Open Journal of Synthesis Theory and Applications
Vol.2 No.1(2013), Article ID:27192,7 pages DOI:10.4236/ojsta.2013.21001
Intercalation of Amines into Layered Calcium Phosphate and Their New Behavior for Copper Retention from Ethanolic Solution
Departamento de Química, Universidade Estadual de Maringá, Maringá, Brazil
Email: *amlazarin2@uem.br
Received November 10, 2012; revised December 13, 2012; accepted December 22, 2012
Keywords: Layered Compounds; Intercalation Reactions; Adsorption
ABSTRACT
Layered calcium phosphate was intercalated using m-aminobenzoic acid and p-aminobenzoic acid, originating materials named CaP/MABA and CaP/PABA. The products were characterized by elemental analysis, infrared spectroscopy, X-ray diffraction, thermogravimetry and scanning electron microscopy. The amount of attached amino groups surfaces were 14.78 and 7.58 mmol∙g−1 for CaP/MABA and CaP/PABA, respectively. Suspended intercalated phosphate adsorbed copper cation from ethanolic solution at liquid/solid interface.
1. Introduction
Organization of organic species on the surface of inorganic solids is a way to prepare inorganic-organic hybrids [1]. Materials design from layered materials through soft-chemical routes is a promising strategy, since the resulting layered inorganic-organic hybrids may have unique nanostructures controlled by host-guest interactions. A wide variety of layered inorganic-organic hybrids have been prepared by intercalation reactions and their properties and possible applications have been investigated [2].
There is considerable interest in the synthesis of new layered metal phosphonate salts due to their robust and yet flexible structural properties. This class of layered inorganic-organic hybrid compounds presents an inorganic backbone with pendant organic groups on both sides of the central layer. This kind of structural arrangement was first synthesized with tetravalent metals and more recently with a series of divalent cations. Polymeric units, containing the metallic cations between phosphate groups, form the overlapping lamella. Application of these materials includes catalysis, sorption and ion exchange, as well as uses in intercalation chemistry [1-8].
Phosphates are characterized by homogeneous properties, with the advantage in forming many minerals and also biocompounds. This versatility stems tetrahedral phosphate units, whose monomer with great reactivity can condense as oligomers and polymers by forming open or close chains and by enclosing a great variety of gel, crystalline or vitreous solids, in addition to microbial constituents [9].
The present investigation deals with the synthesis, characterization, and adsorption properties of layered calcium phosphate (CaP) intercalated with m-aminobenzoic acid (MABA) and p-aminobenzoic acid (PABA). As the guest molecule contains nitrogen and oxygen atoms from amine and carboxylic groups, these basic centers are potentially useful for cation coordination in ethanol solution, an aspect that is also explored in this study. Layered calcium phosphate intercalated with amines aims to find an efficient material for determination of the metal ions present in ethanol used as fuel for car engines.
2. Experimental
2.1. Preparation Calcium Phosphate (CaP)
Layered CaP was synthesized as described elsewhere [10]. Calcium phosphate was synthesized by slowly adding a dilute solution of calcium chloride dihydrated to a 1.50 mol∙dm−3 dibasic ammonium phosphate solution. The mixture was heated to 360 K. The suspension formed was stirred for 1 h, when the solid started to settle out. This was filtered and dried at 320 K. Finally, the resulting compound was heated at 440 K for 48 hs to eliminate ammonia. The reactions are given by Equations (1) and (2).
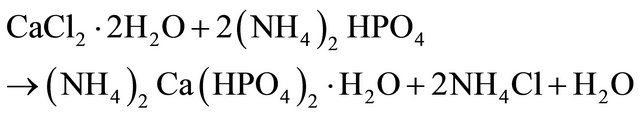 (1)
(1)
 (2)
(2)
2.2. Intercalation Procedure
About 50 mg of CaP was immersed in 20.0 cm3 of 1.0 × 10−3 mol·dm−3 of amino acids (3-amnobenzoic acid or 4- aminobenzoic acid) in aqueous solution, respectively; the suspension was shaken in an orbital mechanical stirrer for 1 h. This time was determined for a constant concentration of amino acids after varying the solid/solution ratios and contact time up to 12 hs in order to obtain the best intercalation condition for this guest molecule. The resulting solid was filtered, washed with doubly-distilled water, and dried at room temperature. Two kinds of host-guest compounds were obtained and abbreviated as CaP/MABA and CaP/PABA, respectively.
