International Journal of Otolaryngology and Head & Neck Surgery
Vol.1 No.1(2012), Article ID:18967,6 pages DOI:10.4236/ijohns.2012.11001
Role of Allergy in Sinonasal Polyposis
1Department of ENT & Head & Neck Surgery, Kasturba Medical College, Mangalore, India
2Department of ENT & Head & Neck Surgery, Dr. Pinnamaneni Siddhartha Institute of Medical Sciences & Research Foundation, Chinoutpally, Gannavaram, India
3Department Of Community Medicine, Dr. Pinnamaneni Siddhartha Institute of Medical Sciences & Research Foundation, Chinoutpally, Gannavaram, India
Email: vennelakalyan@gmail.com
Received March 12, 2012; revised April 17, 2012; accepted May 3, 2012
Keywords: Allergy; Nasal Polyps; Serum IgE; Absolute Eosinophil Count; Tissue Eosinophilia
ABSTRACT
Objectives: The exact role of allergy in sinonasal polyposis is not yet clearly elucidated and is undoubtedly a controversial subject. The study focussed on the association of allergy in nasal polyposis. We aim to determine whether there was a correlation between Serum IgE, absolute eosinophil count, eosinophilic inflammation, and nasal polyps. Methods: A study group of fifty two consecutive patients of nasal polyposis were evaluated prospectively and compared with 26 controls who underwent septoplasty and mimimal FESS or Endoscopic Sphenopalatine artery ligation. Patients were evaluated for presence of allergy with regard to absolute eosinophil count, total serum IGE and tissue eosinophilia and correlations were established. All patients were categorized based on histological evidence of tissue eosinophilia. Results: The incidence of asthma was 5.8% and positive history of allergy was obtained in 40.4% of patients in study group and 7.7% of patients in control group. The statistically significant association was not seen with absolute eosinophil count, Serum IgE and tissue eosinophilia. Tissue eosinophilia was observed in more number of patients with nasal polyposis compared to controls. So clinical significance might be established. Conclusions: Allergy is parameter that is frequently associated with this disease, irrespective of the type of polyp and the age at presentation. Unrecognized and untreated allergy adds to the morbidity of the disease and generally results in poor treatment outcome.
1. Introduction
Nasal polyposis is the the most incapacitating illness of the nasal cavity and paranasal sinuses, of unknown etiology, not mediated by immunoglobulin E (IgE) [1]. Eosinophils seem to play an important role and the condition leads to formation of edematous polyps from sinuses to nasal cavity [1]. It is a chronic inflammatory dis- order of the upper respiratory tract that affects 1% to 4% of the human population. Polyposis is found in wide variety of diseases like cystic fibrosis, chronic rhinosinusitis and aspirin hypersensitivity and has various histological components determined by the basic disease state. The European Position Paper on rhinosinusitis and Nasal Polyps considered nasal polypsis as a type of chronic rhinosinusitis, but recommended excluding from the classification other conditions such as cystic fibrosis, primary ciliary dyskinesia syndrome, and the various forms of autoimmune vasculitis, such as Churg-Strauss syndrome. [1,2] One of the chronic rhinosinusitis subtypes is eosinophilic chronic hyperplastic rhinosinusitis. (ECHRS) [3]. Histologically, ECHRS is manifested by accumulation of eosinophils ,activated mast cells, fibroblasts,and goblet cells. It is frequently associated with the presence of asthma, and this relationship may imply a shared pathophysiology. As with asthma,the presence of activated eosinophils in the sinus tissue is the histologic hallmark of ECHRS [3]. We aimed to study the allergy association in sinonasal polyposis by correlation between certain allergic parameters, absolute eosinophil count, serum IgE, tissue eosinophilia, and nasal polyps. In the clinical assessment of nasal polyps it is considered important to ascertain the extent of the disease for which Lund Mackay Endoscopic staging was used.
2. Patients and Methods
2.1. Patients
A consecutive series of 52 patients with nasal polyposis were evaluated prospectively and compared with 26 patients with no evidence of polyps at the department of Otorhinolaryngology, Kasturba Medical College, Mangalore, India from March 2008 to August 2010. The proceeduers were performed in accordance with the ethical guide lines and had approval by Ethics Committee, Manipal University.
