Open Journal of Applied Sciences
Vol.07 No.01(2017), Article ID:73474,14 pages
10.4236/ojapps.2017.71001
Numerical Analysis of the Magnetization Behavior in Magnetic Resonance Imaging in the Presence of Multiple Chemical Exchange Pools
Kenya Murase
Department of Medical Physics and Engineering, Division of Medical Technology and Science, Faculty of Health Science, Graduate School of Medicine, Osaka University, Osaka, Japan

Copyright © 2017 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 16, 2016; Accepted: January 10, 2017; Published: January 13, 2017
ABSTRACT
The purpose of this study was to demonstrate a simple and fast method for solving the time-dependent Bloch-McConnell equations describing the behavior of magnetization in magnetic resonance imaging (MRI) in the presence of multiple chemical exchange pools. First, the time-dependent Bloch- McConnell equations were reduced to a homogeneous linear differential equation, and then a simple equation was derived to solve it using a matrix operation and Kronecker tensor product. From these solutions, the longitudinal relaxation rate (R1ρ) and transverse relaxation rate in the rotating frame (R2ρ) and Z-spectra were obtained. As illustrative examples, the numerical solutions for linear and star-type three-pool chemical exchange models and linear, star- type, and kite-type four-pool chemical exchange models were presented. The effects of saturation time (ST) and radiofrequency irradiation power (ω1) on the chemical exchange saturation transfer (CEST) effect in these models were also investigated. Although R1ρ and R2ρ were not affected by the ST, the CEST effect observed in the Z-spectra increased and saturated with increasing ST. When ω1 was varied, the CEST effect increased with increasing ω1 in R1ρ, R2ρ, and Z-spectra. When ω1 was large, however, the spillover effect due to the direct saturation of bulk water protons also increased, suggesting that these parameters must be determined in consideration of both the CEST and spillover effects. Our method will be useful for analyzing the complex CEST contrast mechanism and for investigating the optimal conditions for CEST MRI in the presence of multiple chemical exchange pools.
Keywords:
Bloch-McConnell Equations, Multiple Chemical Exchange Pools, Chemical Exchange Saturation Transfer (CEST) Magnetic Resonance Imaging (MRI), Amide Proton Transfer (APT) MRI, Numerical Analysis

1. Introduction
Chemical exchange saturation transfer (CEST) is a novel contrast mechanism for magnetic resonance imaging (MRI) [1] and has been increasingly used to detect dilute proteins via the interaction between labile solute protons and bulk water protons [2] [3] [4] . Moreover, amide proton transfer (APT) imaging, a particular type of CEST MRI that specifically probes labile amide protons of endogenous mobile proteins and peptides in tissue, has been explored for imaging diseases such as acute stroke and tumor, and is currently under intensive evaluation for clinical translation [5] [6] . Furthermore, various CEST agents have been actively developed to detect the parameters that reflect tissue pH and molecular environment and/or to enhance the CEST effect [7] . However, CEST or APT MRI contrast mechanism is complex, depending on not only the concentration of CEST agents or amide protons, exchange and relaxation properties, but also varying with experimental conditions such as magnetic field strength and radiofrequency (RF) power [8] . When there are multiple exchangeable sites within a single CEST system, the CEST contrast mechanism becomes even more complex [9] . Thus, in analyzing the complex CEST contrast mechanism and for investigating the optimal study conditions, numerical simulations are useful and effective [10] [11] . To perform extensive numerical simulations for CEST or APT MRI, it will be necessary to develop a simple and fast method for obtaining the numerical solutions to the time-dependent Bloch-McConnell equations.
The purpose of this study was to present a simple and fast method for solving the time-dependent Bloch-McConnell equations for analyzing the behavior of magnetization in MRI in the presence of multiple chemical exchange pools.
2. Materials and Methods
2.1. Bloch-McConnell Equations in a Two-Pool Chemical Exchange Model
Figure 1 illustrates a two-pool chemical exchange model in which pool A re- presents the bulk water pool. The time-dependent Bloch-McConnell equations in the two-pool exchange model for CEST MRI are given by [10] [11]
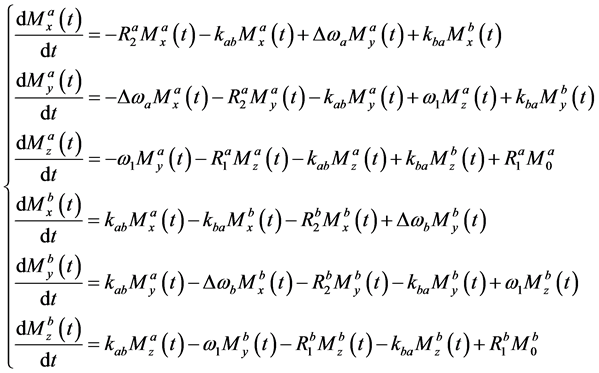 , (1)
, (1)
Figure 1. Illustration of a two-pool chemical exchange model. kab and kba represent the exchange rates from pool A to pool B and from pool B to pool A, respectively.
where superscripts a and b show the parameters in pool A and pool B, respectively. For example, 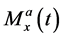 ,
,  , and
, and 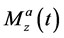 denote the x, y, and z components of the magnetization in the rotating frame in pool A at time t, respectively.
denote the x, y, and z components of the magnetization in the rotating frame in pool A at time t, respectively.  and
and 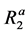 denote the longitudinal and transverse relaxation rates, i.e., the reciprocals of the longitudinal
denote the longitudinal and transverse relaxation rates, i.e., the reciprocals of the longitudinal  and transverse relaxation times
and transverse relaxation times  in pool A, respectively.
in pool A, respectively. 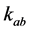 denotes the exchange rate from spins in pool A to those in pool B, whereas
denotes the exchange rate from spins in pool A to those in pool B, whereas 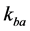 denotes that from spins in pool B to those in pool A.
denotes that from spins in pool B to those in pool A. 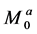 and
and 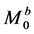 denote the thermal equilibrium z magnetizations in pool A and pool B, respectively.
denote the thermal equilibrium z magnetizations in pool A and pool B, respectively. 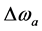 and
and 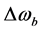 are given by
are given by 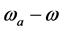 and
and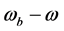 , respectively, where
, respectively, where  and
and 