2.3. Characterization
Calcium and phosphorus [11,12] elemental analyses were performed by atomic absorption spectroscopy using a Perkin Elmer atomic absorption spectrometer, model 5100, and spectrophotometric methods using a Shimadzu spectrophotometer, model MultiSpec-1501.
The amount of m-aminobenzoic acid and p-aminobenzoic acid intercalated into calcium phosphate was determined by nitrogen elemental analysis on a PerkinElmer Analyzer 2400 series H CHNS/O apparatus.
X-ray diffraction patterns were obtained with nickelfiltered CuKa (0.154 nm) radiation in the interval of 2˚ to 65˚ at a speed of 0.033˚ s−1 and a step of 0.050˚ on a Shimadzu XD3-A diffractometer (30/20 kV/mA).
Infrared spectra of the samples were acquired on a Perkin-Elmer FTIR spectrophotometer, model 1600, by using pressed KBr pellets in the 4000 - 400 cm−1 range with 4 cm−1 of resolution.
Thermogravimetric curves were recorded using a DuPont model 1090 B apparatus coupled to a model 951 thermobalance, by heating samples from room temperature to 1273 K at a heating rate of 0.16 K∙s−1 in an argon flow of 1.67 cm3∙s−1. The samples varied in weight from 15.0 to 30.0 mg.
Scanning electron microscopy (SEM) images were obtained for samples dispersed on a double-faced conducting tape adhered to an aluminum support. The samples were coated with gold using a Balzer MED 020 lowvoltage sputtering apparatus. The measurements were carried out on a JEOL JSM-T300 scanning electron microscope.
Copper (II) concentration in the supernatant solutions was determined by atomic absorption spectroscopy using a Perkin-Elmer atomic spectrometer, model 5100.
The UV-Vis spectra of the amines into layered calcium phosphate adsorbed with copper (II) were obtained in Nujol on a Beckman DU 640 spectrophotometer.
2.4. Adsorption Isotherms
The adsorption isotherms for CuCl2 in ethanol solutions and pH = 7 were performed by using the batchwise method. For each isotherm, a series of samples containing 100 mg of CaP intercalated with m-aminobenzoic or p-aminobenzoic was shaken for 3 hs, as previously established, in a orbital bath with variable concentrations of each metal halide at a constant temperature of 298 ± 1 K. The concentration of the metal ion in solution in equilibrium with the solid phase was determined by atomic adsorption spectrometry. The amount of cations adsorbed, Nf, was determined by applying the equation: Nf = (Ni − Ns)/m, where m is the mass of the adsorbent and Ni and Ns are the initial and the equilibrium amounts of the metal in the solution phase in number of moles, respecttively. The cation/basic centers interactive process data at the solid/liquid interface were fitted to a modified Langmuir model:
 (3)
(3)
where Cs is the cation concentration (mmol·dm−3) remaining in solution after equilibrium, Nf as defined before (mmol∙g−1), Ns the amount of cation for monolayer formation (mmol∙g−1) and b a parameter associated with the equilibrium constant for reaction.
3. Results and Discussion
3.1. Characterizations
Calcium and phosphorus elemental analysis determinations for the synthesized compound gave 26.3% and 17.2%, respectively, values which are very close to the expected amounts, 26.5% and 17.1%, for the proposed formula Ca(H2PO4)2. Based on these values, the corresponding molar amounts of these elements were calculated to give phosphorus to calcium ratio equal to two. The quantity of MABA and PABA intercalated into CaP was determined through the 20.69% and 10.61% of nitrogen atoms of this guest molecule, respectively, which corresponds to 14.78 and 7.58 mmol∙g−1. The mechanism of intercalation of amine into the inorganic layers presumes the protonation of the basic centers by the acidic P-OH groups located in the inorganic matrix, in a typical acid-base interaction. On entrance of the amine into the lamellar cavity, it interacts with the monohydrogenphosphate group (O3P-OH), enabling proton transfer to the base to form the organic cation. This species is electrostatically bonded to the inorganic sheets with amine in the original free interlamellar space [13-15].
