Open Journal of Synthesis Theory and Applications
Vol.05 No.02(2016), Article ID:72357,9 pages
10.4236/ojsta.2016.52002
Thermal Stability and Crystallinity Study of Polystyrene/SiO2 Nano-Composites Synthesis via Microwave-Assisted In Situ Polymerization
Nikesh Samarth1*, Linchon Mehta1, Vinayak Kamble1, Malhari Kulkarni2, Prakash Mahanwar1
1Department of Polymer and Surface Engineering, Nathalal Parekh Marg, Mumbai, India
2Polymer Engineering, Maharashtra Institute of Technology, Pune, India

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 7, 2016; Accepted: April 14, 2016; Published: April 29, 2016
ABSTRACT
Serials of polystyrene/SiO2 Nano composites (PS/SiO2) with different content of inorganic fillers were successfully prepared by the in situ bulk radical polymerization of styrene under microwave irradiation. The effect of the amount of Nano SiO2 on the properties of the PS/SiO2 Nanocomposites along with the average relative molecular masses (Mn, Mz and Mw) was investigated by thermal analysis and X-Ray Diffraction (XRD). Their structural model was proposed on the basis of the Optical Microscopy, FTIR (Fourier Transform Infrared) analysis, differential scanning calorimetry (DSC), gel permeation chromatography (GPC) and X-Ray Diffraction (XRD). The dispersion of nanoparticles in Polystyrene is observed in the magnified image. The effect of microwave irradiation power on molecular weight of polystyrene was also studied. It was found that, the microwave assisted reaction needs less time as compare to conventional polymerization and found to be in between 10 to 15 min.
Keywords:
Nanocomposites, In-Situ Polymerization, Bulk Polymerization, Nano SiO2

1. Introduction
The use of microwave irradiation in synthesis of organic compounds has become increasingly popular within the pharmaceutical and academic areas, because it is a new emerging technology for enhanced synthesis reactions and development [1] . Presently, temperature driven organic reactions take place by either of two ways: Conventional heating or microwave-accelerated heating. In the first type, all the reactants are slowly activated by a conventional external heat source through which heat is driven into the substance passing via walls and ultimately to the solvent and reactants. This is a slow and inefficient method for transferring heat into the reacting system. In the second type, microwaves couple directly with the molecules of the entire reaction mixture which leads to rapid rise in the reaction temperature. Since this type of heating is independent of material conductivity instantaneous localized heating can be achieved. It is considered as the new synthetic route for polymerization of monomers [2] [3] .
Before discussing the synthesis route and properties of nano-composites one has to take into account the effect of small size of nano-particles. Nano-composites are new class of composites derived from the ultrafine inorganic particles having dimensions typically in the range of few micrometers that are dispersed in the polymer matrix homogeneously [4] . Due to outstanding properties, such kinds of materials are seeking the attention of academic and industrial researchers. These materials advantages of both polymers and ceramics or glasses having combined properties such as flexibility, toughness, ease processing, hardness, durability and thermal stability [5] .
Organic/inorganic based composites have gained considerable attention and are been significantly studied over a long time. Among the inorganic materials, SiO2 is viewed as being very important among both biological and synthetic materials. The silica has extremely large specific surface area, which enhances the connection between the filler and the polymer matrix [6] . It is well known that the thermal stabilities of the polymeric materials could be improved by addition of inorganic nano-fillers [7] . However, the inorganic nano-fillers have poor dispersibility in the polymer matrix because their surface properties. So, the newer methods are applied for improved dispersion of such inorganic fillers.
To achieve uniform properties in one step process with minimum possible impurities and defects, in situ reactions are being used more to produce different polymers. Free- radical polymerization being the most important technique in the industry is largely focused to be made via in situ reactions [8] . For example, different polymerization reactions such as free-radical polymerization of methyl methaacrylate with benzoyl peroxide as initiator [1] , copolymerization of styrene and methyl methaacrylate with dibenzoyl peroxide as initiator [9] , preparation of nano-composites of polystyrene/silica [10] [11] and composites of polystyrene/flyash [12] have been studied under in situ reactions.
