Pharmacology & Pharmacy
Vol.5 No.7(2014), Article
ID:46785,11
pages
DOI:10.4236/pp.2014.57070
Dietary Magnesium Intake Related to C-Reactive Protein in Newly Diagnosed Coronary Heart Disease Patients at Middle Zone, Gaza Strip. A Hospital Based Study
Jehad H. El-Hissi1, Adham I. Ahmed2*, Ihab M. Al-Masri3, Mazen A. El-Sakka3, Atef A. Masad4, Ahmed A. Najem5
1Faculty of Medicine, Al-Azhar University, Gaza, Palestine
2Al-Aqsa Martyr’s Hospital, Ministry of Health, Dier El-Balah, Palestine
3Faculty of Pharmacy, Al-Azhar University, Gaza, Palestine
4Faculty of Science, The Islamic University, Gaza, Palestine
5Faculty of Intermediate Studies, University of Palestine, Gaza, Palestine
Email: *Rn.Adham@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 February 2014; revised 15 May 2014; 4 June 2014
ABSTRACT
Coronary Heart Disease (CHD) is a highly prevalent disease all over the world. Magnesium (Mg) plays a role in CHD but it is still unclear. C-Reactive Protein (CRP) is an inflammatory marker which may rise in CHD. Aim of study: To assess the impact of dietary Mg intake and its level in relation to CRP among newly diagnosed CHD at middle zone of Gaza Strip. Methodology: Patients (n = 140) with confirmed CHD, 50 ± 10 years, presented in the cardiac care unit at Aqsa Martyr’s Hospital between 1 April 2012 and 30 December 2012, were enrolled in this cross-sectional study after taking consent. ECG, clinical status, and cardiac markers were used to confirm diagnosis by cardiologist. Food frequency questionnaire was used to assess Mg intake and calcium intake in addition to measurement of its level in serum. CRP latex slide was used for measurement of CRP. Results: Mg intake and serum Mg were inversely associated with risk of CHD. Mean of serum Mg among cases (1.80) was lower than controls (2.41) (P = 0.001). Percent of positive CRP was higher in cases (32.9%) than controls (12.9%) (P = 0.005). Mean of serum Mg was (1.96 ± 0.47) for positive CRP which was lower than the mean of serum Mg (2.15 ± 0.44) for negative CRP. Conclusion: Newly diagnosed patients with CHD have a positive CRP, low serum and low Mg intake, and low serum Mg was associated with elevated CRP.
Keywords:Coronary Heart Disease, C-Reactive Protein, Magnesium, Dietary Behavior, Life Style

1. Introduction
Cardiovascular disease (CVD) is considered as the first leading cause of death in the world [1] . In 2008, according to the WHO, 17 million died due to CVDs, therefore it is considered as the top-leading cause of death among non-communicable diseases worldwide [2] .
Mg has a very important role in proper functioning of the human body, especially the cardiovascular system. Mg deficiency in the body is associated with different risk factors for CVDs and atherogenesis such as increasing oxidative stress, cytokine synthesis, nitrogen oxides and mediators of inflammation and adhesion molecules on microvascular endothelial cells [3] . A possible role of Mg in the etiology of ischemic heart disease is still not sufficiently clear and it is likely that several mechanisms are involved. Other studies demonstrate that increased intake of dietary Mg may lower blood triglyceride level and increase high-density lipoprotein [4] . Numerous studies pointed to an inverse relationship between Mg intake through diet and the incidence of CVD [5] [6] .
We hypothesized that this association might be explained, in part, by the anti-inflammatory properties of Mg [3] . Few studies revealed that individuals with intakes below the recommended dietary allowances are more likely to have elevated level of an inflammatory marker called CRP, which may contribute to CVD risk [7] .
To clarify these uncertainties, we examined the association between dietary and plasma Mg and risk of CHD among newly diagnosed CHD patients in the middle zone of the Gaza Strip. In addition to evaluate whether the association between Mg and CHD could be explained fully through traditional cardiovascular pathways, we used specific inclusion criteria.