2.2. Methods
Besides a thorough otorhinolaryngological history and examination, all patients were evaluated for presence of allergy by absolute eosinophil count (AEC) and specific titres of Total Serum IgE was estimated from venous blood by the automated chemiluminiscence system. (Chiron Diagnostics) [4]. Endoscopic examination for measurement of poyp size was performed at each visit. Polyp size was rated on a Lund and Mackay fourpoint scale [5,6] (0, absent polyps; 2, polyps in middle meatus only; 3, polyps beyond middle meatus; 4, polyps obstructing the nose). All the patients undervent Functional Endoscopic Sinus Surgery(FESS) and the polyps were removed with microdebrider during FESS.
In all cases, surgically excised polyps were individually fixated, sectioned and histologically evaluated with H&E staining and with Periodic Acid Schiff stain in suspected cases of Allergic fungal sinusitis. The biopsies were taken from the normal nasal mucosa in controls. In patients with radiological evidence of fungal infection like speckled calcification or hyperdense shadows and presence of thick viscid secretions in the the sinuses, fungal smear and culture was attempted. The average number of eosinophils per field was quantified in absolute terms as percentage of the total number of inflammatory cells. Grade 1— <5 per high power field, Grade 2— >5 per high power field [1].
Grade 2 is considered for presence of tissue eosinophilia.
Categorisation in to study groups was based on histological evidence of tissue eosinophilia [3].
1) Eosinophilic chronic hyperplastic rhinosinusitis:Patients with polyps and sinus tissue eosinophilia;
2) Noneosinophilic chronic hyperplastic rhinosinusitis: patients with polyps but without sinus tissue eosinophilia;
3) Patients without polyps but with sinus tissue eosinophilia: Eosinophilic chronic rhinosinusitis;
4) Patients without polyps and without sinus tissue eosinophilia: Non eosinophilic chronic rhinosinusitis.
Statistical analysis was performed using chisquare test, Student’s t Test, Spearmann’s rank correlation and Z Mann Whitney U test. Statistical package version 11.5 was used.
3. Results
Positive history of asthma was identified in 3 of 52 cases (5.8%) which was relatively less and not statistically significant. None of the patients had history of asprin sensitivity or history of congenital respiratory diseases. Allergic symptoms were noticed in 40.4% of patients in study group and only in 7.7% of patients in control group. The estimation of serum eosinophils in peripheral blood was done in 47 cases, 46.8% cases and 28.6% controls showed elevated titres and the association was not significant. Serum levels of IgE were frequently elevated in patients with clinical history of allergy. Figure 1 illustrates the correlation between Serum IgE and clinical history of allergy. About 90% of the study population with history of allergy reported elevated titres and the association was statistically significant. (p = 0.044). Our study observed that 50% of controls i.e., in patients without polyps and with history of allergy, showed elevated serum IgE titres which was not statistically significant. Even levels were increased in study and control groups without history of allergy.