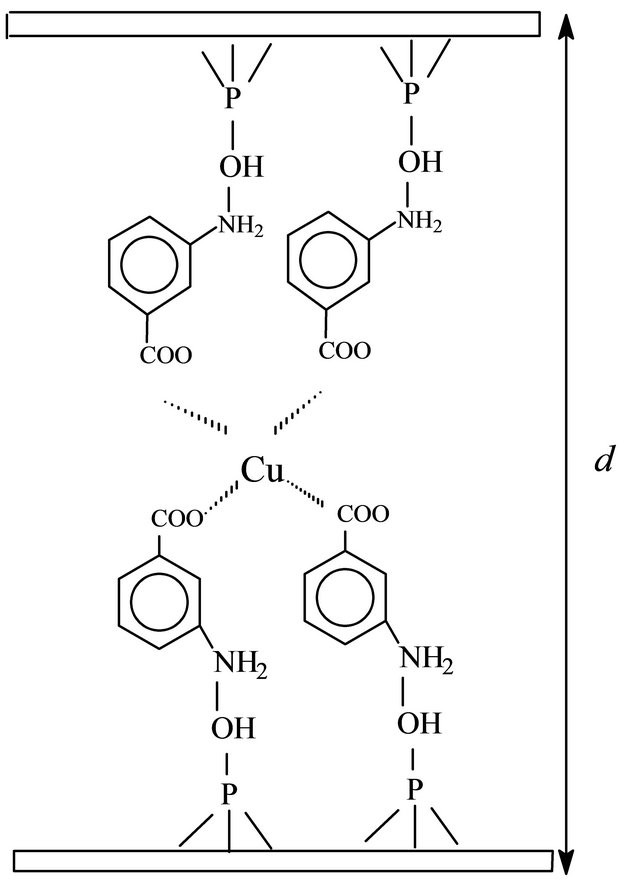
A proposed intercalation scheme for m-aminobenzoic acid and p-aminobenzoic acid inside the free inorganic host cavity is shown in Figure 1. The layered structural feature of the crystalline calcium phosphate displays an interlayer distance which depends on the size of the cation or molecule intercalated into the host. Thus as X-ray powder diffractogram is a good tool for observing the change in the interlayer distance, in order to follow the uptake process.
The X-ray diffraction powder patterns for the synthetic
 (a)
(a)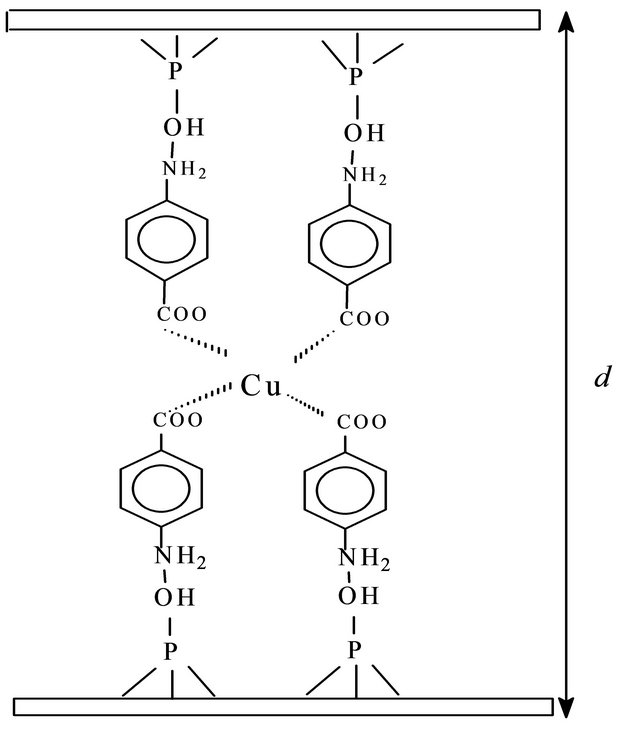 (b)
(b)
Figure 1. Schematic representation for cation adsorption on CaP intercalated with MABA (a) and PABA (b).
