Crystal Structure Theory and Applications
Vol.05 No.02(2016), Article ID:66239,7 pages
10.4236/csta.2016.52002
Estimation of Nucleation Thermodynamical Parameters of La2Sr2CuO4 (LSCO) and YBa2Cu2O7−δ (YBCO) Crystallizing from High Temperature Solution
Shahida Akhter*, Deba Prasad Paul
Department of Physics, University of Chittagong, Chittagong, Bangladesh

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 March 2016; accepted 2 May 2016; published 5 May 2016
ABSTRACT
The growth kinetics of LSCO and YBCO single crystals from high temperature solution of LSCO-CuO solute-solvent and YBCO-CuO solute-solvent systems has been investigated. Based on regular solution model and classical nucleation theory, the thermodynamical data investigated for the systems are used to determine the nucleation parameters: interfacial free energy, metastable zone-width, volume free energy, critical energy barrier for nucleation and radius of critical nucleus for LSCO and YBCO which leads to the understanding of the nucleation phenomena of LSCO and YBCO.
Keywords:
LSCO, YBCO, Interfacial Energy, Metastable Zone-Width

1. Introduction
The important renewable method of growing single crystals of La2Sr2CuO4 (LSCO) and YBa2Cu2O7−δ (YBCO) is the high temperature solution using LSCO-CuO [1] - [3] and BaO-CuO [4] - [6] as flux respectively. A satisfactory flux should have high solubility for the solvent and an appreciable change of solubility with temperature. The widely used flux is the LSCO-CuO mixed in the ratio 0.23:0.77 for LSCO [7] and BaO-CuO mixed in the ratio 0.28:0.72 for YBCO [8] . The binary phase diagram of the solute-solvent system is very essential to grow LSCO and YBCO crystals from flux technique. The high temperature solution growth techniques require the knowledge of the essential nucleation thermodynamical parameters like interfacial energy, metastable zone- width, volume free energy, critical energy barrier for nucleation and radius of crystal nucleus for the growth of good quality bulk single crystals. Up to now, there have been no reports in the literature regarding these key parameters. Here an attempt has been made to estimate these parameters theoretically to understand the role of these nucleation parameters for the crystallization of LSCO from LSCO-CuO flux and YBCO from BaO-CuO flux. According to the assumption of regular solution model, the nucleation process is considered to be homogeneous in nature and in the nucleation process solid and liquid phases coexist together. In this paper the regular solution model has been adopted to calculate the nucleation parameters of LSCO and YBCO for the first time.
2. Nucleation Thermodynamical Parameters
2.1. Interfacial Energy
The interfacial energy  is the interface between the growing crystal and the surrounding mother phase in important role in the nucleation of crystals. It is well known that for all crystal-solution interfaces, it is difficult to predict theoretically the interfacial energy with sufficient accuracy. Also, it is more difficult to determine
is the interface between the growing crystal and the surrounding mother phase in important role in the nucleation of crystals. It is well known that for all crystal-solution interfaces, it is difficult to predict theoretically the interfacial energy with sufficient accuracy. Also, it is more difficult to determine 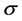 by direct unambiguous experiments from high temperature solution growth. Nielson and Sohnel [9] reported the relationship between interfacial energy
by direct unambiguous experiments from high temperature solution growth. Nielson and Sohnel [9] reported the relationship between interfacial energy  and solubility for the nucleation of electrolyte crystals in aqueous solution. Later, nucleation experiments have been employed to determine
and solubility for the nucleation of electrolyte crystals in aqueous solution. Later, nucleation experiments have been employed to determine  from supersaturated solutions and the relationship between interfacial energy and solubility have been given for low temperature solution growth system by many researchers [10] - [12] . Quit recently, many workers have reported theoretical derivations of more refined expressions for the linear dependence between interfacial energy and solubility [13] . The interfacial energy can be determined experimentally from data on nucleation and growth kinetics, and from contact angle measurements. Consequently, estimation of interfacial energy from physico-chemical data has drawn considerable attention [14] . Bennema and Sohnel [15] based on the regular solution theory have derived an expression for the relationship between the interfacial energy and solubility as
from supersaturated solutions and the relationship between interfacial energy and solubility have been given for low temperature solution growth system by many researchers [10] - [12] . Quit recently, many workers have reported theoretical derivations of more refined expressions for the linear dependence between interfacial energy and solubility [13] . The interfacial energy can be determined experimentally from data on nucleation and growth kinetics, and from contact angle measurements. Consequently, estimation of interfacial energy from physico-chemical data has drawn considerable attention [14] . Bennema and Sohnel [15] based on the regular solution theory have derived an expression for the relationship between the interfacial energy and solubility as
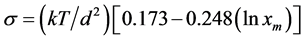 (1)
(1)
where 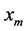 is the mole function of solute; T is the temperature in Kelvin; d is the inter ionic distance or ionic diameter and k is a Boltzmann constant. In the present work this expression is used to calculate the interfacial energy of LSCO crystal from the solubility data of the LSCO-CuO binary phase diagram (Figure 1) and of YBCO crystal of BaO-CuO quasi-binary phase diagram (Figure 2).
