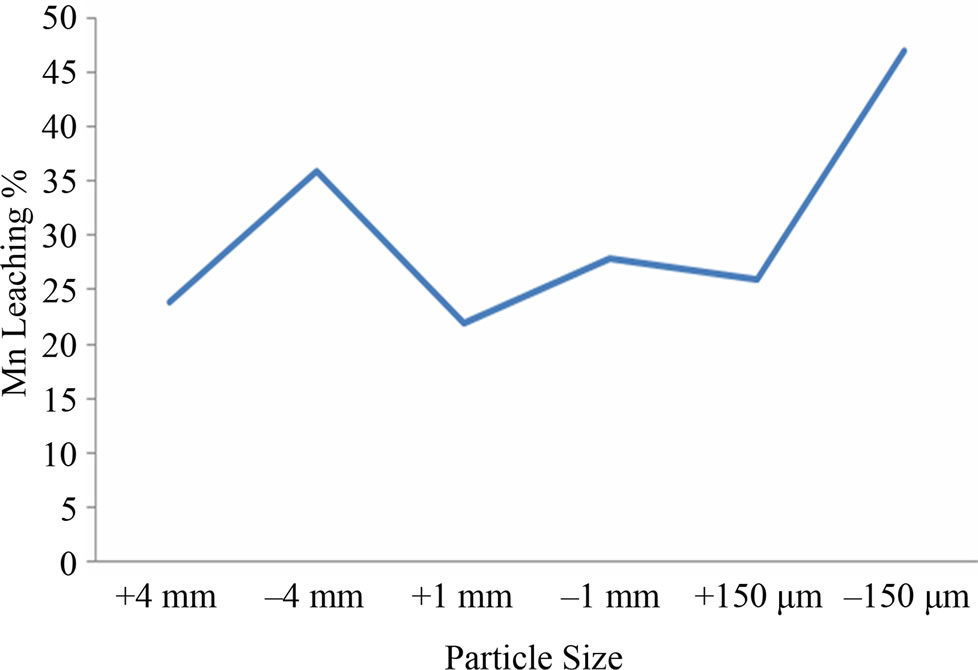 (b)
(b)
Figure 2. (a) and (b) particle size and pulp density optimization for Mn leaching.
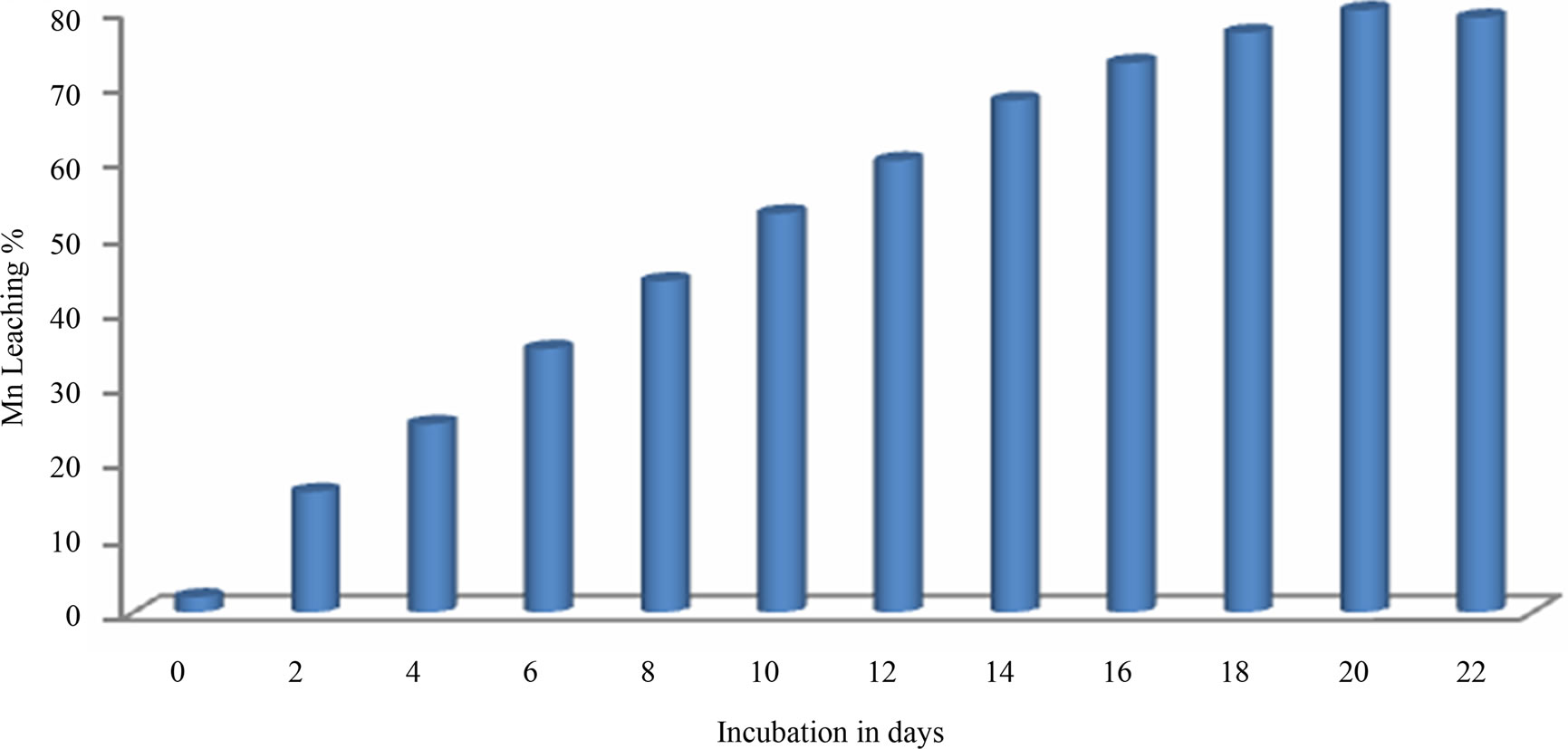
Figure 3. Mn leaching kinetics at initial pH- 5.5 particle size –150 μm, pulp density 10%, temperature 35˚C and agitation 200 rpm.
hydrometallurgical techniques, to recover metals from low grade manganese ore, spent batteries and heavy metal polluted soil, economically.
REFERENCES
- B. P. Xin, D. Zhang, X. Zhang, W. Feng and L. Li, “Bioleaching Mechanism of Co and Li from Spent LithiumIon Battery by the Mixed Culture of Acidophilic Sulfur Oxidizing and Iron-Oxidizing Bacteria,” Bioresource Technology, Vol. 100, No. 24, 2009, pp. 6163-6169. doi:10.1016/j.biortech.2009.06.086
- C. Acharya, R. N. Kar and L. B. Sukla, “Studies on Reaction Mechanism of Bioleaching of Manganese Ore,” Minerals Engineering, Vol. 16, No. 10, 2003, pp. 1027-1030. doi:10.1016/S0892-6875(03)00239-5
- A. P. Das, L. B. Sukla, N. Pradhan and S. Nayak, “Manganese Biomining: A Review,” Bioresource Technology, Vol. 102, No. 16, 2011, pp. 7381-7387. doi:10.1016/j.biortech.2011.05.018