compound and the intercalated form with amines are shown in Figure 2. Sharp peaks indicated the crystalinity of the resulting solids, and the intense peak at 2q = 12.8˚ corresponds to an interlayer distance of 712 pm for the original lamellar compound [16,17], as shown in Figure 2(a). As expected, the interlayer distance increases when this inorganic support is suspended in the aqueous solution containing dissolved amines molecules, to give, in the present case, a sharp peak at 2q = 5.6˚ that corresponds to an interlayer distance of the 1578 pm, which is nearly double the value of the original lamellar compound, as shown in Figures 2(b) and 2(c).
Accommodation of the molecules into the free space of the cavity has been previously discussed [18,19]. In the present case, as the intercalation occurred, the entrance of m-aminobenzoic acid and p-aminobenzoic acid caused a net expansion of 866 pm. Under such conditions, the experimental values suggested that MABA and PABA are perpendicular orientation to the inorganic layer, to form a bilayer arrangement in the cavity, as considered in the Figure 1. A similar behavior was also observed in the intercalation of aromatic amines into crystalline a-titanium hydrogenphosphate [20].
The mechanism of intercalation of an aromatic amine into the inorganic layers presumes the protonation of the basic centers by the acidic P-OH groups located in the inorganic matrix, which is supported by infrared spectrophotometry. The infrared spectra are shown in Figure 3. The infrared spectra indicate that the amines interact strongly with the proton of the P-OH groups. The OH band at 1250 cm−1 in spectra except indicates partial saturation of the host. The presence of OH group on

Figure 2. X-ray diffraction patterns of calcium phosphate (a) and intercalated with MABA (b) and PABA (c).
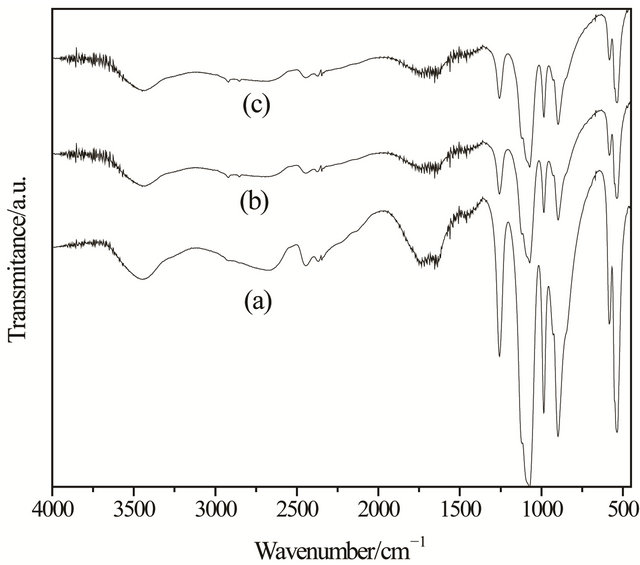
Figure 3. Infrared spectra of (a) precursor phosphate and their intercalated forms with MABA (b) and PABA (c).
phosphate in the lamellar calcium phosphate exhibits stretching at 3400 cm−1 and weak deformation at 1630 cm−1 bands in the infrared spectrum. The intense characteristic stretching bands for phosphate groups are located at 1033 and 1010 cm−1 [16]. However, after MABA and PABA intercalation the bands attributed to OH vibrations remain in the spectrum, with a decrease in intensity, mainly the deformation band as observed before for other systems [21] and no clear evidence of NH vibration bands at 3580 and 3077 cm−1 could be attributed.
The scanning electron microscopic (SEM) photograph of the calcium phosphate and also for the sample intercalated with m-aminobenzoic acid and p-aminobenzoic acid were obtained as shown in Figure 4. The crystal morphology of this compound is clearly lamellar, in good agreement with the expected structural characteristics. The morphology of intercalated m-aminobenzoic acid and p-aminobenzoic is very similar to that of the host matrix. These results are very important in order to obtain pillared compounds with a high degree of crystallinity.