is the mole function of solute; T is the temperature in Kelvin; d is the inter ionic distance or ionic diameter and k is a Boltzmann constant. In the present work this expression is used to calculate the interfacial energy of LSCO crystal from the solubility data of the LSCO-CuO binary phase diagram (Figure 1) and of YBCO crystal of BaO-CuO quasi-binary phase diagram (Figure 2).
2.2. Metastable Zone-Width
Crystallization involves two distinct steps: nucleation, which is the birth of a nucleus and crystal growth which
Figure 1. Binary phase diagram of LSCO-CuO.
Figure 2. Quasi-binary phase diagram of YBCO-BaO/CuO.
involves the growth of the existing nucleus. In order to thermodynamically in a non-equilibrium state and is known as metastable state. This state comes to a thermodynamically stable state with small perturbation, which causes the formation of nuclei of a new phase. The formation of such nuclei is limited due to energy barrier are greater than or equal to a critical size. Initial stage of crystallization in supersaturated solution is the formation of nuclei of crystalline phase. Classical homogeneous theories were developed by high and Pound [16] , Nielson [17] and Zettlemoyer [18] describing nucleation as a relaxation process from a metastable state. The relative super saturation S of a solution at a temperature T is
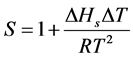 (2)
(2)
where, 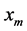 is the heat of solution;
is the heat of solution; 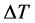 is the super cooling and R is the gas constant.
is the super cooling and R is the gas constant.
The relation between the solubility and the enthalpy of a real solution is given as
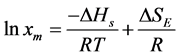 (3)
(3)
where, 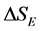 is the excess entropy of mixing.
is the excess entropy of mixing.
From the above equation it is apparent that a plot of 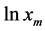 versus 1/T gives a straight line, the slope of which equal to
versus 1/T gives a straight line, the slope of which equal to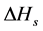 . The value of
. The value of 
2.3. Volume Free Energy
Fluctuations within the supersaturated solution cause the formation of small clusters of molecules, known as embryos. The driving force for the nucleation from the supersaturated solution is

where 
2.4. Critical Energy Barrier
The radius of critical nucleus, and critical energy barrier associated with the critical nucleation for spherical nucleus are related as


3. Results and Discussion
The interfacial energy, metastable zone-width (supercooling temperature), volume free-energy, critical energy barrier and radius of critical nucleus of LSCO and YBCO has been calculated using the different formalism as discussed above and presented in Table 1 and Table 2.
The variation of interfacial energy with temperature is shown in Figure 3 for LSCO. From Figure 3, it can be observed that the interfacial energy increases, with the decrease of temperature of the solution and the trend exhibited with the experimental observation of high temperature solution growth of KTP crystals crystallizing from K6P4O13 flux [19] and in the NdBa2Cu3O7−d (Nd123) system [20] , the strong anistropy in the interfacial energy may be due to the inherent property of LSCO in LSCO-CuO solute- solvent system. The variation of interfacial energy with temperature exhibits similar trend in case of YBCO in BaO-CuO solute-solvent system as shown in Table 2 which is also similar the experimental observation of high temperature solution growth [21] [22] .
The calculated value of metastable zone-width for LSCO at different temperatures is shown in Table 1 and for YBCO at different temperature is shown in Figure 4. From Table 1 for LSCO and Figure 4 for YBCO, it can be concluded that the metastable zone-width is narrow at higher temperature and wide at lower temperature of the solute in the solution. This is very much conformable with the expected prediction. The degree of probability of nucleation depends on the intermolecular distances of the solute particles in the solution and therefore on its concentration. In the literature there is no specific value of metastable zone-width and crystallization tem-
Table 1. Nucleation Thermodynamical parameters of LSCO.
Table 2. Nucleation Thermodynamical parameters of YBCO.
Figure 3. Variation of interfacial energy of LSCO with temperature.
Figure 4. Variation of metastable zone-width of YBCO with temperature.
perature. Different laboratories reported different values of metastable zone-width and crystallizing temperature ranging from 1550 - 1350 K [21] [23] - [25] which is comparable to theoretically calculated values.