2. Subjects and Methods
2.1. Study Design
A case-control study was conducted in Al-Aqsa martyrs’ hospital, Dier Al-Balah. The type of this study design is used widely, often in epidemiology. It is a type of observational study in which two existing groups differing in outcome are identified and compared on the basis of some supposed causal attribute.
2.2. Study Population and Sampling
The target population was hospitalized patients in cardiac care unit with CHD and patients and their fellows without any suspicion of CHD at other departments who are 50 ± 10 years. They were recruited according to the inclusion criteria in the study after getting their consent. A Purposeful, non-random sample included 140 participants divided into two groups according to the eligibility criteria. Participants were allocated into: Group A (n = 70) patients with newly diagnosed with CHD; Group B (n = 70) patients without any suspicion of CHD and their fellows.
Selection Criteria
Subjects who were eligible to participate in the study were those who met the following criteria.
2.3. Inclusion Criteria
• 2.3.1. Cases
• Patients diagnosed with acute CHD who are 50 ± 10 years.
• Evident ischemic ECG abnormalities.
• 2.3.2. Controls
• Patients without CHD.
• Patients who 50 ± 10 years.
• No any history of health problem related to the heart.
• Normal ECG.
• 2.3.3. Exclusion Criteria (for Cases and Controls)
• Those diagnosed with congestive heart failure, gastrointestinal disease, liver or kidney diseases, diabetes mellitus, hyperthyroidism or hyperparathyroidism.
• Patients post recent acute infection, recent trauma or surgical intervention.
• Those who were taking insulin preparations, vitamin-mineral supplements, hormone replacement therapy, or intake of any medication may cause hypomagnesaemia or hypomagnesaemia and also pregnant or lactating women.
2.4. End-Point Ascertainment and Definitions
CHD occurs when a substance called plaque builds up in coronary arteries that lead to arteriosclerosis which is leading-cause of myocardial ischemia and myocardial infarction [8] . Participants included were divided into cases and controls, and defined by cardiologists depending on clinical status, ECG, and biochemical investigations included cardiac markers. Normal Mg level is (1.8 - 2.3) mg/dL. Hypomagnesaemia was considered when serum Mg level below (<1.8) mg/dL. It was categorized into three levels including mild (1.6 - 1.8 mg/dL), moderate (1.3 - 1.5 mg/dL), and sever (<1.2 mg/dL) [9] . Serum CRP level was determined using slide agglutination test and reference value was (up to 6 mg/L) [10] .
2.5. Assessment of Dietary Magnesium Intake
Data on usual diet was ascertained by using the food frequency questionnaires. For each food item, each participant was asked how often, on average, he/she had consumed a specified portion size over the past month. We made a list of the richest food of Mg available depending on the United States Agriculture Department (USDA) food database [11] . The t test was used to assess the difference of servings consumed among participants.
2.6. Measurement of Biochemical Variables
Serum Mg and Calcium were detected by colorimetric methods in the serum of cases and controls by spectrophotometer machine with a kit from (DiaSys Diagnostic Systems GmbH—Germany) [23] , and also serum CRP was measured by the latex agglutination machine with a kit from (Inmesco GmbH—Germany) CRP latex slide test (serology kit) is used for the semi-quantitative measurement [24] .
2.7. Assessment of Coronary Heart Disease
Cardiologists were selected the new cases with CHD and confirmed the diagnosis of CHD on the basis of the criteria of the WHO (symptoms plus either diagnostic ECG changes or elevated levels of cardiac enzymes) [25] .
2.8. Assessment of Other Factors
Lifestyle and dietary data were derived from the questionnaire administered. Intake of saturated-fat food was assessed by a short fat questionnaire designed by Australian Journal of Public Health [12] . Average of Mg rich food intake was computed with the use of a semi quantitative food-frequency questionnaire. The questionnaires were validated by a specialists and face validity was used for other measurements.