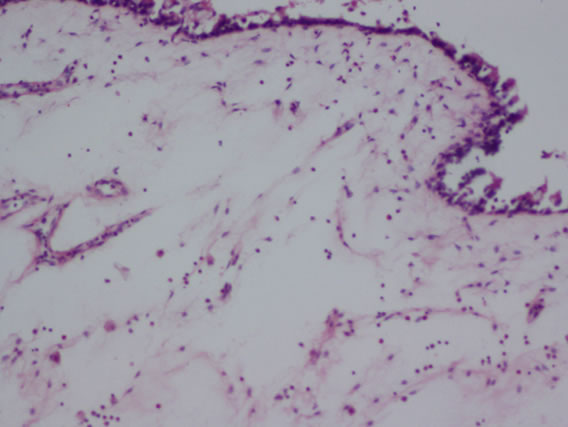
The following histological features (Figure 2) were noted during histopathological examination of polyp specimen
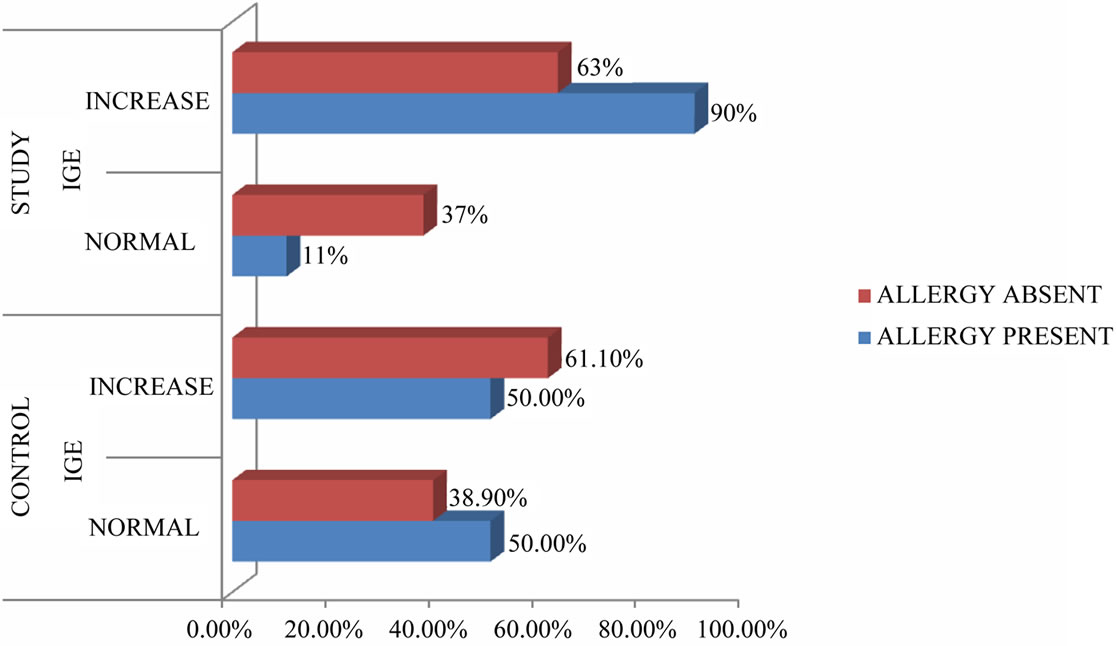
Figure 1. Illustrates the correlation of IgE and allergy.
 (a)
(a)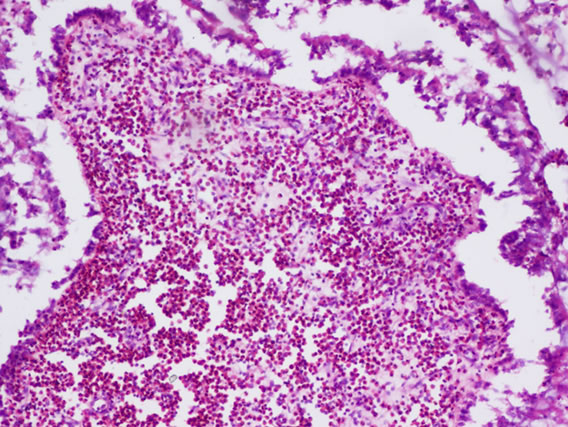 (b)
(b) (c)
(c)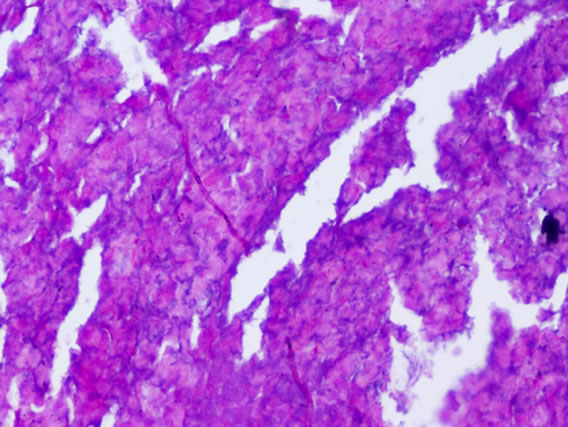 (d)
(d)
Figure 2. (a) Cilated epithelium and oedematous stroma; (b) Eosinophilic infiltration; (c) Squamous metaplasia; (d) Shows fungal hyphae in allergic fungal sinusitis.
and the amount of tissue eosinophilia was estimated.