The calculated value of metastable zone-width for LSCO at different mole-fraction is shown in Figure 5. From Figure 5, it is concluded that metastable zone-width is wide at low concentrations i.e. at low temperature, and narrow at high concentration i.e. at higher temperature of the solute in the solution. The variation of interfacial energy with mole fraction is shown in Table 1 and Table 2 for LSCO and YBCO. It is observed that the interfacial energy increases with the decrease of mole fraction of the solution, which may be due to the inherent property of the flux and trend exhibited in the graph is similar to the experimental observation of high temperature solution growth [22] .
The change in free energy on the formation of a single YBCO molecule at equilibrium has been calculated using the available thermodynamical data. Using the regular solution model the thermodynamical potential of the components associated with the reaction has been calculated. The critical energy, 
For the better understanding of the growth kinetics of LSCO and YBCO crystals, the other nucleation thermodynamical parameters of LSCO and YBCO such as volume free-energy, critical energy, radius of critical energy, energy of formation in the nucleus are calculated for various values of interfacial tension is given in Table 1 and Table 2. The present work provides a comprehensive picture of LSCO crystal from crystallizing LSCO-CuO flux and YBCO crystal from crystallizing BaO-CuO flux. The uncertainty in determining the above nucleation parameters of LSCO and YBCO crystals from high temperature solution growth by experimental method creates interest in determining the above parameters.
Figure 5. Variation of metastable zone-width of LSCO mole fraction (xm).
Figure 6. Variation of critical energy of YBCO with with temperature.
4. Conclusion
The nucleation parameters of LSCO crystal growth from the LSCO-CuO flux and YBCO from BaO-CuO flux at different crystallization temperatures have been calculated. As there are no reports in the literature for the nucleation thermodynamical perameters of LSCO and YBCO crystals, the present theoretically estimated values will be useful for the growth of large size good quality single crystals of LSCO and YBCO.
Cite this paper
Shahida Akhter,Deba Prasad Paul, (2016) Estimation of Nucleation Thermodynamical Parameters of La2Sr2xCuO4 (LSCO) and YBa2Cu2O7–δ (YBCO) Crystallizing from High Temperature Solution. Crystal Structure Theory and Applications,05,17-23. doi: 10.4236/csta.2016.52002
References
- 1. Inoue, T., Hayashi, S., Komatsu, H. and Shimuzu, M. (1987) Growth of (La1–xSrx)2CuO4–δ Crystals from High Temperature Solution. Japanese Journal of Applied Physics, 26, L732.
http://dx.doi.org/10.1143/JJAP.26.L732 - 2. Hidaka, Y., Enomoto, Y., Suzuki, M., Oda, M. and Murakaru, T. (1987) Anisotropie Properties of Superconducting Single-Crystal (La1–xSrx)2CuO4. Japanese Journal of Applied Physics, 26, L377.
http://dx.doi.org/10.1143/JJAP.26.L377 - 3. Chen, C., Watts, B.E., Wanklyn, B.M. and Thomas, P. (1988) The Flux Growth of Crystals of (La,Sr)2CuO4 and (La,Sr)CuO3. Solid State Communications, 66, 611-612.
http://dx.doi.org/10.1016/0038-1098(88)90218-9 - 4. Hidaka, Y., Enomoto, Y., Suzuki, M., Oda, M. and Murakaru, T. (1987) Single Crystal Growth of Lanthanum Alkaline Earth Copper Oxide ((La1–xAx)2CuO4 (A = Barium or Strontium)) and Barium Yttrium Copper Oxide (Ba2YCu3O7–y). Journal of Crystal Growth, 85, 581-584.
http://dx.doi.org/10.1016/0022-0248(87)90025-X - 5. Hayashi, S., Ohno, T., Inoue, T. and Komatsu, H. (1988) Growth of ErBa2Cu3O7–δ Single Crystals from High Temperature Solution. Journal of Crystal Growth, 91, 331-333.
http://dx.doi.org/10.1016/0022-0248(88)90246-1 - 6. Assmus, W. and Schmidbauer, W. (1993) Crystal Growth of HTSC Materials. Superconductor Science and Technology, 6, 555.
http://dx.doi.org/10.1088/0953-2048/6/8/001 - 7. Chen, C. (1992) Progress in Crystal Growth and Characterization. Vol. 24, 244.