The thermogravimetric curves for calcium phosphate and that containing MABA and PABA intercalated are shown in Figure 5. The intercalated compound presented a mass loss in 635 K which corresponds the chemically adsorbed amine elimination and two water molecules for to the formation of calcium pyrophosphate [22]. The curve for the intercalated compound is very similar to those observed for CaP, presenting a difference in percentages at the decomposition stage.
3.2. Adsorption of Copper
Taking into account a property associated with the intercalated crystalline lamellar compound for adsorbing
 (a)
(a) (b)
(b)
Figure 4. SEM photographs of CaP (a) and intercalated with PABA (b).

Figure 5. Thermogravimetric curves of calcium phosphate (a) and intercalated with MABA (b) and PABA (c).
metal ions from ethanol solution, the corresponding isotherm for the selected cations, Cu2+, was investigated. Initially, investigation demonstrated that the original matrix, without MABA and PABA, does not adsorb this cation. The adsorption isotherm of the CaP/MABA and CaP/PABA system is shown in Figure 6. On the contrary, as exemplified for the isotherm of copper on CaP/PABA, the adsorbed data were adjusted to the Langmuir model [23], in which is represented a Cs versus Nf plot to give the slope and from its linearized form, Cs/Nf versus Cs plot, provided the intercept, as shown in Figure 7.
The solid adsorption capacity of metal halide on CaP/MABA and CaP/PABA depends on the nature of the complex formed on the surface and the affinity of any particular attached ligand for the metal. In the present case, the maximum adsorption capacity, Nfmax, for CuCl2 on CaP/MABA and CaP/PABA was 3.92 and 1.96 mmol·g−1, respectively. By considering the equilibrium of complex formation at solid/liquid interface, the average number of ligands bonded and coordinated to the metallic ion (ñ) were determined from the plot of 1/Nf and 1/C. The average number of ligands bonded for CaP/MABA and CaP/PABA was four for metallic ion (Figure 1).
The electronic absorption spectrum of the copper (II) complexed in the intercalated compound is shown in Figure 8. The observed spectrum is very similar to that observed for square planar complexes of Cu(II) on chemically modified silica gel containing pendant chains having amine groups [24] as well as on chemically modified cellulose acetate membranes [25]. The Gaussian components of the bands with peak maxima at 550 and 640 nm correspond to a complex in D4h local crystal field symmetry and the assigned to the 2B1g ® 2Eg and 2B1g ® 2A1g, 2B2g d-d transitions [26-29], respectively.
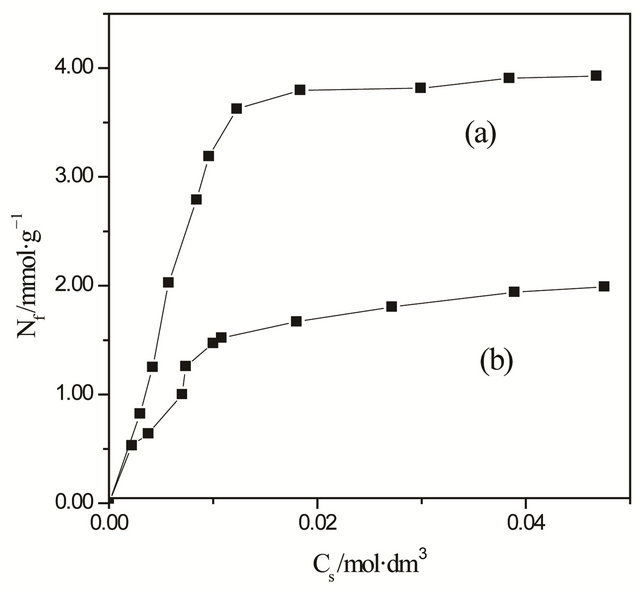
Figure 6. Isotherms of cooper adsorption on the CaP/MABA (a) and CaP/PABA (b).