2 cases of allergic fungal sinusitis were associated with polyps. Figure 3 demonstrated the tissue eosinophilia in our patients from which the following results were derived. About 53.8% of the patients with nasal polyps showed tissue eosinophilia (eosinophil score 2). This percentage was slightly higher than controls (50%) but no significant association was reported .Only clinical significance might be established.
Categorisation of groups was done accordingly. About 53.8% patients had polyps and sinus tissue eosinophilia— Eosinophilic hyperplastic rhinosinusitis, 46.2% patients have noneosinophilic chronic hyperplastic rhinosinusitis, 50% patients have eosinophilic chronic rhinosinusitis, 50% patients have noneosinophilic chronic rhinosinusitis. Table 1 depicted the correlation between tissue eosinophilia and severity of the disease assessed by endoscopic grading. The current study observed that (66.7%) of patients with tissue eosinophilia had total polyp scores ranging from 0 - 2 and 47.4% of patients with tissue eosinophilia had polyp scores between 2 - 4 hnd the correlation was not significant in study population.
Table 2 depicted the statistical correlations between allergic parameters in study and control population which were proved insignificant. About 46.15% of patients with tissue eosinophilia and 42.30% patients without tissue eosinophilia had increased serum IgE levels in study
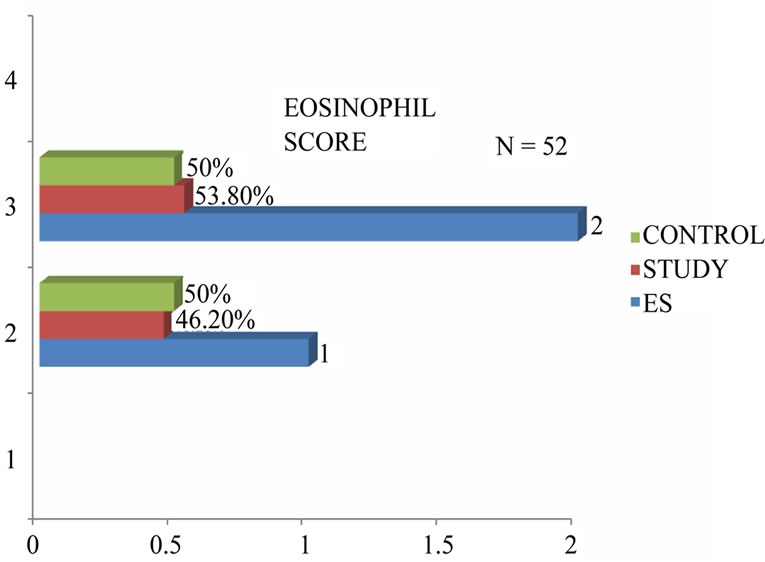
Figure 3. Grading of tissue eosinophilia.
population. MeanValue in study group is 743.90 with tissue eosinophilia, and 998.83 without tissue eosinopilia and the association was not significant. (p = 0.082). In control group, patients with tissue eosinophilia had high increase of serum IgE levels with mean value 1494.62. In study group, patients with tissue eosinophilia had mean absolute eosinophil count 699.91.and 405.47 without tissue eosinophilia. In control group, levels were low comparatively.

Table 1. Correlation between tissue eosinophilia and endoscopic score.

Table 2. Correlation between allergic parameters.
4. Discussion
A history suggestive of a positive reaction to an environmental allergen was obtained in 40.4% of our patients, the positives all had perennial allergy. The most common offenders were dust. Asthma was present in only 5.8% of the patients. The recent studies [7] quoted 73.8% of perennial allergy and 55.4% of seasonal allergy. One of the problems in trying to correlate allergic status with the history is the difficulty in clarifying what constitutes allergic symptoms. Most of the nasal mucosal reactions are stereotyped, irrespective of the pathology. Only history of paroxysmal sneezing, pruritus and mucoid rhinorrhoea, generally relates to allergy. Even more difficult is to elicit a positive history of asthma, especially in the milder forms of the disease. Most patients attribute their chest tightness and discomfort to be due to a chronically blocked nose, most often ignoring these symptoms. Even leading question may fail to elicit a positive answer. This probably explains the lower Incidence of asthma in our study. The notable exception in this study was a total absence of a history of aspirin sensitivity. The literature [8-10] reported that these disorders were found in nonatopic population ,which accounted for only a minority in our study.