2.9. Statistical Analysis
Statistical Package for the Social Sciences (SPSS) program version 19 [13] was used for data analysis which includes cross tabulations of the results and Chi square test for categorical data, odds ratio and the confidence interval was the statistical tool used to assess the association between family members, income per month, and family history, smoking status, exposure to others smoke, and clinical manifestations of hypomagnesaemia. Ttest for quantitative data analysis was used to compare means of serum Mg, Calcium and CRP levels between cases and controls. P value was used for measuring statistical difference between discrete variables.
2.10. Ethical Consideration
We get all of required ethical approvals including dean of postgraduate studies & research affairs, dean of college of pharmacy, Ministry of Health and informed consent of the participants.
3. Results
3.1. Baseline Characteristics of Participants
Regarding the gender, it was noticed that the percent of male gender (62.1%) was higher than females (37.9%) among all the participants and among cases and controls. However, no statistically significant difference was found between cases and controls regarding the gender of participants (P = 0.222).
The mean ages of study population were 51.69 ± 5.7 years and 49.86 ± 6.8 years for case and controls, respectively. The mean age of male participants was 50.36 (±6.5) years and for females was 51.45 (±6.0) years. Participants’ age was divided into three categories one of them is less than 45 years old, from 45 to 55 years and more than 55 years. The majority of participants’ age was from 45 - 55 years (48.6%) as shown in Table1
Education level of participants was categorized into illiterate, basic, secondary, “diploma or bachelor” and “master or PhD”. According to the results obtained in Table 2, the percent of participants who have “diploma or bachelor” degree was the highest among participants (41.4%). On the other hand, the percent of cases having
*Statistically significant.
Table 2. Life style of participants.
*Statistically significant.
“diploma or bachelor” degree (27.1%) was lower than controls (57.7%) and this difference was found to be statistically significant (P = 0.001, Table 1).
The employment status was divided into 3 groups included employed, unemployed and self-employed participants. According to the results shown in Table 2, most of the participants were unemployed (42.9%). Among cases, the percent of unemployment (50.0%) was higher than controls (35.7%), however, the difference was not statistically significant (P = 0.16, Table 1).
Family income data was analyzed in a way to determine income per capita per day. Income per capita was grouped into two categories according to the poverty line endorsed by The World Bank: those with income of less than 2 American dollars ($) per day and those with 2 $ per day or more [14] .
Table 1 shows that the percentage of participants with two or more dollars per day (65.7%) is higher than other category (34.3%). In cases, the percent of participants with two or more dollars per day (55.7%) was higher than those with daily income less than two dollars (44.3%). Nevertheless, the percent of cases with less than two dollars daily (44.3%) were higher than controls (24.3%) and this difference was statistically significant (P = 0.01).
The number of family members was categorized into three groups: less than five, from five to ten and more than ten members as shown in Table1 The highest group of family members was between five to ten (68.6%).
The percentages of cases living with family of 5 - 10 members (71.4%) and more than 10 members (10.0%) were higher than controls (65.7% and 1.4%, respectively) and this difference was statistically significant (P = 0.024).
As shown in Table 2, the percent of smokers among cases (55.7%) was higher than controls (31.4%) and this difference was statistically significant (P = 0.004).
The percent of participants exposed to other’s smoke (30.7%) was found to be lower than non-exposed persons (69.3%). However, Table 4 shows that the percent of exposure to other’s smoke among cases (42.9%) was higher than controls (31.4%) and this difference was statistically significant (P = 0.002).
The data collected in Table 2 show that the majority of participants (43.6%) were consuming moderately saturated fat-rich food. The percent of participants who consumed saturated fat-rich food in “low to moderate” level was (35.7%), whereas those consumed “Moderate to High” level was (20.7%). In this study, the percent of cases who consumed “Moderate to High” saturated fat (28.6%) were higher than controls (12.9%). On the other hand, the percent of controls who consumed “Low to Moderate” saturated fat were (42.9%) higher than cases (28.6%) and these differences were found to be statistically significant (P = 0.045).
Hypertension
The collected data in Table 3 show that the majority of participants had a family history of high blood pressure “Hypertension” (58.2%). However, family history of hypertension among cases (72.9%) was much higher than among controls (44.3%) and this disparity was statistically very significant (P = 0.001).
Table 3. Family history of participants.