The differential equations given by Equation (1) can be combined into one vector equation (homogeneous linear differential equation) [11] :

where

and

where T in Equation (3) denotes the matrix transpose. According to Koss et al. [12] , the matrix A can be given by

where E is the evolution matrix [12] and C is the constant-term matrix. Furthermore, E is given by

In the case of A given by Equation (4), R is reduced to

where

and

K in Equation (6) is given by

where I is a 3-by-3 identity matrix and 

The solution of Equation (2) can be given by [11]

where t represents the so-called saturation time and 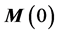


2.2. Linear Three-Pool Chemical Exchange Model
Figure 2(a) illustrates a linear three-pool chemical exchange model in which pool A represents the bulk water pool. In this case, R and K are given by [12]

and


Figure 2. Illustration of three-pool chemical exchange models. (a) and (b) show linear and star-type three-pool chemical exchange models, respectively. As in the case of kab, kac, kca, kbc, and kcb represent the exchange rates from pool A to pool C, from pool C to pool A, from pool B to pool C, and from pool C to pool B, respectively.
respectively. 
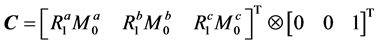
2.3. Triangular Three-Pool Chemical Exchange Model
Figure 2(b) illustrates a triangular three-pool chemical exchange model in which pool A represents the bulk water pool. In this case, K is given by [12]

2.4. Linear Four-Pool Chemical Exchange Model
Figure 3(a) illustrates a linear four-pool chemical exchange model in which pool A represents the bulk water pool. In this case, R and K are given by [12]

and

respectively. Rd in Equation (17) is given by Equation (8) in which the subscript a and superscript a are replaced by d. C is given by

2.5. Star-Type Four-Pool Chemical Exchange Model
Figure 3(b) illustrates a star-type four-pool chemical exchange model in which pool A represents the bulk water pool. In this case, K is given by [12] .


Figure 3. Illustration of four-pool chemical exchange models. (a), (b), and (c) show linear, star-type, and kite-type four-pool chemical exchange models, respectively. As in the case of kab, kad, kda, kcd, and kdc represent the exchange rates from pool A to pool D, from pool D to pool A, from pool C to pool D, and from pool D to pool C, respectively.