The single limiting factor in using the absolute eosinophil count as a criteria to diagnose allergy is the high percentage of false positives and false negatives. Parasitic infections and the tropical eosinophilia syndrome are important differential diagnosis in this part of the world that increase the level of eosinophils. Several authors in their studies [11] stressed that eosinophils are not pathognomic of allergy, sometimes their presence may even indicate a non allergic origin. As a single test, only elevated eosinophil count has little differentiating value. It cannot differentiate well between allergic and intrinsic rhinitis. This is especially so when other parameters of allergy are negative and only eosinophils are increased in the blood.
Although serological testing has proved useful in patients with allergic symptoms, its role is not well defined. Titres are reported to be variably elevated in patients with clinical history of allergy. The literature [12] has documented that that a change in the amount of CD4 and CD8 lymphocytes and an increased level of local IgE contribute to nasal polyposis, but the results should be confirmed in more extensive studies including cytokine analyses [12]. According to some authors the levels of IgE vary widely among patients with atopic diseases and normal people and level is age dependant [13]. To definitely rule out atopy, estimation of specific IgE by RAST or skin tests for allergy are required.
There is no unanimity among authors in method of quantification of eosinophils. Some authors used quantification in absolute values and some others in relative values. In the present study, no statistical association was appreciated between tissue eosinophilia and nasal polyposis but clinical significance was established. More over, incidence of tissue eosinophilia was noticed in patients without polyps. There is geographical variation with regard to the incidence of allergy that might have influenced results in current study. However the literature [1,14,15] supports tissue eosinophilia as striking feature of nasal polyposis.
It is obvious from other studies [1,16-19] that the percentage of allergy positive patients vary widely. Clinical significance was reported with respect to allergic parameters as majority of the study population showed positive parameters for allergy. The study lacks specificity as a more definite parameter for diagnosis of allergy like Skin prick test, Radio allegro sorbent test or RAST could not be carried out. A limitation has to be clarified here. The first is about the controls we selected. Patients with rhino sinusitis with absence of polyps were selected. Epidemiologically, allergic rhinitis is more common than non allergic type, accounting for the increased values of allergic parameters in the controls in our study.
Previous authors fail to stress the fact that a significant number of nasal polyp patients have tested positive for allergic parameters as in our study. Glance at the statistics of our patients was instructive. The effect of therapeutic intervention for allergy on the recurrence of polyps could not be evaluated in our study as the duration of the study was limited and the follow up was only 14 months, an insufficient period for most recurrences to manifest, as allergy is a chronic disease with exacerbations and remissions system. We feel from the light of all the evidence, that it is indeed worthwhile screening for allergy in patients with polyps from the limited parameters used, a pathogenetic mechanism cannot be established for allergy. Yet, there is a clinical significant association. Ignoring this association, can lead to an unfavorable outcome as has been evidenced in our study.
5. Conclusion
Nasal polyposis is a disease of multifactorial etiology. The exact initiating or trigger event at the cellular level has not yet been identified. Much of the research studies have concentrated on isolating factors that are manifest clinically,which may predispose to this condition. Quite often even the isolation of such a factor does not necessarily imply pathogenic association, as they may be only present coincidentally. Our study supports the hypothesis of scarcely relevant role of allergy in pathogenesis of nasal polyps. Allergy is a parameter that has to be confirmed by haematological, cytological and immunological methods. Unrecognized and untreated allergy adds to the morbidity of the disease and generally results in poor outcome.
REFERENCES
- L. Garin, M. Armengot, J. R. Alba and C. Carda, “Correlations between Clinical and Histological Aspects of Nasal Polyposis,” Acta Otorhinolaryngol, Vol. 59, No. 7, 2008, pp. 315-320.