In this work we report a single step process for the synthesis of nano-composites SiO2/PS through in situ polymerization of different concentrations of SiO2 in polystyrene. The nanostructure, composition and molecular weight were also evaluated.
2. Experimental
2.1. Material
Styrene (Commercials Grade) was supplied by local supplier, India used as the monomer. Nano SiO2 having size range 1 - 2 nm was procured from local supplier. AIBN (α,α’-Azobisisobutyronitrile) having M.W: 164.21 and 99% assay was purchased from SAS Chemical, Mumbai. Toluene and methanol was purchased from S.D Fine chemicals, Mumbai.
2.2. Method-Polymerization of Styrene with SiO2 Microwave Irradiation
2.2.1. Microwave Reactor Details
The Microwave reactor used in the present work was MAS-II, SINEO microwave chemistry technology, china as shown in Figure 1. Dynamically adjustable Microwave power between 0 - 1000 W to generate non-pulse and continuous microwave heating based on the temperature of the reaction mixture. The other configuration of Micro- wave Reactor was IR temperature sensor which supports internal and surface tem- perature monitoring of reactant. High precision IR temperature sensor provides high sensitivity and no time lags, generating a safe and convenient operating platform for microwave organic synthesis application.
2.2.2. Microwave Heating Reaction Procedure
Bulk polymerisation was carried out in closed borosilicate reaction vessel (250 mL capacity) in a microwave oven capable of magnetic stirring and heating according to the temperature settings. The MW power input was optimized by varying the power from 50 - 400 W in order to study the effect of MW power on conversion of reaction. However the temperature was checked from time to time to ensure it has not exceeded its range. Hence, an Infrared thermometer was used to check the temperature of the reaction mixture accurately. Power settings were based on adjustments according to temperature of reaction needed. The mixture was kept for 10 - 15 minute cycles [13] [14] [15] . Figure 2 shows the entire scheme of the process carried out.
The optimal condition for Polymerization of styrene was achieved at a reaction time of 10 to 15 min and temperature of 90˚C with AIBN as a catalyst. The following MW
Figure 1. Microwave reactor for Chemical synthesis (MAS II Sineo microwave Technology Pvt. Ltd.).
Figure 2. Schematic diagram of whole synthesis process of Microwave assisted PS.
power were used; 400, 600 keeping other parameters constant (mole ratio, temperature, rpm, time). Before the starting of reaction the nano SiO2 was mixed in liquid styrene monomer in order to ensure better dispersion followed by the addition of initiator. The photograph of the nano-composite prepared is shown in the Figure 3. Effect of increasing the power on reaction conversion will be examined. The MW power giving maximum conversion in minimum time will be finalized.
2.3. Testing and Characterization
2.3.1. Fourier Transform Infra-Red Spectroscopy Analysis (FTIR)
The synthesized samples along with raw material were analyzed using a Bruker ALPHA (Eco-ATR) FTIR spectrometer. The transmission mode was used to obtain the IR of the samples. The scan range used was 4500 cm−1 to 600 cm−1. The FTIR of MWPS@400W 0% SiO2 was done.
2.3.2. Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (Q 100 DSC, TA instruments Ltd., India) characterization was done in the temperature range of −30˚C to 200˚C as per the ASTM E 2161-08 Standard Terminology Relating to Performance Validation in Thermal Analysis to study the crystallization and melting behavior of the composite [16] . The samples were first heated from −30˚C to 200˚C to remove the residual thermal stress in the samples and then held at 200˚C for 3 min prior to cooling to room temperature followed by further heating (second heating cycle) till 200˚C. Scanning rate was maintained constant at 10˚C/min for both heating and cooling cycle with nitrogen gas purge rate at 50 ml/min. Crystallization temperature (Tc) was determined from cooling scan while melting temperature from the second heating scan.