2.6. Kite-Type Four-Pool Chemical Exchange Model
Figure 3(c) illustrates a kite-type four-pool chemical exchange model in which pool A represents the bulk water pool. Although there are no chemical exchanges between pool C and pool D in the star-type four-pool chemical exchange model (Figure 3(b)), there are exchanges between them in the kite-type model (Figure 3(c)). In this case, K is given by [12]

It should be noted that mass balance imposes the following relationship between the exchange rates 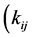


and

2.7. Calculation of R1ρ, R2ρ, and Z-Spectra
The longitudinal relaxation rate in the rotating frame 

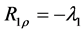
The transverse relaxation rate in the rotating frame 

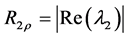
The CEST effect has usually been analyzed using the so-called Z-spectrum [11] . Thus, we calculated the Z-spectrum by using the following equation:

where 


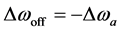

2.8. Simulation Studies
Because we have already treated a two-pool chemical exchange model in our previous paper [11] , we treated three-pool and four-pool chemical exchange models in this study.
First, we considered the three-pool exchange model consisting of bulk water (pool A) and two labile proton pools (pool B and pool C) as illustrative examples. In this case, we assumed that the longitudinal 








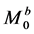
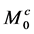
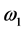
For four-pool exchange models, we simulated one nuclear Overhauser effect site (pool D) in addition to the above bulk water (pool A) and two labile proton pools (pool B and pool C). We assumed that the longitudinal 





Calculations were performed using MATLAB® (The MathWorks Inc., Natick, MA, USA) on an Intel CoreTM i7-4790 CPU (3.6 GHz) with 8-GB RAM. The matrix exponential and Kronecker tensor product were calculated using the MATLAB® functions “expm” and “kron”, respectively.
3. Results
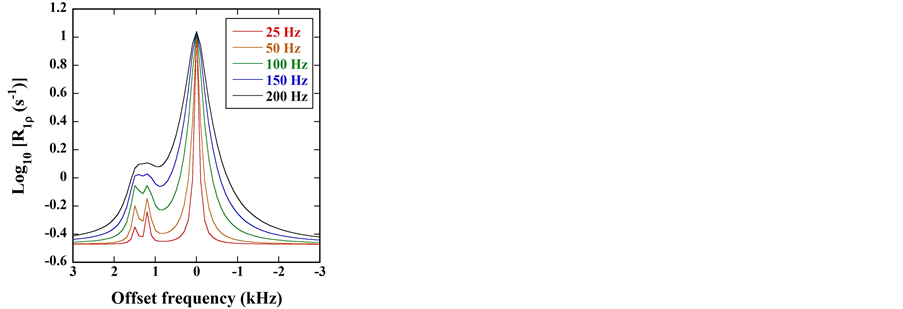
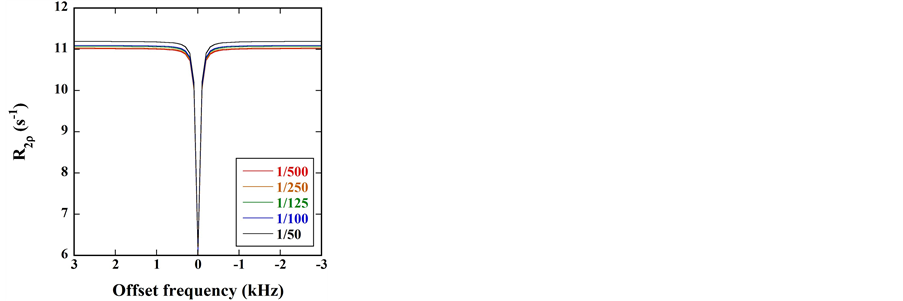
Figure 4 shows the 






Figure 5 shows the 

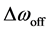


Figure 6 shows the 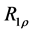

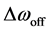





Figure 7 shows the 





Figure 4. (a)

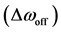

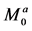

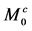




Figure 5. (a)

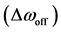







Figure 6. (a)

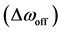




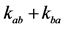


and −1043.7 Hz (−3.5 ppm) in the 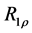

Figure 8 shows the 

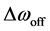






Figure 7. (a)