*Statistically significant.
High blood cholesterol
The results shown in Table 3 demonstrate that there is a statistically significance difference between cases and controls regarding the family history of high blood cholesterol (P = 0.003) as the number of cases with family history of high cholesterol (21) was higher than controls (7).
Heart diseases
The percent of cases with family history of heart disease (48.6%) was statistically much higher than controls (20%) (P = 0.000) as shown in Table3
Obesity
A statistically significant difference was found between cases and controls regarding family history of obesity (P = 0.049) as shown in Table3 The percent among cases (41.4%) is higher than controls (25.7%).
Diabetes mellitus
It’s noticed that the percent of cases with family history of diabetes mellitus (48.6%) is higher than controls (30.0%) and this disparity was found to be statistically significant (P = 0.024, Table 3).
Other diseases
The results of this study revealed that there is no statistically significance difference between cases and controls regarding other diseases such as cancer, gastrointestinal disease, allergies, asthma and psychological disorders (P > 0.05, Table 3).
3.2. Clinical Cutoff Points for Magnesium
3.2.1. Magnesium-Rich Food Intake
Independent t-test was used to compare the means of the number of servings of different types of food rich with Mg. In our study, it was found that cases consumed more servings of cabbage, while controls consumed more servings of molokhia, okra, potatoes and tomatoes. However, there was no statistically significant difference between cases and controls regarding the servings of different vegetables consumed by participants (P > 0.05). Also, the results were revealed that there is no significant difference between cases and controls regarding the means of servings of dates, raisins, banana and grapes consumed by participants (P > 0.05). On the other hand, there was a statistically significant difference between the means of servings consumed of melons and apples.
Regarding to grains, nuts, seeds and legumes the results shown that the number of servings of whole meal bread and cooked bulgur were higher in control than cases. However, only the bulgur intake difference was statistically significant (P < 0.05). On the other hand, the number of servings of white bread was higher in cases than controls, however this difference was not statistically significant (P = 0.761). Regarding the intake of nuts and seeds, it was found that controls consumed more servings of watermelon seeds, pumpkin seeds, sesame seeds; sunflower seeds; cashew; pistachio; peanut; chocolate; tahini in comparison to cases and these differences were found to be statistically significant except for pistachio, peanut and tahini (P > 0.05). On the other hand, the intake of almond was slightly higher in cases than controls but without statistical significance (P = 0.543). It was found that cases consumed more servings of legumes (beans and lentils) than controls; however, this difference was not statistically significant (P > 0.05). There was no significant difference between cases and controls regarding the consumption of coffee (P = 0.828). The mean of servings of cocoa consumed by controls was higher than cases that reached to be statistically significant (P = 0.002). The mean of servings of whole milk consumed by controls was higher than cases and this difference was statistically significant (P = 0.092).
3.2.2. Serum Magnesium
The participants were divided into three groups according to their serum Mg level: low (<1.8) mg/dL, normal (1.8 - 2.3) mg/dL and high (>2.3) mg/dL. The results obtained in revealed 48.6% of cases have low serum Mg level when compared with 4.3% of controls with statistical significant difference (P = 0.001, Table 4). The mean of Mg level among the cases (1.80) was found to be lower than the controls (2.41) and these differences were of high statistical significance (P = 0.001) as shown in Figure 1.
Figure 2 shows the total participants with serum Mg level low (<1.8) mg/dL were 37, hypomagnesmia was categorized into three levels including mild (1.6 - 1.8 mg/dL) represented 45.9%, moderate (1.3 - 1.5 mg/dL) represented 45.9%, and sever (<1.2 mg/dL) represented 8.10%.
3.3. Association between Serum Magnesium and C-Reactive Protein
Table 5 revealed that 22.9% of participants have positive CRP while, 77.1% of them have negative CRP; the
*Statistically significant (P-value < 0.05).