2.3.3. X-Ray Diffraction
The XRD analysis was accomplished to find out the percentage crystallinity and
Figure 3. Photographs of the nano-composite prepared.
orientation in the prepared composite. The A normal focus copper X-ray tube was operated at 30 kV and 15 mA. Sample scanning was done from 2˚ to 80˚ at the rate of 3˚/min. The crystallinity was estimated by applying the peak-area integration method on the data generated by Jade 6.0 software.
2.3.4. Gel Permeation Chromatography
GPC was used for determination of molecular weight and molecular weight distribution. The molecular weight was obtained by running 0.8 weight % polymer in tetrahydrofuran (THF) at 30˚C. THF was used as the eluent phase. Using narrow distribution standards the elution volumes were converted to apparent molecular weights. Agilent 1260 Infinity GPC/SEC System was for determination of Molecular weight.
3. Result and Discussion
The effects of the microwave radiation and different loading of Nano SiO2 were investigated and the results were shown below. The percent conversion of unfilled styrene is near to 60% when the microwave powers used were higher than 400 W. As the bulk polymerization of polystyrene having an exothermic reaction in order to avoid the overshoot of temperature, 400 W was selected the optimum power for further reaction. The effects of the amount of Nano SiO2 added were investigated in the range of 1% to 7% at the interval of 2. It was found that the carbon content (%) of styrene was around 100% when AIBN was added higher than 12 mg. The percent conversion achieved its maximum of 60% when a 1% AIBN was added. Thus it can be said that with higher amount of initiator higher % conversion could be achieved. However, this also induces that lower molecular weight polymers are formed.
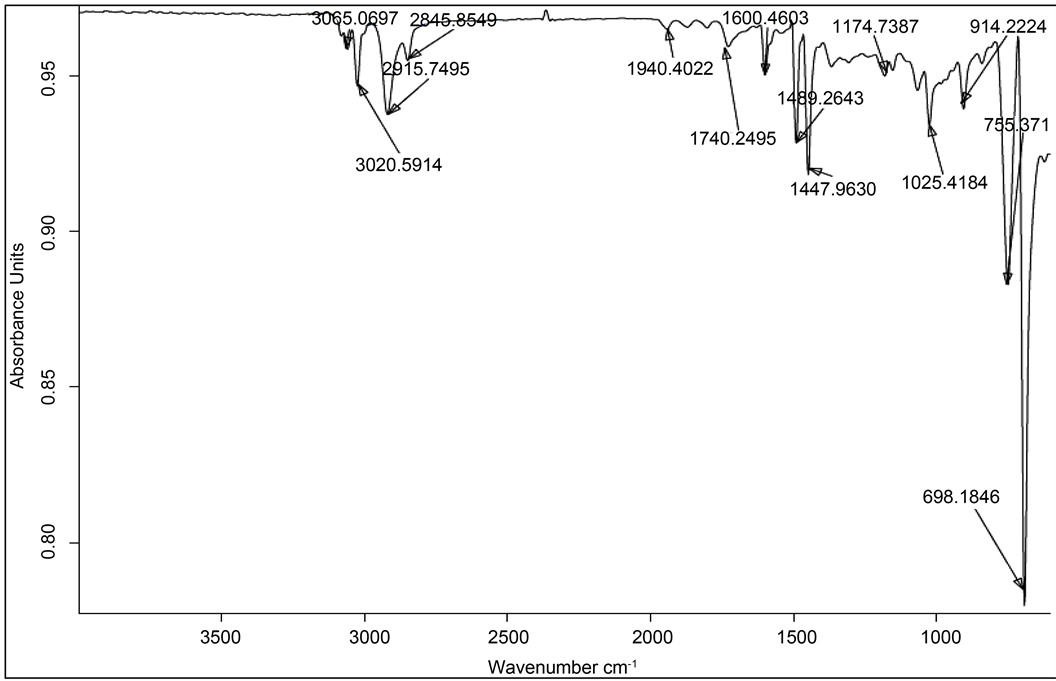
The procedure used in present work is much simpler than the anionic polymerization technique. The resulting product of the free-radical polymerization of styrene under microwave irradiation could be used as nano-composites directly because of the higher conversion of styrene in spite of such a short polymerizing time. The vibration bands corresponding to polystyrene (aromatic C-H stretching vibrations in polystyrene at 2915, 3020, 3086, 755 and 698 cm−1, the aliphatic C-H stretch at 2915 and 2845 cm−1 and C-C vibrations in styrene at 1447 and 1489 cm−1) were all found in the FTIR spectrum of the resulting MWPS (Figure 2). It showed that the polystyrene had been grafted onto the surfaces of the silica nano-particles successfully which could also improve the dispersibility of silica nano-particles in polystyrene or organic solvent such as toluene (Figure 4).