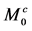


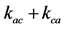

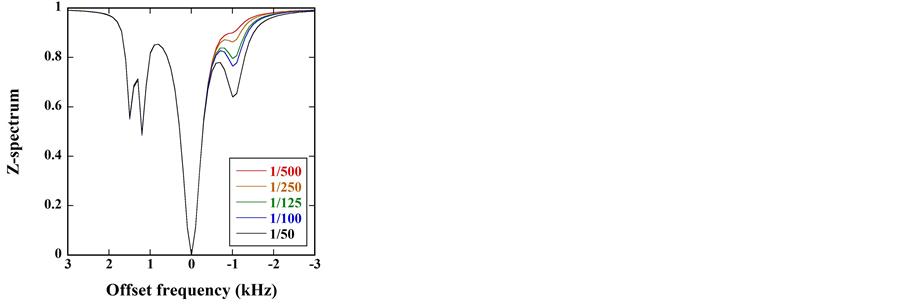
Figure 8. (a)

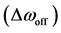








Figure 9 shows the 
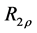


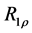

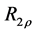

4. Discussion
In this study, we developed a simple equation for solving the time-dependent

Figure 9. (a)











Bloch-McConnell equations in the presence of multiple chemical exchange pools by combining our previous method [11] and the approach presented by Koss et al. [12] . As described in our previous paper [11] , the numerical solutions obtained by our method agreed with the analytical solutions given by Mulkern and Williams [14] . We also compared the solutions obtained by our method for the two-pool exchange model with those obtained using a fourth/fifth-order Runge- Kutta-Fehlberg (RKF) algorithm and found that there was a good agreement between them [11] . These results appear to indicate the validity of our method. Furthermore, the computation time was considerably reduced when using our method (by a factor of approximately 2500 compared to the case when using the RKF algorithm [11] ). Thus, our method can be included in the nonlinear least- squares fitting routine to calculate parameters such as the exchange rate or lifetime of CEST agents [10] .
For calculating the solutions to the time-dependent Bloch-McConnell equations using Equation (12), most computation time is spent calculating the eigenvectors and eigenvalues of matrix A. However, it is necessary to carry out this calculation only once regardless of t in Equation (12). As previously described, in this study, the matrix exponential was computed using the MATLAB® function “expm”, in which a scaling and squaring algorithm with Pade approximation [15] has been used.
In our previous study [11] , we used the two-pool exchange model for CEST or APT MRI as an illustrative example. As pointed out by Woessner et al. [10] , paramagnetic CEST agents often have more than one type of exchangeable proton. For such cases, it is necessary to expand the Bloch-McConnell equations to multi-pool exchange models. Recently, Koss et al. [12] presented a generalized expression for the evolution matrix in the Bloch-McConnell equations in the presence of multiple chemical exchange sites. In their method, the Kronecker tensor product was used [12] . As shown in this study, our method could be easily extended to multi-pool chemical exchange models by modifying matrix A in Equation (12) with use of their approach.
As previously described, 



The spectral dependence of CEST is determined by sweeping the RF irradiation frequency while monitoring the water resonance [1] . As previously described, the CEST effect has usually been analyzed using the so-called Z-spectrum [11] . The Z-spectrum is obtained by plotting the z component of the magnetization of pool A, i.e., bulk water proton 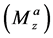


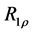
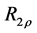


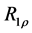





As shown in Figure 6(b) and Figure 8(b), the 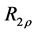