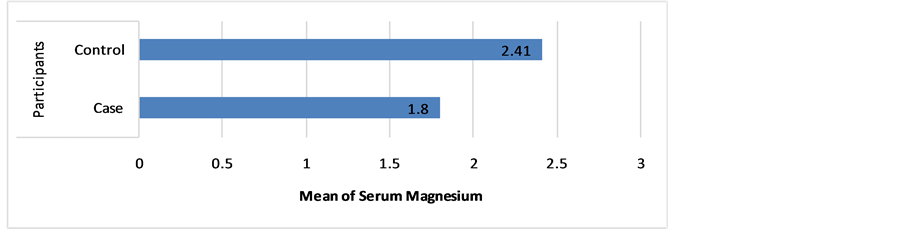
Figure 1. The mean of serum Mg level among participants.
mean of serum Mg was (1.96 ± 0.47) for positive CRP participants which was lower than the mean of serum Mg (2.15 ± 0.44) for negative CRP participants, with statistical significance (P = 0.03).
3.4. Clinical Cutoff Points for C-Reactive Protein
The data collected showed that 32.9% of cases have positive CRP results when compared with 12.9% of controls and this difference was statistically significant (P = 0.005) as shown in Table6
4. Discussion
In this retrospective case control study, we found a modest inverse association between dietary Mg intake and serum Mg in relation to risk of CHD that did reach statistical significance. Percent of cases with low Serum Mg was (48.6%) which is higher than controls (4.3%) and the mean of serum Mg of participants among cases was (1.80 mg/dL) lower than controls (2.41 mg/dL) (P = 0.001). The result of present study agrees with the study of (Altura et al., 2007) that concluded Mg deficiency in the body is associated with different risk factors for CVDs and atherogenesis such as increasing oxidative stress, cytokine synthesis, nitrogen oxides and mediators of inflammation and adhesion molecules on microvascular endothelial cells.
Authors [15] sought to investigate the relationship between dietary Mg intake and mortality due to CVD in population-based sample of Asian adults. Reported findings are based on dietary Mg intake in 58,615 healthy Japanese aged 40 - 79 years, in Japan Collaborative Cohort (JACC) Study. Dietary Mg intake was assessed by a validated food frequency questionnaire administered between 1988 and 1990. During the median 14.7-year follow-up, authors was documented 2690 deaths due to CVD, comprising 1227 deaths from strokes and 557 deaths of CHD. They was concluded that dietary Mg intake was inversely associated with mortality from hemorrhagic stroke in men and with mortality from total and ischemic strokes, CHD, heart failure and total CVD especially in women.

Figure 2. Levels of hypomagnesaemia among participants.
Table 5. Independent t test comparing the means of Mg and CRP.
The deficiency of Mg may be caused by eating cooked and processed food which is considered as a common dietary habit among people of Gaza Strip, this proved by Swaminathan [16] who reported cooking, especially boiling of Mg-rich food results in significant loss of Mg and also [17] who reported that the intake of processed and cooked is highly prevalent in many populations with low Mg intake. Low serum Mg reflects low dietary intake of Mg which was more evident among cases, depending on the literature review there is association between low Mg intake and risk factors for CVD, atherogenesis, increasing mediators of inflammation, and adhesion molecules on microvascular endothelial cells including coronary arteries which provides blood supply to the heart. Furthermore, low dietary Mg intake is associated with increased triglyceride and low-density lipoprotein (LDL) cholesterol levels which are considered as strong indicator for development of CHD.
We found a direct strong relationship between CRP and the development of CHD, in which the percent of cases with positive CRP was (32.9%) higher than controls (12.9%). There was statistically significant relationship that may explained by a role of inflammatory process during the development of CHD. The result of present study agrees with the study of [18] that concluded Mg depletion to be associated with elevated hs-CRP levels, suggesting that hypomagnesaemia and low-grade inflammation are interactive risk factors. In addition, it agrees with the cohort study conducted by [19] which included 1653 adults and revealed a relationship between lower Mg and fiber intakes and increased level of hs-CRP.