Figure 4. FTIR Spectra of Microwave assisted Polystyrene at 400 W (Unfilled PS).
The XRD patterns of PS/SiO2 composite with different percentage of SiO2 loading shown in Figure 5. It can be observed that the pattern of all PS/SiO2 with different SiO2 loading are completely similar and have the same peaks because the SiO2 peak has been overlapped by PS peak. According to this figure, they have only different peak intensities. Existence of silica nano-particles in composite structure is verified by decreasing the intensity of peaks in PS/SiO2 pattern. As shown in Figure 5, the intensity of the highest peak in neat PS pattern has decreased from 2700 to 1500 in PS/ SiO2 composite.
The microstructures of the nano SiO2 in the PS/SiO2 nano-composite are shown in the Figure 6. It can be observed that all the nano-fillers are dispersed in the composite even if some aggregates can be noticed. Further, it can be considered that nano-struc- tures are achieved.
The molecular weight distribution of Polystyrene synthesis via microwave is shown in Table 1. The weight average molecular weight is 80,393 and poly dispersity index is 2341 obtained. The thermal stability properties of the PS/SNs nano-composites were characterized by the differential scanning calorimetry and the results were shown in Table 2. The glass transition temperatures (Tg) of the resulting product of the polymerization of styrene were the lowest. While, the glass transition temperatures of the PS/SNs nano-composites increased with the increasing of the amounts of the Nano
Figure 5. XRD of microwave assisted polystyrene@400W with different loading of Nano SiO2.
Figure 6. Optical micrographs of microwave assisted polystyrene.
Table 1. Molecular weight distribution of Polystyrene synthesized by Microwave.
Table 2. DSC of microwave assisted nano silica filled polystyrene (MWPS) @400 W.
SiO2 in the experimental range. It showed that the thermal stability of the nano-com- posites with chemical bonds between the polymeric matrices and the nano-fillers are better than the pure polymeric materials and those without chemical bonds. Hence it can be concluded that the interface properties had been improved by the microwave assisted reaction.
4. Conclusion
Controlled Microwave irradiations have significant effects in the polystyrene synthesis under solvent-free condition. Irradiating at intense microwave powers namely, higher than 500 W, causes increase in temperature leading to significant decrement in the product yields. Reaction products are easy to use for further application and the method is simple, convenient and eco-friendly. The effect of Nano SiO2 loading was variied and catayst, time, tempreture was kept constant. The effect of Nano SiO2 on reaction was also carried out.In the Polystyrene/Nano SiO2 Nancomposite preparation, the higher Tg was observed for 3% loading of SiO2. Also the percent crystallinity goes decreasing with higher loading of Nano SiO2. The percent conversion of 60% was obtained at 1% loading of AIBN in 15 min at 90˚C. Hence it can be said that higher conversions can be achieved by the proposed method as the proposed method was simpler and more convenient than the other reported methods.
Cite this paper
Samarth, N., Mehta, L., Kamble, V., Kulkarni, M. and Mahanwar, P. (2016) Thermal Stability and Crystallinity Study of Polystyrene/SiO2 Nano-Composites Synthesis via Microwave-Assisted In Situ Polymerization. Open Journal of Synthesis Theory and Applications, 5, 15-23. http://dx.doi.org/10.4236/ojsta.2016.52002
References
- 1. Sinnwell, S. and Ritter, H. (2007) Recent Advances in Microwave-Assisted Polymer Synthesis. Australian Journal of Chemistry, 60, 729-743.
https://doi.org/10.1071/CH07219 - 2. Hayes, B. (2004) Recent Advances in Microwave-Assisted Synthesis. Aldrichimica Acta, 37, 66-76.