- W. Fokkens, V. J. Lund, C. Bachert, P. Clement, P. Hellings, M. Holmstrom, “EAACI Position Paper on Rhinosinusitis,” Rhinology, Vol. 87, 2005.
- S. E. Kountakis, P. Arango, D. Bradley, Z. K. Wade and L. Borish, “Molecular and Cellular Staging for the Severity of Chronic Rhinosinusitis,” Laryngoscope, Vol. 114, No. 11, pp. 1895-1905.
- M. Klink, et al., “Problems in Defining Normal Limits for Serum IgE,” Journal of Allergy and Clinical Immunology, Vol. 85, No. 2, 1990, pp. 440-444. doi:10.1016/0091-6749(90)90153-U
- C. Hopkins, J. P. Browne, R. Slack, V. Lund and P. Browne, “The Lund-Mackay Staging System for Chronic Rhinosinusitis: How Is It Used & What Does It Predict?” Otolaryngology—Head and Neck Surgery, Vol. 137, No. 4, 2007, pp. 555-561. doi:10.1016/j.otohns.2007.02.004
- V. J. Lund and I. S. Mackay, “Staging in Rhinosinusitis,” Rhinology, Vol. 31, 1993, 183-184
- S. M. Houser and K. J. Keen, “The Role of Allergy and Smokings in Chronic Rhinosinusitis and Polyposis,” Laryngoscope, Vol. 118, No. 9, 2008, pp. 1521-1527.
- J. Caplin, T. T. Haynes and J. Spahn, “Are Nasal Polyps an Allergic Phenomenon?” Annals of Allergy, Asthma & Immunology, Vol. 29, 1971, pp. 631-634.
- A. B. D. Lee, D. Lowe and A. Swantson, et al., “Clinical Profile and Recurrence of Nasal Polyps,” The Journal of Laryngology and Otology, Vol. 98, 1984, pp. 783-793. doi:10.1017/S0022215100147462
- B. P. Farrell, “Endoscopic Sinus Surgery: Sinonasal Polyposis and Allergy,” ENT Journal, Vol. 72, 1993, p. 544.
- K. G. Marshall, E. L. Attia and D. Danoff, “Disorders of the Nose and Paranasal Sinuses: Diagnosis and Management,” PSG Publishers, Masachusetts, 2008, pp. 223-227.
- S. Nikalagh, M. G. Boroujerdinia, N. Saki, M. R. Soltan Moradi and F. Rahim, “Immunological Factors in Patients with Chronic Polypoid Sinusitis,” Nigerian Medical Journal, Vol. 19, No. 3, 2010, pp. 316-319.
- StenDreborg, “Allergy Diagnosis,” In: N. Mygind and R. M. Naclerio, Eds., Allergic and Non-Allergic Rhinitis, 1st Edition, W.B. Saunders, Philadelphia, pp. 82-92.
- R. Jankowski, F. Bouchoua, L. Coffinet and J. M. Vignaud, “Clinical Factors Influencing the Eosinophil Infiltration of Nasal Polyps,” Rhinology, Vol. 40, No. 4, 2002, pp. 173-178.
- H. B. Hellquist, “Nasal Polyps Update,” Histopathology, Vol. 17, No. 5, 1996, pp. 237-242.
- V. L. Schram and M. Z. Effron, “Nasal Polyps in Children,” Laryngoscope, Vol. 90, 1980, pp. 1488-1495.
- A. Busuttil, H. Chandrachud, A. I. G. Kerr, et al., “Simple Nasal Polyps and Allergic Manifestations,” Journal of Laryngology and Otology, Vol. 92, No. 6, 1978, pp. 477- 487. doi:10.1017/S0022215100085649
- J. Staikūniene, S. Vaitkus, L. M. Japertiene and S. Ryskiene, “Association of Chronic Rhinosinusitis with Nasal Polyps and Asthma: Clinical and Radiological Features, Allergy and Inflammation Markers,” Medicine (Kaunas), Vol. 44, No. 4, 2008, pp. 257-265.
- M. N. Editorial, “Nasal Polyposis,” The Journal of Allergy and Clinical Immunology, Vol. 86, No. 6, 1990, pp. 827-829.