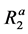





Recently, Koss et al. [12] presented analytical expressions for 
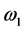
5. Conclusion
We presented a simple and fast numerical method for solving the time-dependent Bloch-McConnell equations in the presence of multiple chemical exchange pools by combining our previous method [11] and the approach presented by Koss et al. [12] . The present method will be useful for analyzing the complex CEST contrast mechanism and for investigating the optimal conditions for CEST MRI in the presence of multiple chemical exchange pools.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Challenging Exploratory Research (Grant No. 25670532) from the Japan Society for the Promotion of Science.
Cite this paper
Murase, K. (2017) Numerical Analysis of the Magnetization Behavior in Magnetic Resonance Imaging in the Presence of Multiple Chemical Exchange Pools. Open Journal of Applied Sciences, 7, 1-14. http://dx.doi.org/10.4236/ojapps.2017.71001
References
- 1. Ward, K., Aletras, A. and Balaban, R. (2000) A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). Journal of Magnetic Resonance, 143, 79-87. https://doi.org/10.1006/jmre.1999.1956
- 2. Goffeney, N., Bulte, J.W.M., Duyn, J., Bryant, L.H. and van Zijl, P.C.M. (2001) Sensitive NMR Detection of Cationic-Polymer-Based Gene Delivery Systems Using Saturation Transfer via Proton Exchange. Journal of American Chemical Society, 123, 8628-8629. https://doi.org/10.1021/ja0158455
- 3. Aime, S., Barge, A., Castelli, D.D., Fedeli, F., Mortillaro, A., Nielsen, F.U. and Terreno, E. (2002) Paramagnetic Lanthanide (III) Complexes as pH-Sensitive Chemical Exchange Saturation Transfer (CEST) Contrast Agents for MRI Applications. Magnetic Resonance in Medicine, 47, 639-648. https://doi.org/10.1002/mrm.10106
- 4. Snoussi, K., Bulte, J.W.M., Gueron, M. and van Zijl, P.C.M. (2003) Sensitive CEST Agents Based on Nucleic Acid Imino Proton Exchange: Detection of Poly(rU) and of a Dendrimer-Poly(rU) Model for Nucleic Acid Delivery and Pharmacology. Magnetic Resonance in Medicine, 49, 998-1005. https://doi.org/10.1002/mrm.10463
- 5. Zhou, J., Lal, B., Wilson, D.A., Laterra, J. and van Zijl, P.C.M. (2003) Amide Proton Transfer (APT) Contrast for Imaging of Brain Tumors. Magnetic Resonance in Medicine, 50, 1120-1126. https://doi.org/10.1002/mrm.10651
- 6. Sun, P.Z., Murata, Y., Lu, J., Wang, X., Lo, E.H. and Sorensen, A.G. (2008) Relaxation-Compensated Fast Multislice Amide Proton Transfer (APT) Imaging of Acute Ischemic Stroke. Magnetic Resonance in Medicine, 59, 1175-1182. https://doi.org/10.1002/mrm.21591
- 7. Maruyama, S., Ueda, J., Kimura, A. and Murase, K. (2016) Development and Characterization of Novel LipoCEST Agents Based on Thermosensitive Liposomes. Magnetic Resonance in Medical Sciences, 15, 324-334. https://doi.org/10.2463/mrms.mp.2015-0039
- 8. Sun, P.Z. (2010) Simultaneous Determination of Labile Proton Concentration and Exchange Rate Utilizing Optimal RF Power: Radio Frequency Power (RFP) Dependence of Chemical Exchange Saturation Transfer (CEST) MRI. Journal of Magnetic Resonance, 202, 155-161. https://doi.org/10.1016/j.jmr.2009.10.012
- 9. Sun, P.Z. (2010) Simplified and Scalable Numerical Solution for Describing Multi-Pool Chemical Exchange Saturation Transfer (CEST) MRI Contrast. Journal of Magnetic Resonance, 205, 235-241. https://doi.org/10.1016/j.jmr.2010.05.004
- 10. Woessner, D.E., Zhang, S., Merritt, M.E. and Sherry, A.D. (2005) Numerical Solution of the Bloch Equations Provides Insights into the Optimum Design of PARACEST Agents for MRI. Magnetic Resonance in Medicine, 53, 790-799. https://doi.org/10.1002/mrm.20408
- 11. Murase, K. and Tanki, N. (2011) Numerical Solutions to the Time-Dependent Bloch Equations Revisited. Magnetic Resonance Imaging, 29, 126-131. https://doi.org/10.1016/j.mri.2010.07.003
- 12. Koss, H., Rance, M. and Palmer, A.G. (2017) General Expression for R_1ρ Relaxation for N-Site Chemical Exchange and the Special Case of Linear Chains. Journal of Magnetic Resonance, 274, 36-45. https://doi.org/10.1016/j.jmr.2016.10.015
- 13. Murase, K. (2013) A Theoretical and Numerical Consideration of the Longitudinal and Transverse Relaxations in the Rotating Frame. Magnetic Resonance Imaging, 31, 1544-1558. https://doi.org/10.1016/j.mri.2013.07.004
- 14. Mulkern, R.V. and Williams, M.L. (1993) The General Solution to the Bloch Equation with Constant RF and Relaxation Terms: Application to Saturation and Slice Selection. Medical Physics, 20, 5-13. https://doi.org/10.1118/1.597063
- 15. Higham, N.J. (2005) The Scaling and Squaring Method for the Matrix Exponential Revisited. SIAM Journal on Matrix Analysis and Applications, 26, 1179-1193. https://doi.org/10.1137/04061101X