- 3. Veldhuis, S. (2011) Use of Microwave Technology in Materials Science, Synthesis, Sintering and “MW-Effect”. Journal Club, IMS Group, University of Twente.
- 4. Hanemann, T. and Szabó, D.V. (2010) Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials, 3, 3468-3517.
- 5. Yu, Y.Y. and Chen, P.K. (2013) Nanocomposites of Polymer and Inorganic Nanoparticles Prepared by Focused Microwave Polymerization for Optical Thin Films Applications. Thin Solid Films, 544, 48-53.
https://doi.org/10.1016/j.tsf.2013.03.140 - 6. Muller, D., Pinheiro, G.K., Bendo, T., Gutiérrez Aguayo, A.J., Barra, G.M.O. and Rambo, C.R. (2015) Synthesis of Conductive PPy/SiO 2 Aerogels Nanocomposites by In Situ Polymerization of Pyrrole. Journal of Nanomaterials, 2015, 1-6.
https://doi.org/10.1155/2015/658476 - 7. Miller, A.C. and Berg, J.C. (2003) Unexpected Behavior between Polystyrene and Untreated and Silane-Treated Glass Beads in Filled Polymeric Composites. Journal of Applied Polymer Science, 89, 521-526.
https://doi.org/10.1002/app.12303 - 8. Zhu, Y.-J. and Chen, F. (2014) Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chemical Reviews, 114, 6462–6555.
- 9. Stange, H., Ishaque, M., Niessner, N., Pepers, M. and Greiner, A. (2006) Microwave-Assisted Free Radical Polymerizations and Copolymerizations of Styrene and Methyl Methacrylate. Macromolecular Rapid Communications, 27, 156-161.
https://doi.org/10.1002/marc.200500640 - 10. Liu, P., Tian, J., Liu, W. and Xue, Q. (2003) Surface Graft Polymerization of Styrene onto Nano-Sized Silica with a One-Pot Method. Polymer Journal, 35, 379-383.
https://doi.org/10.1295/polymj.35.379 - 11. Liu, P. and Su, Z. (2005) Thermal Stabilities of Polystyrene/Silica Hybrid Nanocomposites via Microwave-Assisted In Situ Polymerization. Materials Chemistry and Physics, 94, 412-416.
https://doi.org/10.1016/j.matchemphys.2005.05.023 - 12. Liu, P. and Zhong, W. (2014) Novel Magnetic Crosslinked Composites with Fly Ash as Filler via Facile “One-Pot” In-Situ Radical Bulk Polymerization. Journal of the Taiwan Institute of Chemical Engineers, 45, 1098-1104.
https://doi.org/10.1016/j.jtice.2013.09.029 - 13. Fang, L.J., Han, G. and Zhang, H.Q. (2015) Microwave-Assisted Free Radical Polymerizations, Microwave-Assisted Polymer Synthesis, Volume 274 of the Series Advances in Polymer Science, 87-129.
- 14. Bogdal, D., Bednarz, S. and Matras-Postolek, K. (2015) Microwave-Assisted Synthesis of Hybrid Polymer Materials and Composites, Microwave-Assisted Polymer Synthesis Volume 274 of the Series Advances in Polymer Science, 241-294.
- 15. Ergan, B.T., Bayramoglu, M. and Ozcan, S. (2015) Emulsion Polymerization of Styrene Under Continuous Microwave Irradiation. European Polymer Journal, 69, 374-384.
https://doi.org/10.1016/j.eurpolymj.2015.06.021 - 16. Blaine, R. (2010) Determination of Polymer Crystallinity by DSC. Therm. Anal. TA Instruments, 109 Lukens Drive, New Castle DE 19720, USA, 1-3.