- J. L. Xia, Y. Yang, H. He, X. J. Zhao, C. L. Liang, L. Zheng, C. Y. Ma, Y. D. Zhao, Z. Y. Nie and G. Z. Qiu, “Sulfur Oxidation Activities of Pure and Mixed Thermophiles and Sulfur Speciation in Bioleaching of Chalcopyrite,” Bioresource Technology, Vol. 102, No. 4, 2011, pp. 3877-3882. doi:10.1016/j.biortech.2010.11.090
- H. B. Zhou, W. M. Zeng, Z. F. Yang, Y. J. Xie and G. Z. Qiu, “Bioleaching of Chalcopyrite Concentrate by a Moderately Thermophilic Culture in a Stirred Tank Reactor,” Bioresource Technology, Vol. 100, No. 2, 2009, pp. 515- 520. doi:10.1016/j.biortech.2008.06.033
- K. Bosecker, “Bioleaching: Metal Solubilization by Microorganisms,” FEMS Microbiology Reviews, Vol. 20, No. 3-4, 1997, pp. 591-604. doi:10.1111/j.1574-6976.1997.tb00340.x
- Y. X. Huang, J. B. Cao, X. M. Li, Q. Yang, H. J. Huang, X. Liu and H. Yang, “Study on the Bioleaching Mechanism of Manganse (II) from Manganese-Electrolytic Residue by Manganese-Resistant Strain Fusarium sp.,” Huan Jing Ke Xue, Vol. 32, No. 9, 2011, pp. 2703-2739.
- L. S. Flavio, V. A. Oliveira, D. Guimarães, D. Adelson and A. L. Versiane, “High-Temperature Bioleaching of Nickel Sulfides: Thermodynamic and Kinetic Implications,” Hydrometallurgy, Vol. 105, No. 1-2, 2010, pp. 103- 109. doi:10.1016/j.hydromet.2010.08.006
NOTES
*Corresponding author.