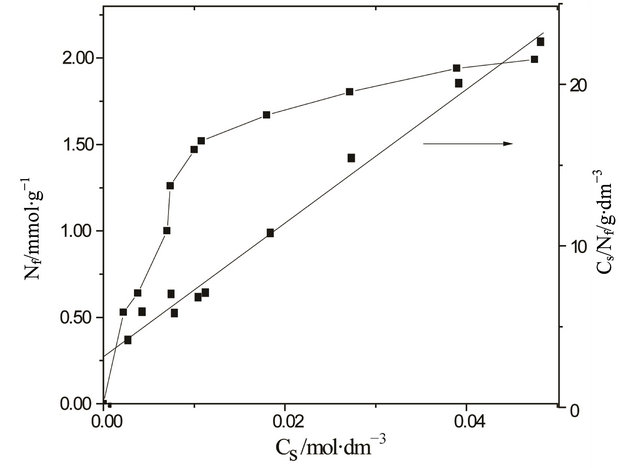
Figure 7. Isotherm for CuCl2 adsorption on CaP/PABA, represented by plot of the cation adsorbed Nf values against concentration Cs. The linearization Cs/Nf versus Cs is given by the straight line.
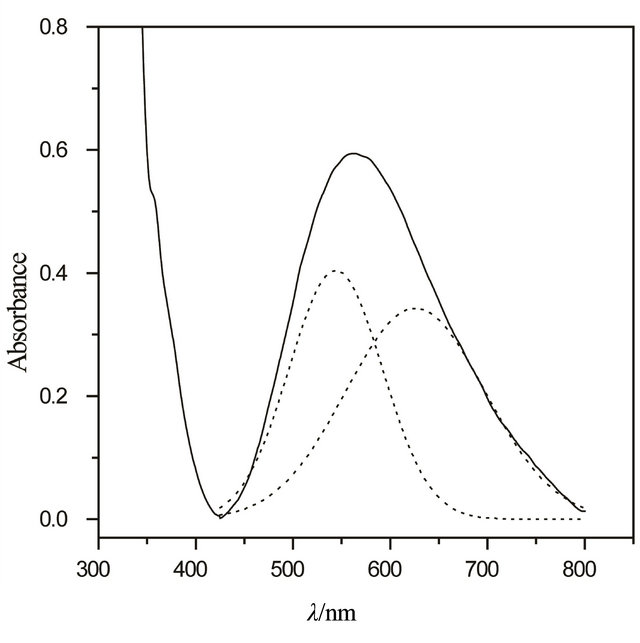
Figure 8. Electronic transition spectrum of the CaP/PABA/ Cu (II).
4. Conclusion
Elemental analysis data enabled the calculation of the molecular formula for the crystalline lamellar Ca(H2PO4)2 compound. The expansion of the basal distance was detected by X-ray diffraction patterns, whose lamellar structure was maintained after intercalation. The availability of the basic centers in the intercalated compound gives to this material the property of adsorbing cations from ethanol solution at the solid/liquid interface, as shown by the isotherm for cations. An additional advantage in the present case is the high functionalization degree exhibited by the material and consequently the large adsorption capacity, resulting from the new preparation procedure used in this work.
5. Acknowledgements
The authors are indebted to CNPq for the financial support.
REFERENCES
- M. Ogawa, “Organized Molecular Assemblies on the Surfaces in Inorganic Solids-Photofunctional InorganicOrganic Supramolecular Systems,” Annual Reports Section C: Physical Chemistry, Vol. 94, No. 1, 1998, pp. 209- 257. doi:10.1039/pc094209
- G. Alberti, T. Bein, et al., “Comprehensive Supramolecular Chemistry,” Pergamon Press, New York, 1996.
- A. Clearfield, “Inorganic Ion Exchange Material,” CRC Press Inc., Boca Raton, 1982.
- A. J. Jacobson, “Intercalation Reaction of Layered Compounds,” In: A. K. Cheetham and P. Day, Eds., Solid State Chemistry Compounds, Clarendon Press, Oxford, 1992, pp. 182-200.