We found an inverse relationship between Mg and CRP, in which 22.9% of participants have positive CRP while, 77.1% of them have negative CRP; the mean of serum Mg was (1.96 ± 0.47) for positive CRP participants which was lower than the mean of serum Mg (2.15 ± 0.44) for negative CRP participants, without statistical significance. The result of present study agrees with the study of Guerrero-Romero & Rodríguez-Morán [20] that concluded low serum Mg levels are independently related to elevated CRP concentration. In addition it agrees with the context of a review article titled with “Mg: Novel Applications in CVD—A Review of the Literature” which authored by [21] focused on the relationship between Mg and CRP and concluded that hypomagnesaemia results in increased CRP and platelet dysfunction, which can lead to thrombosis.
We found a direct strong relationship between Serum Ca and development of CHD, which the percent of cases with low Serum Ca was (7.1%) higher than controls (1.4%) with statistical significance (P = 0.011). The result of present study contradicts with (Lu, et al., 2012) who concluded that the results of their study were inconsistent and the pooled data do not strongly support a significant effect of greater dietary Ca intake on the risk of CAD. On the other hand, many studies revealed association between the effects of hypomagnesmia over Ca in the body that leads to hypocalcaemia. Studies showed that a reduction in extracellular Mg concentration stimulated the secretion of parathyroid hormone (PTH) in the absence of changes in Ca concentration [22] .
5. Conclusion
In conclusion, we found that decreased Mg intake was possibly associated with a higher risk of CHD among adults. Whereas the causal effect of Mg is not certain. In spite of that there is sufficient reason to encourage a balanced diet rich in Mg sources, such as whole grains, nuts, fruits and vegetables which are protective against the risk of CHD.
Acknowledgements
We are very much thankful to the deanship of postgraduate studies & research affairs of Al-Azhar University, deanship of college of pharmacy, ministry of health, Al-Aqsa martyrs’ hospital, special thanks to my uncle in law Mr. Tayseer Ahmed, my friend Mr. Mohammed Najem “Lab. technician” and to the team of Intensive Cardiac Care Unit for their cooperation and assistance in data collection. Finally, I would thank all the patients and volunteers who participated in this study. To all of these individuals I owe many thanks for their insights and unlimited support.
References
- Bernard, J.G., Sliwa, K., Mayosi, B.M. and Yusuf, S. (2010) Theepidemic of Cardiovascular Disease in the Developing World: Global Implications. European Heart Journal, 31, 642-648. http://dx.doi.org/10.1093/eurheartj/ehq030
- WHO (2012) Global Health Observatory (GHO) Deaths from CVD Situation.http://www.who.int/gho/ncd/mortality_morbidity/cvd/en/index.html
- Altura, B.M. and Altura, B.T. (2007) Magnesium: Forgotten Mineral in Cardiovascular Biology and Atherogenesis. In: Nishizawa, Y., Morii, H., Durlach, J., Eds., New Perspectives in Magnesium Research: Nutrition and Health, Springer-Verlag, London, 239-260. http://dx.doi.org/10.1007/978-1-84628-483-0_19
- Singh, R.B., Rastogi, S.S., Sharma, V.K., Saharia, R.B. and Kulsretha, S.K. (1990) Can Dietary Magnesium Modulate Lipoprotein Metabolism? Magnesium and Trace Elements, 9, 255-264.
- Al-Delaimy, W.K., Rimm, E.B. and Willett, W.C. (2004) Magnesium Intake and Risk of Coronary Heart Disease among Men. Journal of the American College of Nutrition, 23, 63-70. http://dx.doi.org/10.1080/07315724.2004.10719344
- Bo, S. and Pisu, E. (2008) Role of Dietary Magnesium in Cardiovascular Disease Prevention, Insulin Sensitivity and Diabetes. Current Opinion in Lipidology, 19, 50-56. http://dx.doi.org/10.1097/MOL.0b013e3282f33ccc
- King, D.E., Mainous 3rd, A.G., Geesey, M.E. and Woolson, R.F. (2005) Dietary Magnesium and C-Reactive Protein Levels. Journal of the American College of Nutrition, 24, 166-171. http://dx.doi.org/10.1080/07315724.2005.10719461
- CDC (2012) About Coronary Artery Disease, USA, 2013. http://www.cdc.gov/heartdisease/coronary_ad.htm
- Hale, A. and Hovey, M. (2013) Fluid, Electrolyte, and Acid-Base Imbalances: Content Review plus Practice Questions (DavisPlus). Davis, Philadelphia, 300-303.