- 8. Dembinski, K., Gervais, M., Odier, P. and Coutures, J.P. (1990) A Non-Polluting Single Crystal Growth Process for YBaCuO and Phase Diagram Studies. Less-Common Metals, 164-165, 177-186.
http://dx.doi.org/10.1016/0022-5088(90)90212-3 - 9. Nielson, A.E. and Sohnel, O. (1971) Interfacial Tensions Electrolyte Crystal-Aqueous Solution from Nucleation Data. Journal of Crystal Growth, 11, 233-242.
http://dx.doi.org/10.1016/0022-0248(71)90090-X - 10. Bennema, P. and Gilmer, G.H. (1993) An Introduction in Crystal Growth. Hartman, P. Ed. (North Holland, Amsterdam, p. 263.
- 11. Sangwal, K. (1989) On the Estimation of Surface Entropy Factor, Interfacial Tension, Dissolution Enthalpy and Metastable Zone-Width for Substances Crystallizing from Solution. Journal of Crystal Growth, 97, 393-405.
http://dx.doi.org/10.1016/0022-0248(89)90221-2 - 12. Sohnel, O. (1982) Electrolyte Crystal-Aqueous Solution Interfacial Tensions from Crystallization Data. Journal of Crystal Growth, 57, 101-108.
http://dx.doi.org/10.1016/0022-0248(82)90254-8 - 13. Mernsmann, A. (1990) Calculation of Interfacial Tensions. Journal of Crystal Growth, 102, 841-847.
http://dx.doi.org/10.1016/0022-0248(90)90850-K - 14. Bennema, P. and Vander Erden, J.P. (1987) Morphology of Crystals. Chap. I, In: Sanagawa, I., Ed., Tokyo, 1-75.
- 15. Bennema, P. and Sohnel, O. (1990) Interfacial Surface Tension for Crystallization and Precipitation from Aqueous Solutions. Journal of Crystal Growth, 102, 547-556.
http://dx.doi.org/10.1016/0022-0248(90)90412-E - 16. Hirth, J.P. and Pound, G.M. (1963) Condensation and Evaporation, Nucleation and Growth Kinetics. Pergamon Press, Oxford.
- 17. Nielson, A.E. (1964) Kinetics and Precipitation. Pergamon Press, New York.
- 18. Zettlemoyer, A.C. (1969) Nucleation. Marcel Dekker Inc., New York.
- 19. Kaweit, M. (1961) über die Kinetik der Phasenbildung in kondensierten Systemen. Zeitschrift für Physikalische Chemie, 28, 245-249.
http://dx.doi.org/10.1524/zpch.1961.28.3_4.245 - 20. Paul, D.P., Jayavel, R. and Subramanian, C. (1999) Investigations on Nucleation Thermodynamical Parameters of NdBa2Cu3O7–δ (Nd123) Crystallizing from High Temperature Solution. Materials Chemistry and Physics, 59, 175-178.
http://dx.doi.org/10.1016/S0254-0584(99)00040-1 - 21. Joseph Kumur, F., Ganesa Moorthy, S., Jayaraman, D. and Subramanian, C.J. (1996) Estimation of Metastable Zone Width, Interfacial Energy and Growth Rates of KTiOPO4 Crystallizing from K6P4O13 Flux by Hot Stage Microscopy. Journal of Crystal Growth, 160, 129-135.
http://dx.doi.org/10.1016/0022-0248(95)00443-2 - 22. Shahida, A. and Deba, P.P. (2004) Estimation of Nucleation Thermodynamical Parameters of La2Sr2CuO4 (LSCO) Crystallizing from High Temperature Solution. Journal of Materials Chemistry and Physics, 88, 41-45.
http://dx.doi.org/10.1016/j.matchemphys.2004.05.047 - 23. Oka, K., Saito, M., Ito, M., Nakane, K., Murata, K., Nshihara, Y. and Unoki, H. (1989) Phase Diagram and Crystal Growth of NdBa2Cu3O7–y. Japanese Journal of Applied Physics, 28, L219-L221.
http://dx.doi.org/10.1143/JJAP.28.L219 - 24. Kumar, J., Thirumavalavan, M., Ramasamy, P., Gnanam, F.D. and Ramasamy, P. (1986) Thermodynamics and Nucleation Behaviour in the System Y2O3-Al2O3-Y3Al5O12. Journal of Physics D: Applied Physics, 19, 1223-1232.
http://dx.doi.org/10.1088/0022-3727/19/7/012 - 25. O’Bryan, H.M. and Connor Jr., P.B. (1996) Growth of Rare-Earth Aluminum Garnets by Floating Zone Technique. American Ceramic Society Bulletin, 45, 578-581.
NOTES
*Corresponding author.