- A. Lerf, “Intercalation Compounds in Layered Host Lattices: Supramolecular Chemistry in Nanodimension,” In: H. S. Nalwa, Ed., Handbook of Nanostructured Materials and Nanotechnology, Academic Press, New York, 2000, pp. 1-152. doi:10.1016/B978-012513760-7/50053-8
- M. Ogawa and K. Kuroda, “Photofunctions of Intercalation Compounds,” Chemical Reviews, Vol. 95, No. 2, 1995, pp. 399-438. doi:10.1021/cr00034a005
- A. Clearfield, “Metal-Phosphonate Chemistry,” Progress in Inorganic Chemistry, Vol. 47, No. 1, 1998, pp. 371-510. doi:10.1002/9780470166482.ch4
- A. Cabreza, M. A. G. Aranha, M. M. Lara, S. Bruque and J. Sanz, “Ab Initio Powder Structure Determination and Thermal Behavior of a New Lead (II) Phenylphosphonate, Pb(O3PC6H5),” Acta Crystallographica Section B: Structural Science, Vol. 52, 1996, pp. 982-988.
- O. G. Silva, M. G. Fonseca and L. N. H. Arakaki, “Silylated Calcium Phosphates and Their New Behavior for Copper Retention from Aqueous Solution,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 301, No. 1-3, 2007, pp. 376-381. doi:10.1016/j.colsurfa.2006.12.072
- C. F. N. Silva, A. M. Lazarin, R. L. Sernaglia and E. I. S. Andreotti, “Synthesis, Characterization and Cation Adsorption of P-Aminobenzoic Acid Intercalated on Calcium Phosphate,” Materials Research Bulletin, Vol. 47, No. 6, 2012, pp. 1539-1543. doi:10.1016/j.materresbull.2012.02.024
- D. M. Poojary, D. Grohol and A. Clearfield, “Synthesis and X-ray Powder Structure of a Novel Porous Uranyl Phenylphosphonate Containing Unidimensional Channels Flanked by Hydrophobic Regions,” Angewandte Chemie International Edition in English, Vol. 34, No. 13-14, 1995, pp. 1508-1510. doi:10.1002/anie.199515081
- J. A. A. Sales and C. Airoldi, “Calorimetric Investigation of Metal Ion Adsorption on 3-Glycidoxypropyltrimethylsiloxane + Propane-1,3-diamine Immobilized on Silica Gel,” Thermochimica Acta, Vol. 427, No. 1-2, 2005, pp. 77-83. doi:10.1016/j.tca.2004.08.015
- C. Airoldi and S. F. Oliveira, “On the Thermochemistry of Intercalation of N-Alkylamines into Alpha-Titanium Hydrogenphosphate,” Structural Chemistry, Vol. 2, No. 1, 1991, pp. 41-46. doi:10.1007/BF00673488
- C. Airoldi and S. Roca, “Calorimetric Study of Intercalation of N-Alkyldiamines into Alpha-Titanium Hydrogenphosphate,” Journal of Materials Chemistry, Vol. 6, No. 12, 1996, pp. 1963-1966. doi:10.1039/jm9960601963
- R.-Q. Zeng, X.-K. Fu and X.-B. Yang, “Intercalation of Non-Aromatic Heterocyclic Amines into Layered Zirconium Glycine-N, N-Dimethylphosphonate,” Chemical Papers, Vol. 64, No.1, 2010, pp. 118-122. doi:10.2478/s11696-009-0091-x
- H-N. Kim, S. W. Keller, T. E. Mallouk and J. Schmitt, “Characterization of Zirconiumphosphate/Polycation Thin Films Grown by Sequential Adsorption Reactions,” Chemistry of Materials, Vol. 9, No. 6, 1997, pp. 1414-1421. doi:10.1021/cm970027q
- C. Airoldi and C. B. A. Lima, “Topotactic Exchange and Intercalation of Calcium Phosphate,” Solid State Sciences, Vol. 6, No.11, 2004, pp. 1245-1250. doi:10.1016/j.solidstatesciences.2004.06.009