- Kao, P.C., Shiesh, S.C. and Wu, T.J. (2006) Serum C-Reactive Protein as a Marker for Wellness Assessment. Annals of Clinical and Laboratory Science, 36, 163-169.
- USDA (2013) Agricultural Research Service. Nutrient Intakes Percent of Population 2 Years Old and over with Adequate Intakes Based on Average Requirement. Community Nutrition Mapping Project.http://www.ars.usda.gov/ba/bhnrc/ndl
- Dobson, A.J., Blijlevens, R., Alexander, H.M., et al. (1993) Short Fat Questionnaire: A Self-Administered Measure of Fat-Intake Behaviour. Australian and New Zealand Journal of Public Health, 17, 144-149.
- IBM (2013) SPSS Statistics, USA, 2013.http://www-01.ibm.com/software/analytics/spss/products/statistics/
- The World Bank (2013) Poverty Headcount Ratio at $2 a Day (PPP) (% of Population), USA, 2005.http://data.worldbank.org/indicator/SI.POV.2DAY
- Zhang, W., Iso, H., Ohira, T., Date, C. and Tamakoshi, A. (JACC Study Group) (2012) Associations of Dietary Magnesium Intake with Mortality from Cardiovascular Disease: The JACC Study. Atherosclerosis, 221, 587-595.
- Swaminathan, R. (2003) Magnesium Metabolism and Its Disorders. Clinical Biochemistry Review, 24, 47-66.
- Bo, S., Durazzo, M. and Guidi, S. (2006) Dietary Magnesium and Fiber Intake, Inflammatoryand Metabolic Parameters in Middle-Aged Subjects from a Population-Based Cohort. The American Journal of Clinical Nutrition, 84, 1062-1069.
- Rodríguez-Morán, M. and Guerrero-Romero, F. (2008) Serum Magnesium and C-Reactive Protein Levels. Archives of Disease in Childhood, 93, 676-680.
- Bo, S., Durazzo, M., Guidi, S., Carello, M., Sacerdote, C., Silli, B., Rosato, R., Cassader, M., Gentile, L. and Pagano, G. (2006) Dietary Magnesium and Fiber Intakes and Inflammatory and Metabolic Indicators in Middleaged Subjects from a Population-Based Cohort. The American Journal of Clinical Nutrition, 84, 1062-1069.
- Romero, G. and Morán, R. (2002) Relationship between Serum Magnesium Levels and C-Reactive Protein Concentration, in Non-Diabetic, Non-Hypertensive Obese Subjects. International Journal of Obesity, 26, 469-474.
- Kupetsky-Rincon, E. and Uitto, J. (2012) Magnesium: Novel Applications in Cardiovascular Disease—A Review of the Literature. Annals of Nutrition and Metabolism, 61, 102-110.
- Agus, Z.S. (1999) Hypomagnesemia. Journal of the American Society of Nephrology, 10, 1616-1622.http://www.ncbi.nlm.nih.gov/pubmed/10405219
- Anonymous (2014) Compact Version—DiaSys Diagnostic Systems GmbH, Germany.http://www.diasys-diagnostics.com/fileadmin/downloads/Print_Material/Katalog/DiaSys_Shortcatalogue_2014_ENG.pdf
- Anonymous (2014) CRP-Latex Agglutination Test—Inmesco GmbH, Germany.http://www.inmesco.de/pdf/customer-support/rapid-latex-slide-test/inmesco-crp-latex-agglutination-test.pdf
- Anonymous (1979) Nomenclature and Criteria for Diagnosis of Ischemic Heart Disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization Task Force on Standardization of Clinical Nomenclature. Circulation, 59, 607-609.
List of Abbreviations
CHD: Coronary Heart Disease Mg: Magnesium ECG: Electrocardiography CRPC: Reactive Protein CVD: Cardiovascular disease
NOTES

*Corresponding author.