- P. Capková and H. Schenk, “Host-Guest Complementarity and Crystal Packing of Intercalated Layered Structures,” Journal of Inclusion Phenomena and Macrocyclic Chemistry, Vol. 47, No. 1, 2003, pp. 1-10. doi:10.1023/B:JIPH.0000003826.01697.42
- R.-Q. Zeng, X.-K. Fu and X.-B. Yang, “Intercalation of Basic Amino Acids into Layered Zirconium Proline-N-Methylphosphonate Phosphate,” Chemical Papers, Vol. 65, No. 5, 2011, pp. 676-681. doi:10.2478/s11696-011-0049-7
- L. M. Nunes and C. Airoldi, “Thermochemical Data on Intercalation of Aromatic Amines into Crystalline α-Titanium Hydrogenphosphate,” Thermochimica Acta, Vol. 435, No. 1, 2005, pp. 118-123. doi:10.1016/j.tca.2004.06.019
- C. A. Pessôa, Y. Gushikem and L. T. Kubota, “Electrochemical Study of Methylene Blue Immobilized in Zirconium Phosphate,” Electroanalysis, Vol. 9, No. 10, 1997, pp. 800-803. doi:10.1002/elan.1140091014
- Y. Murakami and H. Imai, “Synthesis and Thermal Changes of Calcium Phenylphosphate and Calcium Phenylphosphonate,” Journal of the Ceramic Society of Japan, Vol. 100, No. 1160, 1992, pp. 439-443. doi:10.2109/jcersj.100.439
- G. C. Patrucelli, M. A. Meirinho, T. R. Macedo and C. Airoldi, “Crystalline Polysilicate Magadiite with Intercalated n-Alkylmonoamine and Some Correlations Involving Thermochemical Data,” Thermochimica Acta, Vol. 450, No. 1-2, 2006, pp. 16-21. doi:10.1016/j.tca.2006.06.023
- V. N. Zaitsev, V. V. Skopenko and A. K. Trofimchuk, “The Stereochemistry of Coordination Compounds of Cobalt (II) and Copper (II) Fixed on the Surface of Modified Aerosols,” Russian Journal of Inorganic Chemistry, Vol. 29, No. 1, 1984, pp. 700-703.
- A. M. Lazarin, C. A. Borgo and Y. Gushikem, “A Platinum Electrode Coated with a Copper (II) Aminopropyl Complex-Cellulose Acetate Membrane and Its Use for Dissolved Oxygen Reduction,” Journal Membrane Science, Vol. 22, No. 1-2, 2003, pp. 175-184. doi:10.1016/S0376-7388(03)00257-6
- S. M. D. Romanowshi and A. S. Mangrich, “Synthesis and Characterization of New Copper (II) Coordination Compounds with Unsymmetrical N,O-Donor Ligands: Contributions for the Galactose Oxidase Active Site,” Quimica Nova, Vol. 24, No. 5, 2001, pp. 592-598.
- D. Zurita, C. Scheer, J. L. Pierre, and E. Saint-Aman, “Solution Studies of Copper (II) Complexes as Models for the Active Site in Galactose Oxidase,” Journal of the Chemical Society, Dalton Transactions, Vol. 1996, No. 23, 1996, pp. 4331-4336. doi:10.1039/dt9960004331
- Z. D. Matovic, V. D. Miletec, G. Samardzic, E. Pelosi, S. Lanelli and S. Trifunovic, “Square-Planar Copper (II) Complexes with Tetradentate Amido-Carboxylate Ligands. Crystal Structure of Na2[Cu(obap)]2·2H2O. Strain Analysis and Spectral Assignments of Complexes,” Inorganic Chimica Acta, Vol. 358, No. 11, 2005, pp. 3135-3144. doi:10.1016/j.ica.2005.04.025
- V. Muresan, L. S. Sbirna, S. Sbirna, C. I. Lepadatu and N. Muresan, “Transition Metal Complexes with a New Thioamide of the Dibenzofuran Series,” Acta Chimica Slovenica, Vol. 48, No. 3, 2001, pp. 439-443.
NOTES
*Corresponding author.

